摘要/Abstract
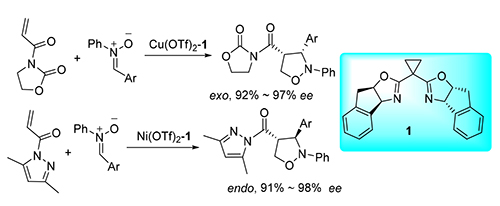
研究了Inda-BOX 1金属络合物为催化剂的两种缺电子烯烃与C,N-二芳基硝酮的不对称环加成反应.研究结果表明,在催化剂存在的条件下,两反应均可得到4-位取代产物.其中N-丙烯酰基噁唑烷酮为亲偶极体时,反应的exo/endo选择性达到100/0,且exo产物的ee值高达97%;N-丙烯酰基-3,5-二甲基吡唑为亲偶极体时,exo/endo选择性为0/100,且endo产物的ee值高达98%.对亲偶极体及硝酮结构与反应选择性之间的关系进行了初步探讨.
关键词: 不对称催化, 1,3-偶极环加成反应, 手性双噁唑啉, N-α,β-不饱和酰基化合物, C,N-二芳基硝酮, 对映体选择性
Asymmetric cycloaddition reactions catalyzed by Inda-BOX 1 metal complex between two kinds of electron-withdrawing alkenes and C,N-diarylnitrone have been studied respectively. Results showed that the 4-substituted products were mainly obtained in both reactions under the best conditions. When N-acryloyl oxazolidinone was used as dipolarophile, the exo/endo selectivity of the reaction was 100/0, and the ee of the exo product was as high as 97%. When N-acryloyl-3,5-dimethyl pyrazole was used as dipolarophile, the selectivity of exo/endo was 0/100, and the ee of endo product was up to 98%. The relationship of the dipolarophile, the structure of nitrone and the selectivity of the reaction was discussed.
Key words: asymmetric catalysis, 1,3-dipolar cycloaddition reaction, chiral dioxazoline, N-α,β-unsaturated acyl compounds, C,N-diarylnitrone, enantioselectivity
PDF全文下载地址:
点我下载PDF
