

1. 江南大学 粮食发酵工艺与技术国家工程实验室,江苏 无锡 214122;
2. 江南大学 生物工程学院,江苏 无锡 214122
收稿日期:2019-11-23;接收日期:2020-04-10;网络出版时间:2020-05-15
基金项目:国家自然科学基金(No. 31571817)资助
摘要:番茄红素作为一种高附加价值的萜类化合物已受到国内外研究者的广泛关注。首先对酿酒酵母Saccharomyces cerevisiae模式菌株S288c和YPH499合成番茄红素的能力进行分析比较,结果表明YPH499更适合作为底盘细胞用于番茄红素的合成。随后比较组成型启动子GPDpr、TEF1pr和诱导型启动子GAL1pr、GAL10pr对番茄红素合成的影响,结果发现以GPDpr、TEF1pr作为番茄红素合成途径基因crtE、crtB和crtI的启动子,摇瓶发酵60 h后,番茄红素产量为15.31 mg/L;以GAL1pr和GAL10pr为启动子时,其产量为123.89 mg/L,提高8.09倍。继续改造甲羟戊酸(MVA)途径,过量表达N-末端截短的关键酶基因tHMG1 (3-羟基-3-甲基戊二酸单酰辅酶A还原酶),番茄红素产量为265.68 mg/L,单位菌体产量72.79 mg/g。文中所设计构建的异源表达番茄红素合成途径的酿酒酵母菌株单位细胞产量高,可以进一步改造和优化后用于番茄红素的工业化生产。
关键词:番茄红素酿酒酵母底盘细胞诱导型启动子单位菌体产量
Construction of a highly efficient synthetic lycopene engineered Saccharomyces cerevisiae
Ling Sun1,2, Junhua Wang1,2, Wei Jiang1,2, Youran Li1,2, Liang Zhang1,2, Zhongyang Ding1,2, Zhenghua Gu1,2, Guiyang Shi1,2, Sha Xu1,2


1. National Engineering Laboratory for Cereal Fermentation Technology, Jiangnan University, Wuxi 214122, Jiangsu, China;
2. School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
Received: November 23, 2019; Accepted: April 10, 2020; Published: May 15, 2020
Supported by: National Natural Science Foundation of China (No. 31571817)
Corresponding author: Sha Xu. Tel/Fax: +86-510-85914371; E-mail: xusha1984@jiangnan.edu.cn.
Abstract: Lycopene, as a high value-added terpene compound, has been widely concerned by researchers at home and abroad. Firstly, the ability of lycopene synthesis of Saccharomyces cerevisiae model strains S288c and YPH499 was analyzed and compared. The results showed that YPH499 was more suitable for lycopene synthesis as yeast chassis. Subsequently, the effects of constitutive promoters GPDpr, TEF1pr and inducible promoters GAL1pr, GAL10pr on Lycopene synthesis were compared. The results showed that when GPDpr and TEF1pr were used as promoters of crtE, crtB and crtI in lycopene synthesis pathway, the production of lycopene was 15.31 mg/L after 60 h fermentation in shaking flask. When GAL1pr and GAL10pr were used as promoters, the production was 123.89 mg/L, which was 8.09 times higher. In addition, the methylvaleric acid (MVA) pathway was further modified to overexpress the key enzyme gene of N-terminal truncation, tHMG1 (3-hydroxy-3-methylglutaryl coenzyme A reductase). The lycopene production was 265.68 mg/L, and the yield per cell was 72.79 mg/g. The Saccharomyces cerevisiae strain designed and constructed in this study can express lycopene in high yield per cell, thus could be used in the industrial production of lycopene after further construction and optimization.
Keywords: lycopeneSaccharomyces cerevisiaeyeast chassisinducible promoteryield per cell
番茄红素是一种深红色类胡萝卜素,其分子结构式为C40H56。作为人体必需的营养物质,番茄红素具有抗氧化性、提高免疫力、预防眼部及心血管疾病等功效,应用广泛。同时番茄红素作为一种很强的抗氧化剂,具有极强的清除自由基的能力,对防治肺癌[1]、胃癌[2]、前列腺癌[3]、卵巢癌[4]、结肠癌[5]等均有显著效果。目前,番茄红素主要由植物提取、化学合成和生物合成3种方法获得。从植物提取公众接受度高,但生长周期长、操作繁琐、收益率低且受环境影响较大;化学合成纯度较高,且不断优化合成路线后产业化比较稳定,但存在低产率及污染环境等问题[6];生物合成法虽会受到菌株遗传稳定性的影响,但因其具有低成本、生产周期短和绿色环保等优点,越来越受到人们的青睐。
酿酒酵母Saccharomyces cerevisiae作为安全的模式微生物(GRAS),遗传可操作性强,在现代工业中已得到了广泛的应用[7-9]。酿酒酵母自身存在甲羟戊酸(MVA)途径(图 1),是番茄红素[10]、青蒿酸[11]等异源萜类物质合成的前体代谢途径。其中番茄红素以甲羟戊酸(MVA)途径产生的IPP (异戊二烯基焦磷酸)和DMAPP (二甲基丙烯焦磷酸)作为前体,经由香叶基香叶基二磷酸合成酶(CrtE)生成香叶基香叶基二磷酸酯(GGPP),在八氢番茄红素合成酶(CrtB)作用下合成Phytoene (八氢番茄红素),之后由八氢番茄红素去饱和酶(CrtI)作用产生一系列中间代谢产物,包括Phytofluene (六氢番茄红素)、ζ-Carotenet (ζ-胡萝卜素)、Neurosporene (链孢红素)和终产物Lycopene (番茄红素)[12] (图 1)。
 |
| 图 1 酿酒酵母合成番茄红素代谢路径 Fig. 1 Metabolic pathway of lycopene in S. cerevisiae. Description of the Mevalonate pathway and metabolic pathway of lycopene in S. cerevisiae. Abbreviations of the pathway intermediates and genes are as follows: IPP isopentenyl diphosphate, DMAPP dimethylallyl diphosphate, FPP farnesyl diphosphate, GGPP geranyl geranyl diphosphate. CrtE (GGPPS) geranylgeranyl diphosphate synthase, CrtB Phytoene synthase, CrtI Phytoene desaturase. The black part of the figure is the MVA pathway, and the red part is the lycopene synthesis pathway |
| 图选项 |
目前已有一些通过构建酿酒酵母细胞工厂进行番茄红素合成的策略[13-15]。浙江大学于洪巍团队[16]对番茄红素合成酶CrtYB进行修饰,与来源于红发夫酵母Xanthophyllomyces dendrorhous的CrtI、CrtE以及酿酒酵母tHMG1基因共表达,构建得到的菌株番茄红素单位细胞产量为24.41 mg/g DCW (1.61 g/L)。天津大学元英进团队[10]对番茄红素合成途径与宿主细胞进行修饰,筛选不同来源的CrtI/B/E基因以得到最佳组合,敲除酿酒酵母CEN.PK2菌株YPL062W基因以增加胞质Acetyl-CoA前体供应,并敲除远端遗传基因ROX1、YJL064W和DOS2及调控转录因子INO2,重组菌在5 L发酵罐中单位细胞产量为55.56 mg/g DCW。武汉大学刘天罡团队[17]通过脂质工程与传统代谢工程结合,利用上调甲羟戊酸(MVA)途径、增强胞质Acetyl-CoA前体供应以及促进辅因子NADPH合成等策略,构建的酿酒酵母产番茄红素单位细胞产量为56.2 mg/g。进一步通过增强三酰甘油(TAG)代谢等方法,最终以酿酒酵母CEN.PK2菌株为底盘细胞构建得到工程菌,番茄红素单位细胞产量为70.5 mg/g DCW,通过高密度培养后达到73.3 mg/g DCW,此时番茄红素产量为2.37 g/L。本研究结合底盘细胞适配性和启动子筛选,利用CRISPR-Cas9技术构建得到高效合成番茄红素的酿酒酵母菌株,进一步改造和优化后可用于番茄红素的工业化生产。
1 材料与方法1.1 材料1.1.1 菌株、质粒和培养基本文涉及的所有质粒和菌株信息详见表 1。大肠杆菌生长用SOB培养基(Super optimal broth):蛋白胨10 g/L,酵母粉5 g/L,氯化钠0.5 g/L,氯化钾0.186 g/L,六水合氯化镁2.207 g/L。酵母生长用YPD培养基:20 g/L葡萄糖、20 g/L胰蛋白胨、10 g/L酵母提取物。配置固体培养基时加入2.0%的琼脂粉。在菌株构建和摇瓶发酵过程中,培养基中添加的氨苄青霉素(Ampicillin)、潮霉素(Hygromycin B)、硫酸诺尔斯菌素(Nourseothricin Sulfate,NTC)、遗传霉素(Geneticin,G418)终浓度分别为100、500、100、500 μg/mL。YPG培养基(20 g/L半乳糖、20 g/L胰蛋白胨,10 g /L酵母提取物)用于诱导表达Cre酶使G418抗性标记丢失。
表 1 质粒和菌株Table 1 Plasmids and strains
| Strains and plasmids | Genotype | Source |
| pY26 | tCYC1-GPDprPTEF1pr-tADH1 | This lab |
| pSH47-Hygr | Cre | This lab |
| PUC57-crtI | crtI | This study |
| PUC57-crtB | crtB | This study |
| PUC57-crtE | crtE | This study |
| YJL064W-ROX1(gRNA) | Double knockout plasmid carrying the PAM sequence of the gene YJL064W and ROX1 | This study |
| Ts-GAL80(XKX) | GAL80,Kanr | This study |
| Ts-IB(GAL1prGAL10pr) | tCYC1-crtI-GAL1prGAL10pr-crtB-tADH1, possessing YJL064W homologous arm | This study |
| Ts-IE(GAL1prGAL10pr) | tCYC1-crtI-GAL1prGAL10pr-crtE-tADH1, possessing ROX1 homologous arm | This study |
| Ts-IB(GPDprTEF1pr) | tCYC1-crtI-GPDprTEF1pr-crtB-tADH1, possessing YJL064W homologous arm | This study |
| Ts-IE(GPDprTEF1pr) | tCYC1-crtI-GPDprTEF1pr-crtE-tADH1, possessing ROX1 homologous arm | This study |
| Ts-ADH5 | ADH5 | This study |
| Ts-tHMG1 | GAL1pr-tHMG1 possessing ADH5 homologous arm | This study |
| S288c | This lab | |
| YPH499 | MATa, his3-△200, leu2-△1, trp1-△63, ura3-52, lys2-801, ade2-101 | This lab |
| S288c-CrtE/B/I | tCYC1-crtI-GPDprTEF1pr-crtB-tADH1::YJL064W tCYC1-crtI-GPDprTEF1pr-crtE-tADH1::ROX1 | This study |
| YPH499-CrtE/B/I | tCYC1-crtI-GPDprTEF1pr-crtB-tADH1::YJL064W tCYC1-crtI-GPDprTEF1pr-crtE-tADH1::ROX1 | This study |
| YPH499GAL80 | YPH499GAL80△::loxp | This study |
| WHtE | YPH499GAL80△::loxp, ERG20-GAL1prGAL10pr-tHMG1::his3, IDI1-GAL1prGAL10pr-tHMG1::trp1, ERG10-GAL1prGAL10pr-tHMG1::lys2, ERG9-GAL1prGAL10pr-ERG8::ura3, ERG13-GAL1prGAL10pr-ERG12::leu2 | This lab |
| YPH499GAL80-CrtE/B/I | YPH499GAL80△::loxp tCYC1-crtI-GPDprTEF1pr-crtB-tADH1::YJL064W tCYC1-crtI-GPDprTEF1pr-crtE-tADH1::ROX1 | This study |
| YPH499GAL80-Galpr-CrtE/B/I | YPH499GAL80△::loxp, tCYC1-crtI-GAL1prGAL10pr-crtB-tADH1::YJL064W tCYC1-crtI-GAL1prGAL10pr-crtE-tADH1::ROX1 | This study |
| WHtE-Galpr-CrtE/B/I | WHtE tCYC1-crtI-GAL1prGAL10pr-crtB-tADH1::YJL064W tCYC1-crtI-GAL1prGAL10pr-crtE-tADH1::ROX1 | This study |
| YPH499GAL80-Galpr-CrtE/B/I-tHMG1 | YPH499GAL80-Galpr-CrtE/B/I, GAL1pr-tHMG1-tCYC1::ADH5 | This study |
表选项
1.1.2 引物实验所用引物均由生工生物工程(上海)股份有限公司合成,如表 2所示。
表 2 引物Table 2 Primers
| Oligo name | Sequence (5′–3′) |
| For construction of tCYC1-crtI-GAL1prGAL10pr-crtB-tADH1, YJL064W homologous arm | |
| YJL064W_LF | CGCGGATCCCTGGCCCTTAAGTTCTTCG (BamHⅠ) |
| YJL064W_LR | gggacgctcgaaggctttGCCCCCTAATGAAGTTTACTCC |
| I/B)tCYC1_F | CAGAACTCTAACGATATTAGGATATAAcatgtaattagttatgtcacgcttac |
| I/B)tCYC1_R | AGCCTTGAATTTAGGAGGaaagccttcgagcgtccc |
| BtCrtI_F | AATTTTTGAAAATTCAATATAAATGTCTGATCAGAAGAAGCAC |
| BtCrtI_R | gtaagcgtgacataactaattacatgTTATATCCTAATATCGTTAGAGTTCTG |
| GAL10pr_F | GTGCTTCTTCTGATCAGACATTTATATTGAATTTTCAAAAATT |
| GAL1pr_R | CCAATAATGGTGGTTGTGACATTATAGTTTTTTCTCCTTGACGTTA |
| PaCrtB_F | TAACGTCAAGGAGAAAAAACTATAATGTCACAACCACCATTATTGG |
| PaCrtB _R | caaacctctggcgaagaattTTAAACAGGTCTTTGCCATAAACC |
| I/B)tADH1_F | GGTTTATGGCAAAGACCTGTTTAAaattcttcgccagaggtttg |
| tADH1_R | GATAGCTCGCTCGATGTGAgctattacgccagctgaattg |
| YJL064W_RF | caattcagctggcgtaatagcTCACATCGAGCGAGCTATC |
| YJL064W_RR IB-YAN-F | CGCGGATCCGCCAAAGAAGACGAAGAAACC (BamHⅠ) CTACGGCAGTGCTCCTAG |
| For construction of tCYC1-crtI-GAL1prGAL10pr-crtE-tADH1, ROX1 homologous arm | |
| ROX1_LF | CGCGGATCCCTCTTGCATTTTCCTTTTCTGC (BamHⅠ) |
| ROX1_LR | gggacgctcgaaggctttTGTTGATTGTCTAACTGCGTTC |
| I/B)tCYC1_F | CAGAACTCTAACGATATTAGGATATAAcatgtaattagttatgtcacgcttac |
| I/E)tCYC1_R | GAACGCAGTTAGACAATCAACAaaagccttcgagcgtccc |
| BtCrtI_F | AATTTTTGAAAATTCAATATAAATGTCTGATCAGAAGAAGCAC |
| BtCrtI_R | gtaagcgtgacataactaattacatgTTATATCCTAATATCGTTAGAGTTCTG |
| GAL10pr_F | GTGCTTCTTCTGATCAGACATTTATATTGAATTTTCAAAAATT |
| I/E)GAL1pr_R | CCATTGCGGTATAAGCCATTATAGTTTTTTCTCCTTGACGTTA |
| TmCrtE_F | TAACGTCAAGGAGAAAAAACTATAATGGCTTATACCGCAATGG |
| TmCrtE_R | caaacctctggcgaagaattTTAGTTTTGCCTGAAAGCGATG |
| I/E)t ADH1_F | CATCGCTTTCAGGCAAAACTAAaattcttcgccagaggtttg |
| I/E)t ADH1_R | CGGAAAGAAGAAATGGAAAAAAAAAAgctattacgccagctgaattg |
| ROX1_RF | caattcagctggcgtaatagcTTTTTTTTTTCCATTTCTTCTTTCCG |
| ROX1_RR IE-YAN-F | CGCGGATCCCGACTATGCTGAAACATTATAC (BamHⅠ) GTTCTTCCTCGTATTGTCTTGC |
| For construction of tCYC1-crtI-GPDprTEF1pr-crtB-tADH1, YJL064W homologous arm and tCYC1-crtI-GPDprTEF1pr- crtE-tADH1, ROX1 homologous arm | |
| BcuⅠ-CrtI-F | GGACTAGTATGTCTGATCAGAAGAAGCAC |
| NotⅠ-CrtB-F | AAGGAAAAAAGCGGCCGCATGTCACAACCACCATTATTGG |
| NotⅠ-CrtE-F | AAGGAAAAAAGCGGCCGCATGGCTTATACCGCAATGG |
| For construction of double knockout plasmid YJL064W-ROX1(gRNA) | |
| gRNA(471 bp)-F | AAGGAGGGTATTCTGGGCCTCCATGTC |
| gRNA(471 bp)-R | TCTGCAGAATTCGTCGACGAGCTCGGTAC |
| HindⅢ-gRNA(471 bp)-F | CCCAAGCTTAAGGAGGGTATTCTGGGCCTCCATGTC |
| gRNA149bp(YJL064W)-F | GTGAGTTCATCTGGGAGCGGgttttagagctagaaatagcaag |
| gRNA302bp(YJL064W)-R | CCGCTCCCAGATGAACTCACgatcatttatctttcactgcggag |
| gRNA149bp(ROX1)-F | AGAGAAGGTAATAGAGGGGGgttttagagctagaaatagcaag |
| gRNA302bp(ROX1)-R | CCCCCTCTATTACCTTCTCTgatcatttatctttcactgcggag |
| gRNA-YAN-F | GCTAAATGTACGGGCGACAGTCAC |
| gRNA-YAN-R | CGCGTTGGCCGATTCATTAATGCAG |
| For construction of knockout expression box of GAL80 | |
| KpnⅠ-GAL80-1 | CGGGGTACCAGGAGCAAGCAACTGACC |
| KpnⅠ-GAL80-3 | CGGGGTACCATTGGGCGTTCTATGAGG |
| SalⅠ-GAL80-2 | ACGCGTCGACAAGCATCTTGCCCTGTGCT |
| SalⅠ-GAL80-4 | ACGCGTCGACGACGGGAGTGGAAAGAACG |
| SalⅠ-XKX-F | ACGCGTCGACAAAATTCTGCGTTCGGTACC |
| SalⅠ-XKX-R | ACGCGTCGACCGATCCTCTGGAGATGAGCTC |
| For construction of GAL1pr-tHMG1-terminal, ADH5 homologous arm | |
| NheI –GAL1pr-F GALpr-R | CTAGCTAGCTTATATTGAATTTTCAAAAATT GTTTTCACCAATTGGTCTGCAGCCATtatagttttttctccttgacgtta |
| tHMG1-F | TAACGTCAAGGAGAAAAAACTATAatggctgcagaccaattggtgaaaac |
| NotⅠ-tHMG1-R | AAGGAAAAAAGCGGCCGCGTTGTAATTCAACCCTGAAGATCTTC |
| SmaⅠ-ADH5-1 | TCCCCCGGGGGGACTACAAGATATTTCTAGC |
| NheⅠ-ADH5-2 | CTAGCTAGCGATGCTTTGATTTTGTAGATATG |
| NotⅠ-ADH5-3 | AAGGAAAAAAGCGGCCGCTCTTTTGTAACGAATTTGATG |
| SmaⅠ-ADH5-4 | TCCCCCGGGGCAAAATACCAAATGTCCAC |
| Generated restriction enzyme sites are underlined and indicated in the table. | |
表选项
1.1.3 主要工具酶和试剂文中所用的2×Taq PCR Master Mix、2×Pfu PCRMaster Mix购自杭州宝赛公司;Phanta? Max Super-Fidelity DNA polymerase购自南京诺唯赞生物科技有限公司;Fast DigestedTM快速限制性内切酶购自美国ThermoFisher Scientific公司;T4 DNA Ligase和pMD19T-simple购自大连TaKaRa公司;硫酸诺尔斯菌素购自上海懋康生物科技有限公司;氨苄青霉素、PEG3350、醋酸锂、潮霉素、遗传霉素G418购自Sigma公司;质粒DNA提取试剂盒、DNA纯化试剂盒和DNA凝胶回收试剂盒购自北京博大泰克生物有限公司;番茄红素(HPLC > =90%,CAS#502-65-8)标准品购自上海源叶生物科技有限公司;鲑鱼精DNA购自北京索莱宝科技有限公司;蛋白胨、酵母粉、琼脂粉购自OXOID公司;三氯甲烷、丙酮为分析纯,甲醇为色谱纯,均购自国药集团化学试剂有限公司。
1.2 方法1.2.1 番茄红素合成途径的构建本实验所选取的表达番茄红素合成途径相关酶的编码基因来源情况如下:香叶基香叶基二磷酸合酶CrtE来源于曼地亚红豆杉(Taxusx media,Tm),八氢番茄红素合成酶CrtB来源于成团泛菌(Pantoea agglomerans,Pa),八氢番茄红素去饱和酶CrtI来源于三孢布拉氏霉菌(Blakeslea trispora,Bt)。基因按照酿酒酵母密码子偏好性进行优化并规避常用酶切位点由苏州金唯智生物科技有限公司合成质粒PUC57-crtI、PUC57-crtB和PUC57-crtE备用。
分别以质粒PUC57-crtI、PUC57-crtB和PUC57-crtE为模板扩增crtI、crtB和crtE片段。以本实验室保藏的酿酒酵母YPH499基因组为模板,扩增双向启动子GAL1prGAL10pr及YJL064W和ROX1两个基因的左右同源臂,并选择BamHⅠ作为YJL064W和ROX1两个基因左右同源臂的酶切位点。分别以质粒pY26和PSH47-Hygr为模板扩增ADH1和CYC1终止子片段。将这些产物纯化后,通过融合PCR,将YJL064W左同源臂与CYC1终止子片段融合,YJL064W右同源臂与ADH1终止子片段融合,crtI、crtB与启动子GAL1prGAL10pr融合,根据凝胶电泳显示融合片段大小正确之后,将上述3组片段纯化再进行融合PCR,得到YJL064W-tCYC1-crtI-GAL1prGAL10pr- crtB-tADH1-YJL064W,最后通过TA克隆,连接至载体pMD19T-simple,得到质粒Ts-IB (GAL1prGAL10pr)。用同样的方法构建Ts-IE (GAL1prGAL10pr)、Ts-IB(GPDprTEF1pr)和Ts-IE (GPDprTEF1pr)质粒,所有质粒送上海生工测序。
1.2.2 重组菌的筛选本研究利用的CRISPR-Cas9基因编辑系统是一个双质粒系统,Cas9蛋白和向导gRNA分别由两个游离质粒在酿酒酵母菌株中表达以进行相应基因的敲除与敲入。转化酿酒酵母时,待转化目的物包括gRNA质粒和线性化的基因表达盒通过醋酸锂转化法转化至酿酒酵母,利用同源重组机制实现组装[18]。在固体培养基冷却至合适温度时,添加500 μg/mL G418用于敲除GAL80基因的转化子筛选,添加100 μg/mL NTC用于转化酿酒酵母Cas9质粒后转化子的筛选;同时添加100 μg/mL NTC和500 μg/mL Hygromycin B用于转化酿酒酵母番茄红素合成途径表达盒后转化子的筛选。转化平板于30 ℃培养箱中倒置培养待长出转化子,经菌落颜色变化(合成番茄红素的菌株显深黄色或深红色)和菌落PCR初步筛选,再通过基因组验证获得整合表达番茄红素工程菌株。
1.2.3 重组菌发酵实验将平板活化得到的单克隆工程菌株接种于YPD液体培养基中,30 ℃、200 r/min振荡培养至对数生长中期(36 h左右),然后以OD600=0.5的初始菌体浓度转接于含50 mL YPD液体培养基的250 mL锥形瓶中,30 ℃、200 r/min振荡培养,定时取样对重组菌生长趋势、合成番茄红素水平、消耗葡萄糖情况以及生成副产物甘油、乙酸和乙醇情况进行测定。
1.2.4 番茄红素的提取对发酵过程中提取的发酵液样品进行快速提取,具体方法如下所述:分别各取两等份1 mL发酵液,离心收集菌体,无菌水清洗后,其中一份菌体55 ℃烘干至恒重,称重计算菌体干重;另一份用于产物提取。向待提取番茄红素的离心管中加入等体积的玻璃珠(0.5 mm)和1 mL氯仿,振荡破碎2 min,冰浴1 min,10 000 r/min离心1 min,取上清液;黑暗条件下放置至细胞萃取完全后,离心取适量体积萃取液用氮吹仪将氯仿快速吹干,用丙酮复溶过0.22 μm有机膜后用超高效合相色谱(UPC2)分析番茄红素产量。
1.2.5 番茄红素的含量测定使用Waters超高效合相色谱仪(Ultra Performance Convergence Chromatography,UPC2)对番茄红素进行含量测定。检测条件为:配备PDA检测器的ACQUITY UPC2系统和专用色谱柱Viridis? HSS C18 SB Column 100A,1.8 μm;流动相为20%甲醇和80% CO2,流速1 mL/min,柱温40 ℃,检测波长450 nm,背压13.793 MPa (2 000 psi),进样量2 μL,重复3次测定;番茄红素标准品用于定量分析。将规格为20 mg的番茄红素标品用丙酮充分溶解,并加入0.05 g的2, 6-二叔丁基-4-甲基苯酚(BHT)作为保护剂[19],-70 ℃避光保存备用。并用丙酮稀释为不同浓度的标准样品后通过超高效合相色谱仪(UPC2)测定以制作标准曲线。
1.2.6 胞外代谢物的检测发酵液中葡萄糖、甘油、乙酸和乙醇的浓度利用高效液相色谱(赛默飞,UltiMate3000)进行测定。以Bio-RadHPX-87H为色谱柱,5%的稀硫酸溶液作为流动相,流速为0.8 mL/min,柱温50 ℃,示差检测器。
2 结果与分析2.1 番茄红素合成途径表达盒的构建构建番茄红素合成途径表达盒如图 2所示,将构建得到的质粒Ts-IB(GAL1prGAL10pr)和Ts-IE(GAL1prGAL10pr)分别用BamHⅠ酶切,结果如图 3A所示,所得条带大小均与理论值4 722 bp/ 2 701 bp和4 735 bp/2 701 bp相符。同理,Ts-IB(GPDprTEF1pr)和Ts-IE(GPDprTEF1pr)用BamHⅠ酶切,凝胶电泳结果如图 3B所示,所得条带大小均与理论值5 180 bp/2 701 bp和5 192 bp/2 701 bp相符,测序结果表明表达盒已成功构建。
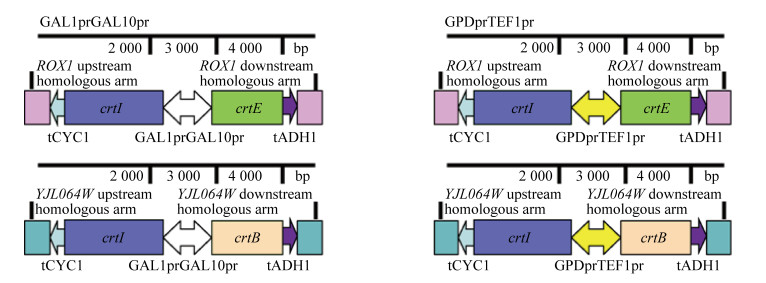 |
| 图 2 番茄红素合成途径表达盒 Fig. 2 Lycopene synthesis pathway expression cassette. Description of two-way bidirectional promoters controlling the expression of key genes crtI, crtB and crtE in the lycopene synthesis pathway. The left of the figure is the inducible promoters to control the expression of lycopene synthesis pathway while the right part is constitutive promoters |
| 图选项 |
 |
| 图 3 番茄红素合成途径表达盒的酶切验证 Fig. 3 Verification of the expression cassette of lycopene synthesis pathway by restriction enzyme digestion. (A) Enzyme digestion verification of plasmid Ts-IB(GAL1prGAL10pr) and Ts-IE(GAL1prGAL10pr). (B) Digestion verification of plasmid Ts-IB(GPDprTEF1pr) and Ts-IE(GPDprTEF1pr). (C) PCR validation of double-knocking plasmid YJL064W-ROX1(gRNA). M: DL 15 000 DNA marker; all lanes are digested by BamHⅠ |
| 图选项 |
已有研究表明ROX1作为一种转录因子,可抑制甲羟戊酸途径和麦角固醇合成酶;而YJL064W基因的敲除可以提高红没药烯和类胡萝卜素的产量[20]。另外,刘天罡团队[17]研究发现可通过增加八氢番茄红素去饱和酶(CrtI)基因拷贝数提高番茄红素的产量。根据以上分析,本研究利用CRISPR-Cas9技术在ROX1和YJL064W两个基因的开放阅读框(ORF),分别同时敲入crtE-crtI和crtB-crtI两组基因,实现crtI基因的双拷贝和番茄红素合成途径的异源表达。
2.2 不同底盘细胞对番茄红素途径表达水平的影响异源路径与底盘细胞的适配性是高效利用微生物作为细胞工厂的关键因素之一[10]。本研究以酿酒酵母菌株S288c与YPH499为底盘细胞,在YJL064W和ROX1两个基因开放阅读框(ORF)分别同时敲入tCYC-crtI-GPDprTEF1pr-crtB-tADH1和tCYC1-crtI-GPDprTEF1pr-crtE-tADH1两组表达盒,得到酿酒酵母工程菌株S288c-CrtE/B/I和YPH499-CrtE/B/I。对重组菌进行摇瓶发酵实验,提取发酵液样品,按照上述条件用UPC2进行定性和定量分析。结果如图 4所示,两株菌的生长能力与番茄红素合成能力均有较大差异(图 4)。发酵60 h,YPH499-CrtE/B/I菌体生长能力明显低于S288c-CrtE/B/I (图 4A)。酿酒酵母S288c- CrtE/B/I在发酵60 h后番茄红素产量为4.11 mg/L,YPH499-CrtE/B/I番茄红素产量为15.31 mg/L,是前者的3.7倍,说明酿酒酵母YPH499虽然较模式菌株S288c菌体生长能力低,但产物积累能力更强,因此更适合作为底盘细胞合成番茄红素。
 |
| 图 4 底盘细胞对重组菌合成番茄红素的影响(A:菌体生长曲线(OD600);B:工程菌摇瓶发酵生产番茄红素) Fig. 4 Effect of chassis hosts on synthesis of lycopene by engineered S. cerevisiae. (A) Typical profiles observed for cell growth (OD600). (B) Lycopene production in Shake flask fermentation using engineered S. cerevisiae |
| 图选项 |
2.3 比较启动子对番茄红素途径表达水平的影响本研究进一步比较启动子对控制番茄红素合成途径表达的影响。首先敲除GAL80基因使半乳糖诱导型启动子GAL1pr和GAL10pr实现组成型表达[21]。在此基础上,分别转化组成型启动子GPDpr和TEF1pr和半乳糖诱导型启动子GAL1pr和GAL10pr控制番茄红素合成途径表达盒,获得YPH499GAL80-CrtE/B/I和YPH499GAL80-Galpr- CrtE/B/I两株酿酒酵母工程菌。摇瓶发酵3 d,结果如图 5所示,两株菌生长速率差别不大(图 5A),但合成番茄红素的水平明显存在巨大差异(图 5B)。当以GAL1pr和GAL10pr控制番茄红素合成途径表达时,番茄红素产量在发酵60 h即可达到123.89 mg/L,产量提高8.09倍。如图 5C所示,4株工程菌发酵48 h后,发酵液颜色即呈现明显差别。相比其他3株菌发酵液呈现黄色,YPH499GAL80-Galpr- CrtE/B/I发酵液呈现出明显的红色。结果表明以YPH499菌株作为底盘细胞,半乳糖诱导型启动子GAL1pr和GAL10pr控制表达番茄红素合成途径效果更佳。对YPH499GAL80-Galpr-CrtE/B/I胞外代谢物包括发酵液中葡萄糖、甘油、乙酸、乙醇的浓度进行测定(图 5D),结果表明,发酵液中葡萄在12 h–30 h之间被快速消耗用于菌体生长,整个过程基本没有乙酸产生,但存在一定量的副产物乙醇和少量的甘油。
 |
| 图 5 启动子对重组菌合成番茄红素的影响(A:不同启动子条件下的菌体生长曲线;B:工程菌摇瓶发酵生产番茄红素;C:番茄红素在不同菌株中的积累;D:发酵过程中的胞外代谢产物和葡萄糖浓度变化曲线) Fig. 5 Effect of promoters on synthesis of lycopene by engineered S. cerevisiae. (A) The effect of different promoters on cell growth. (B) Lycopene production in Shake flask fermentation using engineered S.cerevisiae. (C) Accumulation of lycopene in different strains. (D) The concentration of extracellular metabolites including glucose concentration, glycerol concentration, and ethanol concentration in the fermentation of YPH499GAL80-Galpr-CrtE/B/I |
| 图选项 |
2.4 过量表达tHMG1对番茄红素合成能力的影响为了进一步提高番茄红素产量,以本实验室构建的一株将甲羟戊酸(MVA)途径基因全部上调的重组菌株WHtE作为宿主细胞(数据未发表)异源表达番茄红素合成途径。结果如图 6所示,摇瓶发酵60 h,番茄红素的产量并没有显著提高,仅为12.34 mg/L,且菌株生长受到严重抑制,推测可能基因改造过多对菌株生长造成影响反而不利于产物合成。文献报道tHMG1作为甲羟戊酸(MVA)途径的关键酶可以有效解除麦角固醇的反馈抑制[22]。如图 6B所示,当只过表达甲羟戊酸(MVA)途径的关键酶基因tHMG1时,最终番茄红素产量达到265.68 mg/L。此时,单位菌体番茄红素产量达到72.79 mg/g,而菌体干重仅为3.65 g/L。
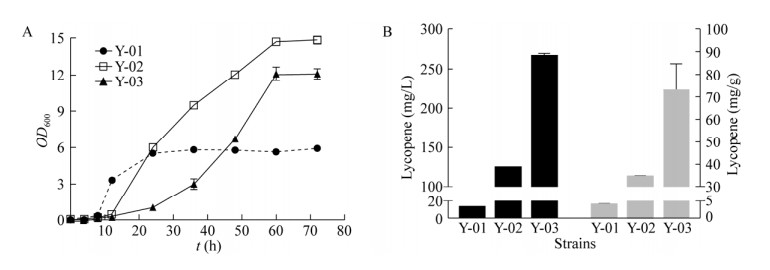 |
| 图 6 调控MVA途径对重组菌合成番茄红素的影响 Fig. 6 Effect of regulation MVA pathway on synthesis of lycopene in S. cerevisiae. (A) Growth curve of different strains. (B) Lycopene production in Shake flask fermentation of different strains. (Y-01, Y-02 and Y-03 are engineered strains WHtE-Galpr-CrtE/B/I, YPH499GAL80-Galpr-CrtE/B/I and YPH499GAL80-Galpr-CrtE/B/I-tHMG1, respectively) |
| 图选项 |
3 讨论本研究发现,选择合适的底盘细胞能够极大地提高产物合成效率。选择酿酒酵母YPH499作为底盘细胞,仅表达外源番茄红素合成途径,番茄红素产量达到15.31 mg/L,是S288c的3.7倍。李霞等[23]分别以酿酒酵母INVSc1和CEN.PK2-1C为底盘细胞,发酵120 h,番茄红素产量分别为0.025 mg/L和0.052 mg/L,说明不同遗传背景酿酒酵母的番茄红素合成能力不同,与本研究结果相符。同时,启动子作为启动基因转录调控的元件,其活性强弱直接影响基因的表达水平,进而影响代谢途径转化效率和目标产物产量[24]。本研究结果表明在GAL双向启动子调控下,番茄红素产量达到123.89 mg/L,相比组成型强启动子调控的菌株产量提高8.09倍。现有研究表明,在敲除GAL80的前提下,GAL启动子虽然不受半乳糖的调控,但是其转录强度随着葡萄糖浓度的降低而提高[10]。在发酵过程中,前期由于GAL启动子活性较低,番茄红素积累量较低,随着葡萄糖的消耗,GAL启动子的转录强度逐渐提高,产物大量积累。
为了进一步增强番茄红素合成途径的代谢通量,过表达甲羟戊酸途径的关键酶调控基因tHMG1,最终番茄红素产量达到265.68 mg/L,单位菌体产量为72.79 mg/g DCW。经过改造,单位菌体产量已经达到较高水平,但产量仍然较低的原因是由于发酵结束菌体干重仅为3.65 g/L。由于番茄红素积累在微生物胞内,不会主动分泌到培养基中,因此单位细胞产量是一个重要指标。后续的改造重点在于增加菌体积累,通过流加碳源、优化培养基组分、碳氮比等方法进行高密度培养[10]。综上所述,本研究构建了一株番茄红素单位菌体产量较高的菌株,后续将对发酵条件进行优化,对重组菌进行高密度培养,提高细胞浓度,以实现番茄红素的高产。
参考文献
| [1] | Rakic JM, Liu C, Veeramachaneni S, et al. Lycopene inhibits smoke-induced chronic obstructive pulmonary disease and lung carcinogenesis by modulating reverse cholesterol transport in ferrets. Cancer Prev Res, 2019, 12(7): 421-431. DOI:10.1158/1940-6207.CAPR-19-0063 |
| [2] | Han H, Lim JW, Kim H. Lycopene inhibits activation of epidermal growth factor receptor and expression of Cyclooxygenase-2 in gastric cancer cells. Nutrients, 2019, 11(9): 2113. DOI:10.3390/nu11092113 |
| [3] | Li DB, Chen LN, Zhao WR, et al. MicroRNA-let-7f-1 is induced by lycopene and inhibits cell proliferation and triggers apoptosis in prostate cancer. Mol Med Rep, 2016, 13(3): 2708-2714. |
| [4] | Xu J, Li YF, Hu HY, et al. Effects of lycopene on ovarian cancer cell line SKOV3 in vitro: Suppressed proliferation and enhanced apoptosis. Mol Cell Probes, 2019, 46: 101419. DOI:10.1016/j.mcp.2019.07.002 |
| [5] | Langner E, Lemieszek MK, Rzeski W, et al. Lycopene, sulforaphane, quercetin, and curcumin applied together show improved antiproliferative potential in colon cancer cells in vitro. J Food Biochem, 2019, 43(4): e12802. DOI:10.1111/jfbc.12802 |
| [6] | Clinton S. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr Rev, 1998, 56(2): 35-51. |
| [7] | Dai ZB, Liu Y, Zhang XN, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng, 2013, 20: 146-156. DOI:10.1016/j.ymben.2013.10.004 |
| [8] | Dai ZB, Liu Y, Huang LQ, et al. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol Bioeng, 2012, 109(11): 2845-2853. DOI:10.1002/bit.24547 |
| [9] | Han JY, Seo SH, Song JM, et al. High-level recombinant production of squalene using selected Saccharomyces cerevisiae strains. J Ind Microbiol Biotechnol, 2018, 45(4): 239-251. |
| [10] | Chen Y, Xiao WH, Wang Y, et al. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact, 2016, 15: 113. |
| [11] | Zhang CB, Liu JJ, Zhao FL, et al. Production of sesquiterpenoid zerumbone from metabolic engineered Saccharomyces cerevisiae. Metab Eng, 2018, 49: 28-35. DOI:10.1016/j.ymben.2018.07.010 |
| [12] | Cunningham XF Jr, Sun Z, Chamovitz D, et al. Molecular structure and enzymatic function of lycopene cyclase from the Cyanobacterium Synechococcus sp. strain PCC7942. Plant Cell, 1994, 6(8): 1107-1121. |
| [13] | Yamano S, Ishii T, Nakagawa M, et al. Metabolic engineering for production of β-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotechnol Biochem, 1994, 58(6): 1112-1114. DOI:10.1271/bbb.58.1112 |
| [14] | Shi B, Ma T, Liu TL, et al. Systematic metabolic engineering of Saccharomyces cerevisiae for lycopene overproduction. J Agric Food Chem, 2019, 67(40): 11148-11157. DOI:10.1021/acs.jafc.9b04519 |
| [15] | Bahieldin A, Gadalla NO, Al-Garni SM, et al. Efficient production of lycopene in Saccharomyces cerevisiae by expression of synthetic crt genes from a plasmid harboring the ADH2 promoter. Plasmid, 2014, 72: 18-28. DOI:10.1016/j.plasmid.2014.03.001 |
| [16] | Xie WP, Lv XM, Ye LD, et al. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab Eng, 2015, 30: 69-78. DOI:10.1016/j.ymben.2015.04.009 |
| [17] | Ma T, Shi B, Ye ZL, et al. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng, 2019, 52: 134-142. DOI:10.1016/j.ymben.2018.11.009 |
| [18] | Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol, 2002, 350: 87-96. DOI:10.1016/S0076-6879(02)50957-5 |
| [19] | Xu F, Yuan QP, Dong HR. Determination of lycopene and β-carotene by high-performance liquid chromatography using sudan I as internal standard. J Chromatogr B, 2006, 838(1): 44-49. |
| [20] | ?zaydin B, Burd H, Lee TS, et al. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab Eng, 2013, 15: 174-183. DOI:10.1016/j.ymben.2012.07.010 |
| [21] | Peng BY, Wood RJ, Nielsen LK, et al. An Expanded Heterologous GAL Promoter Collection for Diauxie-Inducible Expression in Saccharomyces cerevisiae. ACS Synth Biol, 2018, 7(2): 748-751. DOI:10.1021/acssynbio.7b00355 |
| [22] | Donald KA, Hampton RY, Fritz IB. Effects of Overproduction of the Catalytic Domain of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase on Squalene Synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol, 1997, 63(9): 3341-3344. DOI:10.1128/AEM.63.9.3341-3344.1997 |
| [23] | Li X, Zhao JY, Lou XD, et al. Optimization of the adaptability of Saccharomyces cerevisiae producing lycopene. Food Fermentat Ind, 2018, 44(6): 24-29 (in Chinese). 李霞, 赵金雨, 娄兴丹, 等. 酿酒酵母合成番茄红素的适配性优化. 食品与发酵工业, 2018, 44(6): 24-29. |
| [24] | Keasling JD. Synthetic biology and the development of tools for metabolic engineering. Metab Eng, 2012, 14(3): 189-195. |
