摘要/Abstract
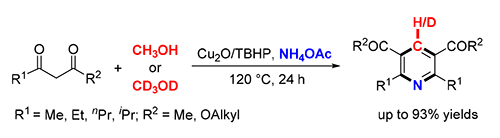
首次发展了一个铜催化的1,3-二羰基化合物、甲醇和乙酸铵的串联氧化形式环加成反应,能以中等到优异的产率合成一系列对称的2,3,5,6-四取代吡啶.反应中,甲醇既可作为反应溶剂,又可作为产物中吡啶环骨架的碳合成子.初步机理研究表明,该反应可能经历了一个自由基历程,且甲醇中甲基的碳氢键断裂为反应的决速步骤.该合成方法具有操作简单、环境友好等优点.
关键词: 铜, 氧化环化, 吡啶, 甲醇
A copper-catalyzed oxidative formal cycloaddition of 1,3-dicarbonyl compounds, methanol and ammonium acetate was first demonstrated, affording symmetrical 2,3,5,6-tetrasubstituted pyridines in moderate to excellent yields. Methanol was employed as the carbon synthon as well as the reaction solvent. The preliminary mechanistic studies revealed that the reaction underwent a radical pathway and the C(sp3)-H bond cleavage of methanol was the rate-determining step. This method is operationally simple and environmentally friendly.
Key words: copper, oxidative annulation, pyridine, methanol
PDF全文下载地址:
点我下载PDF
