牟春晓, 孟慧, 朱立麒, 陈振海


扬州大学兽医学院, 江苏高校动物重要疫病与人兽共患病防控协同创新中心, 江苏 扬州 225009
收稿日期:2020-11-09;修回日期:2021-02-05;网络出版日期:2021-04-19
基金项目:国家自然科学基金(31802225);江苏高校优势学科建设工程资助项目;江苏高校动物重要疫病与人兽共患病防控协同创新中心
*通信作者:陈振海, E-mail: zhenhai@yzu.edu.cn.
摘要:[目的] 枯草芽孢杆菌能有效诱导肠道黏膜免疫应答,但活化黏膜下树突状细胞(DC)的具体机制不完全清楚。[方法] 本研究首先用不同浓度枯草芽孢杆菌刺激小鼠肠上皮CMT93细胞,用荧光定量PCR和ELISA检测细胞因子表达水平,然后将枯草芽孢杆菌刺激细胞的培养上清与小鼠骨髓源树突状细胞(BMDC)进行共孵育,用流式细胞术检测BMDC活化标志,最后用RNA干扰技术证明IL-33在活化BMDC中的作用。[结果] 枯草芽孢杆菌能显著刺激CMT93细胞分泌IL-6、IL-33和IFN-γ等细胞因子,对刺激IL-33表达呈现剂量依赖性;枯草芽孢杆菌刺激CMT93细胞产生的细胞因子能活化BMDC,在RNA干扰IL-33基因表达和枯草芽孢杆菌刺激后,CMT93细胞培养上清活化BMDC的能力显著降低。[结论] 本研究结果表明枯草芽孢杆菌刺激肠上皮细胞产生的IL-33在BMDC活化中具有重要作用。
关键词:枯草芽孢杆菌肠上皮细胞白细胞介素33树突状细胞
The important role of IL-33 secreted from Bacillus subtilis-stimulated intestinal epithelial cells in mouse dendritic cell activation
Chunxiao Mou, Hui Meng, Liqi Zhu, Zhenhai Chen


Jiangsu University Co-innovation Center for the Prevention and Control of Important Animal Infectious Diseases and Zoonose, College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, Jiangsu Province, China
Received: 9 November 2020; Revised: 5 February 2021; Published online: 19 April 2021
*Corresponding author: Zhenhai Chen, E-mail: zhenhai@yzu.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (31802225), by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and by the Jiangsu Co-Innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses
Abstract: [Objective] Bacillus subtilis can induce efficient intestinal mucosal immune responses, but the detailed mechanism for dendritic cell activation remains to be defined. [Methods] Mouse intestinal epithelial CMT93 cells were stimulated with different concentra amyloliquefaciens tions of Bacillus subtilis and the cytokine expression was measure by quantitative RT-PCR and ELISA. After incubation with the cytokine-containing cell culture medium, activation of mouse bone marrow-derived dendrites (BMDC) was analyzed by flow cytometry and the role of IL-33 in BMDC activation was confirmed by RNA interference. [Results] Bacillus subtilis could significantly stimulate CMT93 cells to secret IL-6, IL-33 and IFN-γ, and induce the IL-33 selection in a dose-dependent manner. The cytokine-containing cell medium could significantly activate BMDC. After interfering IL-33 gene expression and stimulation with Bacillus subtilis, however, the ability of CMT93 cell medium for BMDC activation was significantly reduced. [Conclusion] These data suggest that IL-33 from Bacillus subtilis-stimulated mouse intestinal epithelial cells plays an important role in the dendritic cell activation.
Keywords: Bacillus subtilisintestinal epithelial cellsIL-33dendritic cells
枯草芽孢杆菌是广泛存在的益生菌,具有较强的抗逆性[1-2],口服进入机体肠道后能拮抗病原菌粘附[2]、促进黏膜淋巴组织发育和诱导黏膜IgA抗体反应,因此是良好的黏膜免疫增强剂[3-4],但目前对其调节黏膜免疫的机制尚不明确。
树突状细胞(dendritic cell,DC)作为天然免疫系统的“哨兵”,不仅能在黏膜组织捕获抗原,而且活化后能向淋巴结迁移,刺激T、B淋巴细胞免疫应答[5-7]。尽管肠道DC能利用其树突摄取肠黏膜的枯草芽孢杆菌,迁移至引流淋巴组织激发免疫反应[8],但口服枯草芽孢杆菌进入肠道后接触的主要是肠上皮细胞,如何有效活化黏膜下层的DC尚不明确,因此研究枯草芽孢杆菌活化DC的具体机制具有重要意义。
肠上皮细胞作为肠道抵抗病原的第一道屏障,能通过模式识别受体识别病原相关分子模式[9],分泌多种细胞因子(IL-6、IL-5、IFN-γ和IL-33),从而活化黏膜下DC、巨噬细胞和淋巴细胞。IL-33是IL-1家族的一种细胞因子[10-11],不仅能作为免疫佐剂促进抗体应答反应和病原清除[12],还能上调黏膜下DC表面共刺激分子的表达,促进DC分泌促炎细胞因子[13-14]。尽管多种黏膜免疫增强剂都能刺激IL-33表达[15],但口服枯草芽孢杆菌能否刺激肠上皮细胞产生IL-33及其在活化黏膜下层DC中的作用尚不清楚。为此,本研究先用枯草芽孢杆菌刺激小鼠肠上皮细胞系,然后将细胞培养上清与小鼠骨髓源树突状细胞(bone marrow-derived dendritic cell,BMDC)进行共孵育,利用RNA干扰技术证明IL-33在活化BMDC中发挥重要作用,为阐明枯草芽孢杆菌促进黏膜免疫应答的具体机制提供了科学依据。
1 材料和方法 1.1 材料与试剂 枯草芽孢杆菌168购自武汉淼灵生物公司;小鼠结肠癌细胞系(CMT93)由扬州大学阴银燕老师惠赠;4–6周龄C57BL/6J小鼠购自扬州大学实验动物中心;RPMI 1640培养基、DMEM培养基和胎牛血清购自美国Hyclone公司;青霉素、链霉素购自北京索莱宝科技有限公司;小鼠重组粒细胞-巨噬细胞集落刺激因子(rmGM-CSF)和小鼠重组白介素4 (rmIL-4)购自南京巴傲德生物科技公司;脂质体(Lipofectamine 3000)购自赛默飞世尔科技公司;PE标记羊抗小鼠CD40单克隆抗体、FITC标记羊抗小鼠MHC Ⅱ单克隆抗体、PE标记羊抗小鼠CD80单克隆抗体、FITC标记羊抗小鼠CD86单克隆抗体及对应的同型对照抗体购自美国BD公司;细胞活力检测试剂盒(CCK-8剂盒)和小鼠IL-33 ELISA检测试剂盒购自武汉博士德生物工程有限公司;细胞RNA提取试剂盒购自宝生物工程(大连)有限公司;反转录试剂盒和荧光定量PCR酶购自南京诺唯赞生物科技股份有限公司。
1.2 枯草芽孢杆菌对CMT93细胞的毒性检测 将枯草芽孢杆菌168接种于LB培养基中37 ℃培养过夜,离心收集细菌,无菌PBS离心洗2次后涂琼脂平板,37 ℃培养过夜后进行菌落计数,用PBS悬浮备用。将含10%胎牛血清(FBS)和2%青霉素、链霉素的DMEM分装96孔板,加入CMT93细胞,5×104个细胞/孔,CO2培养箱37 ℃培养至满层。按1×105、1×104、1×103 CFU/孔加入枯草芽孢杆菌,37 ℃孵育24 h。加入CCK-8溶液,10 μL/孔,37℃避光孵育2 h,用酶标仪测定OD450值。细胞活性(%)=(OD枯草芽孢杆菌– OD细胞培养基)/(OD阴性孔–OD细胞培养基)×100。
1.3 枯草芽孢杆菌刺激与细胞因子ELISA检测 将含10% FBS和2%青霉素、链霉素的DMEM分装12孔板,接种CMT93细胞,5×105个细胞/孔,37 ℃培养至细胞满层。按1×105 CFU/孔加入枯草芽孢杆菌,37 ℃孵育24 h。收获细胞培养上清液,按照ELISA试剂盒说明书检测IL-6、IFN-γ、IL-12、IL-1β和IL-33表达水平。
1.4 IL-33表达荧光定量PCR检测 如前进行CMT93细胞培养,长满单层后加入枯草芽孢杆菌,接种剂量分别为1×106、1×105和1×104 CFU/孔,孵育24 h后分别收集细胞和培养液。细胞培养上清10000 r/min离心10 min,用0.22 μm滤膜过滤后备用。用细胞RNA提取试剂盒提取细胞RNA,用PrimeScriptTM RT Mix反转录试剂盒反转录得到cDNA。用荧光定量PCR检测IL-33转录水平,正、反向引物序列分别为5′-GTTCTGGAGACTACAGCGCA-3′和5′-TTTG CCGGGGAAATCTTGGA-3′,内参(GAPDH)正、反向引物分别为5′-GGCTGTATTCCCCTCCATC G-3′和5′-CCAGTTGGTAACAATGCCATGT-3′。PCR程序为:恒温段95 ℃ 30 s;循环段95 ℃ 10 s,60 ℃ 30 s,40个循环;熔解段95 ℃ 15 s,60 ℃ 60 s,95 ℃ 15 s。
1.5 小鼠BMDC制备与培养 颈椎脱臼处死C57BL/6J小鼠,无菌分离股骨和胫骨。注射器吸取RPMI 1640培养基冲洗骨髓至无菌离心管中,1500 r/min离心10 min,弃上清。沉淀细胞加入1 mL红细胞裂解液混匀,孵育1 min后加入5 mL RPMI 1640培养基,1500 r/min离心10 min,弃上清。细胞用含10% FBS、10 ng/mL rmGM-CSF、10 ng/mL rmIL-4和1%青霉素、链霉素的RPMI 1640培养基重悬,接种6孔细胞培养板,CO2培养箱37 ℃培养60 h后换液,继续培养5 d,收集未活化的BMDC。
1.6 BMDC活化标志的流式细胞术检测 将BMDC接种24孔板,5×105细胞/mL,用上述细胞培养液培养24 h后收集细胞,1500 r/min离心10 min,弃上清。细胞用适量PBS洗涤2次,每孔细胞用100 μL PBS重悬。按照使用说明书分别加入PE标记羊抗小鼠CD40单克隆抗体、FITC标记羊抗小鼠MHCII单克隆抗体、PE标记羊抗小鼠CD80单克隆抗体、FITC标记羊抗小鼠CD86单克隆抗体以及对应的同型对照抗体,4 ℃孵育30 min,PBS清洗2次后进行流式细胞术检测。
1.7 siRNA干扰CMT93细胞的IL-33基因表达与检测 用网络在线软件(http://rnaidesigner.thermofisher.com/rnaiexpress/insert.do)设计3条小鼠IL-33 siRNA序列,靶向序列分别为AGACTC CGTTCTGGCCTCACCATAA(222)、GCTGCGT CTGTTGACACATTGAGCA(384)和ACACAGA CATCTGGCTGCATGCCAA(643),经过预实验筛选发现siRNA 384的干扰效果最佳。将CMT93细胞接种6孔板,按照Lipofectamine 3000说明书进行siRNA384转染,30 pmol/孔。转染后36 h收集细胞,接种12孔板,待细胞贴壁后加入枯草芽孢杆菌,1×106 CFU/孔。CO2培养箱37 ℃孵育24 h后收集细胞培养上清,用ELISA试剂盒检测IL-33表达水平。按1.6方法分离培养未成熟BMDC,与CMT93细胞培养液孵育24 h后收集BMDC,用流式细胞术检测进行BMDC活化表型检测。
1.8 数据处理及分析 数据均采用平均值±标准误表示。采用SPSS 17.0软件对数据进行统计分析,采用单因子方差分析进行差异显著性检验。
2 结果和分析 2.1 枯草芽孢杆菌对CMT93细胞的毒性检测 从图 1可以看出,与不同剂量的枯草芽孢杆菌孵育24 h后,CMT93细胞的活性无明显改变。
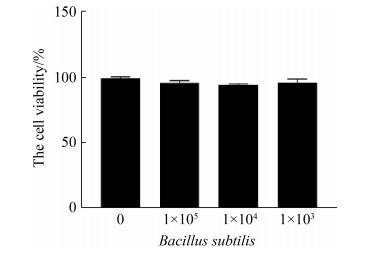 |
| 图 1 枯草芽孢杆菌对CMT93细胞活性的影响 Figure 1 The influence of Bacillus subtilis 168 on CMT93 cell viability. The CMT93 cells viability were detected by CCK-8, compared with the control. *: P < 0.05, **: P < 0.01. |
| 图选项 |
2.2 枯草芽孢杆菌刺激CMT93细胞的细胞因子表达检测 将枯草芽孢杆菌168与CMT93细胞共孵育24 h,收集培养上清用ELISA检测IL-6、IFN-γ和IL-33表达水平。从图 2可知,枯草芽孢杆菌刺激能显著促进CMT93细胞分泌IL-6、IFN-γ和IL-33,但不能显著促进IL-12和IL-1β表达。
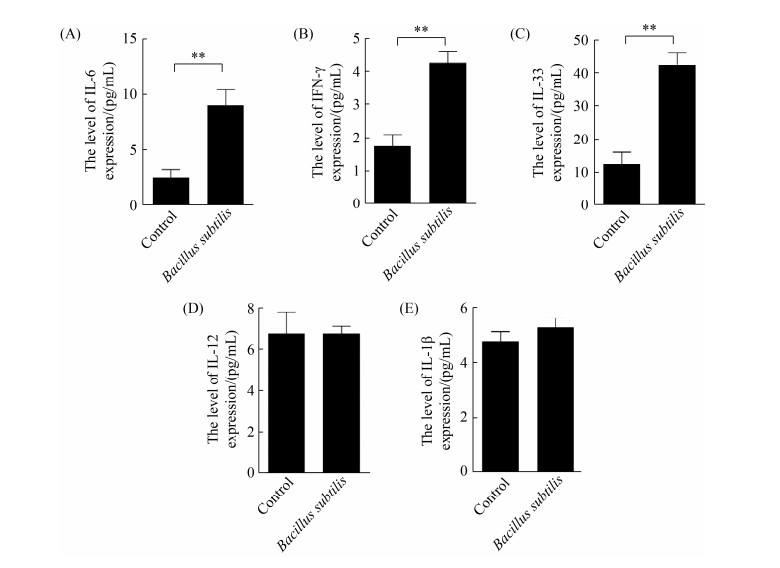 |
| 图 2 枯草芽孢杆菌刺激CMT93细胞的细胞因子表达检测 Figure 2 Detection of cytokine expression in Bacillus subtilis-stimulated CMT93 cells. The protein expression level of IL-6 (A), IFN-γ (B), IL-33 (C), IL-12 (D) and IL-1β (E) in Bacillus subtilis-stimulated CMT93 cells were detected, compared with the control. *: P < 0.05, **: P < 0.01. |
| 图选项 |
2.3 枯草芽孢杆菌刺激CMT93表达IL-33的剂量依赖性 用不同浓度枯草芽孢杆菌与CMT93细胞进行共孵育,24 h后分别收集细胞和培养上清,分别用荧光定量PCR和ELISA检测IL-33表达水平。从图 3可以看出,枯草芽孢杆菌刺激CMT93细胞的IL-33表达具有明显的剂量依赖性。
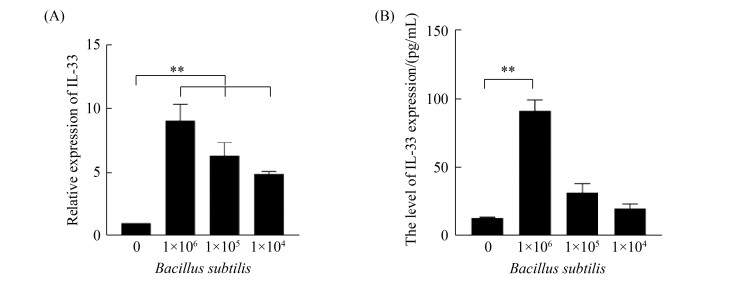 |
| 图 3 枯草芽孢杆菌刺激CMT93细胞表达IL-33的剂量依赖性 Figure 3 Dose-dependent IL-33 expression in Bacillus subtilis-stimulated CMT93 cells. The relative mRNA expression of IL-33 (A) and protein expression level of IL-33 (B) in Bacillus subtilis-stimulated CMT93 cells were detected, compared with the control. *: P < 0.05, **: P < 0.01. |
| 图选项 |
2.4 细胞因子激活BMDC的表型检测 用不同浓度枯草芽孢杆菌刺激CMT93细胞,收集培养上清与BMDC共孵育24 h,用流式细胞术进行激活表型检测。从图 4可以看出,与空白对照组相比,枯草芽孢杆菌刺激CMT93细胞产生的细胞因子能上调BMDC的CD40 (A)、CD80 (B)、CD86 (C)和MHCII (D)表达,并具有枯草芽孢杆菌剂量依赖性。
 |
| 图 4 细胞因子激活BMDC的表型检测 Figure 4 Detection of BMDC activation after cytokine stimulation. The expression of CD40 (A), CD80 (B), CD86 (C) and MHCII (D) on the surface of BMDC were detected by flow cytometric, compared with the control. *: P < 0.05, **: P < 0.01. |
| 图选项 |
2.5 siRNA抑制CMT93细胞的IL-33表达检测 将IL-33基因特异siRNA转染CMT93细胞,转染后24 h用高剂量枯草芽孢杆菌刺激,用ELISA检测细胞培养上清中的IL-33表达水平。结果与正常CMT93细胞相比,siRNA转染细胞培养上清中的IL-33表达量显著降低(图 5)。
 |
| 图 5 siRNA干扰CMT93细胞的IL-33表达检测 Figure 5 Detection of IL-33 expression in siRNA-transfected CMT93 cells. Compared with the control. *: P < 0.05, **: P < 0.01. |
| 图选项 |
2.6 IL-33表达抑制对活化BMDC的影响 在IL-33基因特异性siRNA转染后,用高剂量枯草芽孢杆菌刺激CMT93细胞,流式细胞术检测BMDC活化表型。结果与正常CMT93细胞培养液相比,IL-33基因特异性siRNA转染细胞在枯草芽孢杆菌刺激后,其培养上清上调BMDC活化的能力降低(图 6)。
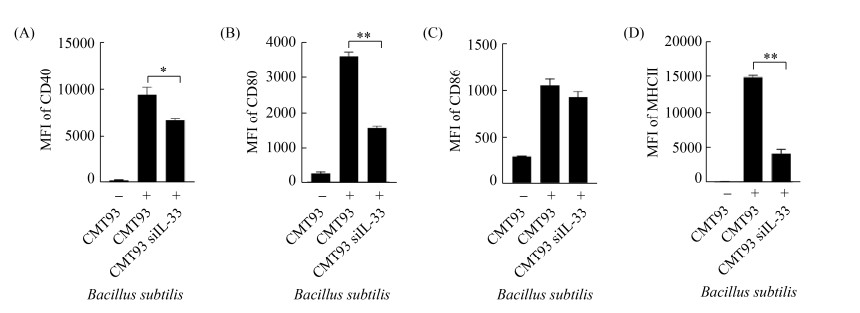 |
| 图 6 干扰IL-33基因表达对激活BMDC的影响 Figure 6 The effect of IL-33 interference on BMDC activation. The expression of CD40 (A), CD80 (B), CD86 (C) and MHCII (D) on the surface of BMDCs were detected by flow cytometric, compared with the control. *: P < 0.05, **: P < 0.01. |
| 图选项 |
3 讨论 黏膜疫苗接种可以同时诱导黏膜和全身免疫反应[16-17],但黏膜免疫系统的复杂性和屏障作用制约着黏膜疫苗的研发。枯草芽孢杆菌具有黏膜免疫增强作用,其表面展示的病毒抗原能有效诱导黏膜局部和全身免疫应答[18],但具体的免疫应答机制还有待进一步研究。枯草芽孢杆菌168是全基因组最早测序的枯草芽孢杆菌[19],已被用作黏膜免疫佐剂[20]。例如,口服枯草芽孢杆菌168分泌的抗菌肽(sublancin)能激活小鼠肠道巨噬细胞、减轻免疫抑制和诱导平衡的Th1和Th2免疫应答[21]。口服和鼻内接种表面表达或吸附梭状芽孢杆菌FliD抗原的枯草芽孢杆菌168都能诱导小鼠产生抗原特异性IgA抗体[22-23]。然而,枯草芽孢杆菌增强黏膜免疫应答的具体机制还有待深入研究。
枯草芽孢杆菌增强黏膜免疫应答的可能机制包括黏膜下DC伸出树突直接摄取枯草芽孢杆菌从而活化DC[8, 18],或者通过刺激肠上皮细胞分泌细胞因子间接激活DC[24]。为了阐明这一问题,本研究用枯草芽孢杆菌168刺激小鼠肠上皮细胞系[25-26],结果显示枯草芽孢杆菌能显著刺激CMT93细胞分泌IFN-γ、IL-6和IL-33等细胞因子,而且具有枯草芽孢杆菌剂量依赖性。将含细胞因子的CMT93细胞培养液与BMDC进行共孵育,结果显示能显著活化BMDC,提示枯草芽孢杆菌可能通过刺激肠上皮细胞产生细胞因子活化DC。
IFN-γ、IL-6和IL-33都是能活化DC的细胞因子[24],其中IL-33是新发现的IL-1家族成员,不仅参与局部炎症、过敏反应和免疫应答[27-28],还能促进DC分化成熟[11-12, 15, 29]。为了阐明枯草芽孢杆菌刺激肠上皮细胞分泌的IL-33在激活DC中的作用,本研究先用siRNA干扰CMT93细胞的IL-33基因表达,经枯草芽孢杆菌刺激后,再用细胞培养上清与BMDC进行共孵育,结果显示细胞培养上清活化BMDC的能力显著降低,提示IL-33是活化BMDC的主要细胞因子,枯草芽孢杆菌可能通过刺激肠上皮细胞分泌IL-33来活化黏膜下DC。
References
| [1] | Tjalsma H, Antelmann H, Jongbloed JDH, Braun PG, Darmon E, Dorenbos R, Dubois JYF, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, van Dijl JM. Proteomics of protein secretion by Bacillus subtilis: separating the "secrets" of the secretome. Microbiology and Molecular Biology Reviews, 2004, 68(2): 207-233. DOI:10.1128/MMBR.68.2.207-233.2004 |
| [2] | Song M, Hong HA, Huang JM, Colenutt C, Khang DD, Nguyen TVA, Park SM, Shim BS, Song HH, Cheon IS, Jang JE, Choi JA, Choi YK, Stadler K, Cutting SM. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine, 2012, 30(22): 3266-3277. DOI:10.1016/j.vaccine.2012.03.016 |
| [3] | Hinc K, Stasi?oj? M, Pi?tek I, Peszyńska-Sularz G, Isticato R, Ricca E, Obuchowski M, Iwanicki A. Mucosal adjuvant activity of IL-2 presenting spores of Bacillus subtilis in a murine model of Helicobacter pylori vaccination. PLoS ONE, 2014, 9(4): e95187. DOI:10.1371/journal.pone.0095187 |
| [4] | Liang JF, Fu J, Kang HH, Lin J, Yu QH, Yang Q. The stimulatory effect of TLRs ligands on maturation of chicken bone marrow-derived dendritic cells. Veterinary Immunology and Immunopathology, 2013, 155(3): 205-210. DOI:10.1016/j.vetimm.2013.06.014 |
| [5] | Chang SY, Ko HJ, Kweon MN. Mucosal dendritic cells shape mucosal immunity. Experimental & Molecular Medicine, 2014, 46(3): e84. |
| [6] | Short KR, Brooks AG, Reading PC, Londrigan SL. The fate of influenza A virus after infection of human macrophages and dendritic cells. The Journal of General Virology, 2012, 93(Pt 11): 2315-2325. |
| [7] | Kitani A, Xu L. Regulatory T cells and the induction of IL-17. Mucosal Immunology, 2008, 1(Suppl 1): S43-S46. |
| [8] | Huang LL, Qin T, Yin YY, Gao X, Lin J, Yang Q, Yu QH. Bacillus amyloliquefaciens SQR9 induces dendritic cell maturation and enhances the immune response against inactivated avian influenza virus. Scientific Reports, 2016, 6: 21363. DOI:10.1038/srep21363 |
| [9] | Yousefi B, Eslami M, Ghasemian A, Kokhaei P, Salek Farrokhi A, Darabi N. Probiotics importance and their immunomodulatory properties. Journal of Cellular Physiology, 2019, 234(6): 8008-8018. DOI:10.1002/jcp.27559 |
| [10] | Hodzic Z, Schill EM, Bolock AM, Good M. IL-33 and the intestine: The good, the bad, and the inflammatory. Cytokine, 2017, 100: 1-10. DOI:10.1016/j.cyto.2017.06.017 |
| [11] | Park SH, Kim MS, Lim HX, Cho D, Kim TS. IL-33-matured dendritic cells promote Th17 cell responses via IL-1β and IL-6. Cytokine, 2017, 99: 106-113. DOI:10.1016/j.cyto.2017.07.022 |
| [12] | Rose WA, Okragly AJ, Patel CN, Benschop RJ. IL-33 released by alum is responsible for early cytokine production and has adjuvant properties. Scientific Reports, 2015, 5: 13146. DOI:10.1038/srep13146 |
| [13] | Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. European Journal of Immunology, 2011, 41(6): 1675-1686. DOI:10.1002/eji.201041033 |
| [14] | Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. Journal of Allergy and Clinical Immunology, 2009, 123(5): 1047-1054. DOI:10.1016/j.jaci.2009.02.026 |
| [15] | Bol-Schoenmakers M, Braber S, Akbari P, de Graaff P, van Roest M, Kruijssen L, Smit JJ, van Esch BCAM, Jeurink PV, Garssen J, Fink-Gremmels J, Pieters RHH. The mycotoxin deoxynivalenol facilitates allergic sensitization to whey in mice. Mucosal Immunology, 2016, 9(6): 1477-1486. DOI:10.1038/mi.2016.13 |
| [16] | Eslani M, Baradaran-Rafii A, Ahmad S. Cultivated limbal and oral mucosal epithelial transplantation. Seminars in Ophthalmology, 2012, 27(3/4): 80-93. |
| [17] | Sohi H, Ahuja A, Ahmad FJ, Khar RK. Critical evaluation of permeation enhancers for oral mucosal drug delivery. Drug Development and Industrial Pharmacy, 2010, 36(3): 254-282. DOI:10.3109/03639040903117348 |
| [18] | Mou CX, Zhu LQ, Xing XP, Lin J, Yang Q. Immune responses induced by recombinant Bacillus subtilis expressing the spike protein of transmissible gastroenteritis virus in pigs. Antiviral Research, 2016, 131: 74-84. DOI:10.1016/j.antiviral.2016.02.003 |
| [19] | Paik SH, Chakicherla A, Hansen JN. Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of, sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168. The Journal of Biological Chemistry, 1998, 273(36): 23134-23142. DOI:10.1074/jbc.273.36.23134 |
| [20] | Liu YK, Zhang J, Wang S, Guo Y, He T, Zhou R. A novel adjuvant "sublancin" enhances immune response in specific pathogen-free broiler chickens inoculated with Newcastle disease vaccine. Journal of Immunology Research, 2019, 2019: 1016567. |
| [21] | Wang S, Huang S, Ye QH, Zeng XF, Yu HT, Qi DS, Qiao SY. Prevention of cyclophosphamide-induced immunosuppression in mice with the antimicrobial peptide sublancin. Journal of Immunology Research, 2018, 2018: 4353580. |
| [22] | Potocki W, Negri A, Peszyńska-Sularz G, Hinc K, Obuchowski M, Iwanicki A. The combination of recombinant and non-recombinant Bacillus subtilis spore display technology for presentation of antigen and adjuvant on single spore. Microbial Cell Factories, 2017, 16(1): 1-11. DOI:10.1186/s12934-016-0616-2 |
| [23] | Potocki W, Negri A, Peszyńska-Sularz G, Hinc K, Obuchowski M, Iwanicki A. IL-1 fragment modulates immune response elicited by recombinant Bacillus subtilis spores presenting an antigen/adjuvant chimeric protein. Molecular Biotechnology, 2018, 60(11): 810-819. DOI:10.1007/s12033-018-0117-0 |
| [24] | Stagg AJ. Intestinal dendritic cells in health and gut inflammation. Frontiers in Immunology, 2018, 9: 2883. DOI:10.3389/fimmu.2018.02883 |
| [25] | Algieri F, Garrido-Mesa J, Vezza T, Rodríguez-Sojo MJ, Rodríguez-Cabezas ME, Olivares M, García F, Gálvez J, Morón R, Rodríguez-Nogales A. Intestinal anti-inflammatory effects of probiotics in DNBS-colitis via modulation of gut microbiota and microRNAs. European Journal of Nutrition, 2020: 1-15. DOI:10.1007/s00394-020-02441-8 |
| [26] | Choudhry N, Petry F, van Rooijen N, McDonald V. A protective role for interleukin 18 in interferon γ-mediated innate immunity to Cryptosporidium parvum that is independent of natural killer cells. The Journal of Infectious Diseases, 2012, 206(1): 117-124. DOI:10.1093/infdis/jis300 |
| [27] | Pastorelli L, De Salvo C, Vecchi M, Pizarro TT. The role of IL-33 in gut mucosal inflammation. Mediators of Inflammation, 2013, 2013: 608187. |
| [28] | Takatori H, Makita S, Ito T, Matsuki A, Nakajima H. Regulatory mechanisms of IL-33-ST2-mediated allergic inflammation. Frontiers in Immunology, 2018, 9: 2004. DOI:10.3389/fimmu.2018.02004 |
| [29] | Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. European Journal of Immunology, 2009, 39(12): 3331-3342. DOI:10.1002/eji.200939472 |
