摘要/Abstract
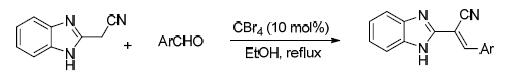
2-苯并咪唑-3-芳基丙烯腈衍生物具有广谱的生物活性,同时还是一类重要的合成中间体,在有机合成领域具有广泛的应用.以芳醛和2-氰甲基苯并咪唑为原料,乙醇为溶剂,在四溴化碳促进下,高效合成了2-(1H-苯并[d]咪唑)-3-芳基丙烯腈.反应在回流条件下搅拌5~10 min即可完成,以74%~96%的产率得到目标产物.该方法为2-苯并咪唑-3-芳基丙烯腈衍生物的制备提供了反应条件温和、后处理方便和底物适用范围广的合成策略.
关键词: 苯并咪唑, 四溴化碳, 丙烯腈, Knoevenagel缩合
2-(1H-Benzo[d] imidazol-2-yl)-3-arylacrylonitrile derivatives not only exhibit a variety of important biological activities, but also are important intermediates in organic synthesis. The CBr4-promoted reaction of aromatic aldehydes with 2-(1H-benzo[d] imidazol-2-yl) acetonitrile to obtain 2-(1H-benzo[d] imidazol-2-yl)-3-arylacrylonitriles was developed. Structurally diverse 2-(1H-benzo[d] imidazol-2-yl)-3-arylacrylonitriles were obtained in moderate to good yields (74%~96%) under mild conditions. This method has the advantages of operational simplicity and wide substrate scope.
Key words: benzimidazol, carbon tetrabromide, acrylonitrile, Knoevenagel condensation
PDF全文下载地址:
点我下载PDF
