陈华海, 赵昌会, 朱家良, 尹业师


湘南优势植物资源综合利用湖南省重点实验室, 湖南南岭地区植物资源研究开发湖南省工程研究中心, 湖南科技学院化学与生物工程学院, 湖南 永州 425199
收稿日期:2020-08-30;修回日期:2020-10-30;网络出版日期:2021-02-18
基金项目:中央引导地方科技发展专项(2019XF5067);湖南省自然科学基金(2020JJ2016,2019JJ40092,2019JJ40091)
*通信作者:尹业师, Tel/Fax: +86-746-6381164;E-mail: yinyeshi@126.com.
摘要:[目的] 银杏提取物在防治心血管系统和神经系统疾病方面发挥重要功能。鉴于肠道菌群已被认定为一个新兴的药物作用靶标,研究银杏双黄酮和银杏内酯与人体肠道菌群之间的相互作用具有非常重要的意义,这将为进一步理解银杏提取物的功能和作用机制奠定基础。[方法] 本研究使用人体肠道菌群体外批量发酵、细菌总量测定、细菌16S rDNA高通量测序、气相色谱和液相色谱检测等方法,对银杏双黄酮和银杏内酯B单独或复合在体外与人体肠道菌群的相互作用进行研究。[结果] 银杏双黄酮和银杏内酯B单独添加对人体肠道菌群总量、肠道菌群结构组成和短链脂肪酸产量没有显著影响。但有意思的是,复合添加银杏双黄酮和银杏内酯B后,Coriobacteriaceae科和Cupriavidus属细菌的比例显著升高,Gemella菌细菌比例显著降低。功能基因预测分析发现,编码K00076、K12143、K07716和K00220的基因在复合添加银杏双黄酮和银杏内酯B后显著富集。K00076和K00220是氧化还原酶,催化CH-OH供体基团的电子转移,可能参与银杏双黄酮和银杏内酯B的代谢和修饰。HPLC检测发现,人体肠道菌群体外对银杏双黄酮和银杏内脂B的降解修饰率分别为70%和35%左右。[结论] 体外复合添加银杏双黄酮和银杏内酯B可显著改变肠道某些细菌的丰度。同时,体外研究表明肠道菌群具有代谢修饰银杏双黄酮和银杏内酯B的功能。
关键词:银杏双黄酮银杏内酯B人体肠道菌群
Study the interactions between ginkgetin and ginkgolide B with human gut microbiota in vitro
Huahai Chen, Changhui Zhao, Jialiang Zhu, Yeshi Yin


Key Laboratory of Comprehensive Utilization of Advantage Plants Resources in Hunan South, Hunan Engineering Research Center for Research and Development of Plant Resources in Nanling Area, College of Chemistry and Bioengineering, Hunan University of Science and Engineering, Yongzhou 425199, Hunan Province, China
Received: 30 August 2020; Revised: 30 October 2020; Published online: 18 February 2021
*Corresponding author: Yeshi Yin, Tel/Fax: +86-746-6381164; E-mail: yinyeshi@126.com.
Foundation item: Supported by the Project for Guiding Local Science and Technology Development by the Central Government (2019XF5067) and by the Hunan Natural Science Foundation (2020JJ2016, 2019JJ40092, 2019JJ40091)
Abstract: [Objective] Ginkgo biloba extract plays an important role in the prevention and treatment of cardiovascular and nervous system diseases. In consideration of the fact that gut microbiota has been identified as a new drug target, it is of great significance to study the interactions between ginkgetin and ginkgolide B with human gut microbiota, which will lay a foundation for further understanding the function and mechanism of Ginkgo biloba extract. [Methods] In this study, batch fermentation, bacterial amount detection, 16S rDNA high-throughput sequencing, GC and HPLC measurement were used to study the interactions between ginkgetin and/or ginkgolide B with human gut microbiota in vitro. [Results] Neither ginkgetin nor ginkgolide B alone had a significant effect on the total amount of gut microbiota, the composition of intestinal flora and the production of short chain fatty acids by the gut microbiota. However, when ginkgetin and ginkgolide B were added in combination, the proportion of bacterial represented by the family Coriobacteriaeae and genus Cupriavidus increased significantly, while the proportion represented by the genus Gemella decreased significantly. Functional gene prediction analysis found that genes encoding K00076, K12143, K07716, and K00220 significantly enriched in the presence of ginkgetin and ginkgolide B. Moreover, K00076 and K00220 are oxidoreductases that catalyze the transfer of electrons from the CH-OH donor group and may be involved in the metabolism and modification of ginkgetin and ginkgolide B. The degradation and modification rates of ginkgetin and ginkgolide B by human gut microbiota in vitro were ~70% and ~35%, respectively. [Conclusion] The relative percentage of some intestinal bacteria was significantly changed by the combined addition of ginkgetin and ginkgolide B in vitro. Meanwhile, human gut microbiota has the function on metabolism or modifying ginkgetin and ginkgolide B.
Keywords: ginkgetinginkgolide Bhuman gut microbiota
银杏为银杏科银杏属植物,是一种寿命极长的古老树种,最早曾出现在侏罗纪和白垩纪时期,为少数基本保持了2亿年前生态特征并幸存下来的植物之一,有“活化石”之称[1]。因银杏为中国独有,其他国家的银杏树均直接或间接从中国引进,所以银杏又被称为中国国宝和植物界的“大熊猫”。银杏化学成分丰富,具有多种生物活性,但主要活性成分以银杏黄酮和银杏内酯为主。银杏叶和白果分别被《中国药典》收录,已经被报道具有辅助降血脂、辅助降血压、增强免疫力、辅助保护肝损伤、提高缺氧耐受力、抗氧化、保护胃黏膜和改善记忆力等保健功能[2]。有临床研究表明,银杏对脑梗死[3-4]、冠心病[5-7]和糖尿病[8-9]等具有一定的防治作用。市场上也已有多种银杏提取物相关产品出售,如用于心血管系统疾病防治的银杏达莫注射液和血络通胶囊;用于脑神经系统疾病防治的金纳多注射液和达纳康片剂等。
人体肠道菌群具有免疫调控[10]、参与物质代谢和营养吸收[11]等重要功能,被报道与多种疾病的发生发展密切相关[12]。同时,肠道菌群也可以参与某些药物的代谢与修饰,从而影响药物的药效和毒副作用[13-16]。因此,人体肠道菌群已经被认定为多种疾病防治的重要靶标[17-21]。药物,包括很多非抗生素类药物被报道具有调控肠道菌群结构的功能[22-24]。Maier等研究发现1000多种常用药物中,24%的非抗菌素类药物具有抑制肠道细菌生长的功能,其中40种药物具有广谱抑菌活性[24]。而且多种药物可能通过调控肠道菌群影响疾病防治效果,如二甲双胍通过改变二型糖尿病患者肠道菌群而达到治疗效果[25];低聚甘露酸钠通过调控肠道菌群并抑制肠道细菌氨基酸引起的神经炎症,从而缓解阿尔茨海默症[26]。还有一些药物需要与肠道菌群共同发挥作用才能发挥更好的疗效[27]。如肠道细菌和肿瘤内细菌可参与调节药物的有效性和毒性,促进或削弱多种化疗药物的抗癌效果和毒副作用[28]。由于中草药多以口服为主,其药代动力学的影响和药效的发挥与肠道菌群关系密切[29],中草药与肠道菌群的相互作用研究已经成为新的研究热点之一。
虽然银杏提取物的生理功能已有较多研究,但其作用机制还有待进一步研究。银杏提取物与肠道菌群的相互作用研究可能会是一个新的突破口。肉鸡和仔猪饲养实验表明,银杏叶提取物可能通过抑制大肠杆菌生长和促进乳酸杆菌繁殖改善动物生长性能[30-32]。Choi等研究发现肠道菌群可以影响银杏叶提取物在小鼠体内的生物利用度[33]。张磊艺等对糖尿病大鼠进行研究发现,银杏叶提取物可能通过促进肠道有益菌生长、影响脂质代谢,从而达到调脂目的[34]。Chen等发现一种水溶性银杏多糖可能通过增加乳酸菌种类的丰富性,修复抑郁症小鼠的肠道菌群失衡,从而表现出缓解抑郁的功效[35]。然而,到目前为止,关于银杏提取物与人体肠道菌群相互作用的研究还鲜见报道。鉴于中医药多以复方、多成分药物配伍使用为主,本研究不仅研究了单独使用银杏双黄酮或银杏内酯B在体外对人体肠道菌群的调控作用,也研究了2种药物复合使用后对人体肠道菌群的影响。同时,本研究也对肠道菌群代谢修饰银杏双黄酮和银杏内酯B的情况进行了初步分析。这将为进一步阐明中医药的分子作用机制奠定基础。
1 材料和方法 1.1 试验材料 银杏内酯B和银杏双黄酮购于成都普瑞法科技开发有限公司,产品纯度大于98%。
1.2 志愿者粪便样品收集 本试验共收集12名成年健康志愿者(男女各6名)粪便样品。志愿者年龄介于20岁至60岁之间。所有志愿者的日常饮食均是中国传统食物,无素食主义者。采样前至少3个月未曾服用抗生素或有过医院治疗经历等,没有出现过腹泻等胃肠不适症状。
1.3 体外发酵培养基配制 根据本实验室前期工作经验选择在体外对人体肠道菌群有较好模拟效果的VIS培养基[36-37]。具体培养基配方如下(g/L):可溶性淀粉8.0,胰蛋白胨3.0,蛋白胨3.0,酵母提取物4.5,粘液素0.5,L-半胱氨酸盐酸盐0.8,3号胆盐0.4,血红素0.05,吐温80 1 mL,氯化钾2.5,氯化钠4.5,六水氯化镁4.5,六水氯化钙0.2,磷酸二氢钾0.4,刃天青10,微量元素溶液2 mL。微量元素包括(g/L):七水硫酸镁3.0,二水氯化钙0.1,四水氯化锰0.32,七水硫酸铁0.1,七水硫酸钴0.18,七水硫酸锌0.18,五水硫酸铜0.01,六水氯化镍0.092。将培养基pH调节至6.5,定容后高压灭菌,然后分装到20 mL无菌离心管中,每管5 mL。根据培养基中是否添加药物,将培养基分为4组,分别为VIS组(没添加任何药物的对照组)、VIS-T (培养基中添加了终浓度16 μg/mL的银杏双黄酮)、VIS-B组(培养基中添加了终浓度16 μg/mL的银杏内酯B)、VIS-T-B (培养基中添加了终浓度16 μg/mL的银杏双黄酮和16 μg/mL的银杏内酯B)。
1.4 粪菌悬液制备 称取志愿者粪便样品5 g溶解入50 mL高压灭菌后的PBS中(0.1 mol/L,pH 7.0),充分混匀后制成10% (W/V)粪便匀浆。使用4层纱布过滤后,将上清液转移到20 mL离心管中备用。
1.5 体外发酵与样品收集 将分装后的培养基放入厌氧工作站中静置8 h。将制备好的粪菌悬液分别接种入VIS、VIS-T、VIS-B和VIS-T-B培养基,每4.5 mL培养基中接种500 μL (5%)粪菌悬液,摇匀后放置在厌氧工作站中静置培养。分别在接种后24 h和48 h各取样1.5 mL,10000 r/min离心2 min后,将沉淀和上清液分别保存到–30 ℃冰箱,其中沉淀用于细菌总量测定和DNA测序,上清液用于短链脂肪酸、银杏双黄酮和银杏内酯B的含量测定。
1.6 细菌总量测定 将细菌沉淀用无菌水洗涤后,用无菌水重悬混匀,10倍稀释后使用分光光度计测量OD600吸光值。
1.7 细菌基因组DNA提取 采用QIAamp粪便细菌基因组DNA提取试剂盒,根据试剂盒说明书对粪便样本和发酵样品进行细菌基因组DNA提取。
1.8 细菌16S rDNA V3-V4区高通量测序与分析 将提取的细菌基因组DNA样品送到杭州利贞生物医药科技有限公司采用Illumina Miseq进行二代测序。扩增引物为338F (5?-ACTCCTACGG GAGGCAGCA-3?)和806R (5?-GGACTACHVGGG TWTCTAAT-3?)。测序获得的序列首先使用QIIME和Mothur软件进行质检和OTUs分类(以97%相似性为分类阈值)。每个OTU的代表序列使用RDP分类器和SILVA数据库进行细菌分类注释。对获得的OTU表和细菌分类注释表,采用上海盈飞生物科技有限公司开发的细菌多样性分析软件包(http://amplicon.vgenomics.cn:9000/)进行分析。分析模块主要包括α多样性分析、β多样性分析、细菌组成分析、LEfSe分析和PICRUST细菌基因功能预测分析。原始序列数据已提交到NCBI数据库(PRJNA60746)。
1.9 短链脂肪酸含量测定 发酵液上清液采用岛津气相色谱仪GC plus 2010进行短链脂肪酸含量分析。分析方法参照本实验室前期发表的文章[37-38]。使用DB-FFAP型气相色谱柱(0.32 mm×30 m×0.5 μm)和氢气火焰离子化检测器,以巴豆酸为内标物,以乙酸、丙酸、丁酸、异丁酸、戊酸和异戊酸为标准品进行检测。气相色谱的进样口参数为:进样量1 μL、温度250 ℃、载气类型为N2、吹扫流量3.0 mL/L、压力54.2 kPa、分流比为8:1。线速的控制模式:线速度28.1 cm/s、总流量16.1 mL/min、色谱柱流量1.46 mL/min。
1.10 肠道菌群对银杏双黄酮和银杏内酯B的降解分析 参考Mesbah等的方法[39]使用Vertex C18 (4.6 mm×250 mm,5 μm)色谱柱对发酵液中银杏双黄酮和银杏内酯的含量进行HPLC检测。色谱柱温度30 ℃,进样量10 μL,流速1.0 mL/min。银杏双黄酮的检测波长为330 nm;流动相为0.1%磷酸水溶液(A相)和乙腈(B相);0–10 min,55% A和45% B;10–20 min,45% A和55% B。银杏内酯B的检测波长为220 nm,流动相为水: 甲醇: 异丙醇=72.5:17.5:10.0。分别称取1 mg银杏双黄酮和银杏内酯B于1 mL甲醇中,并稀释成一系列浓度梯度,制作标准曲线。利用对照组(未加菌液)与实验组中银杏双黄酮和银杏内酯B的浓度差异,计算降解修饰率。
1.11 统计分析 实验数据通过IBM SPSS Statistics 20.0软件对各组数据之间进行独立样品t检验。P < 0.05被认为具有显著差异。
2 结果和分析 2.1 银杏双黄酮和银杏内酯B对细菌总量的影响 使用分光光度计法对发酵后细菌OD600值进行测定,结果发现无论是发酵后24 h还是发酵后48 h,银杏双黄酮和银杏内酯B对细菌OD600值均没有显著影响。在发酵后24 h VIS、VIS-T、VIS-B和VIS-T-B组OD600值分别为2.10、2.32、2.03和2.37。发酵后48 h的OD600值分别为2.29、2.57、2.18和2.58。虽然发酵后48 h的OD600值较发酵后24 h有所增加,但增加幅度不大(图 1)。
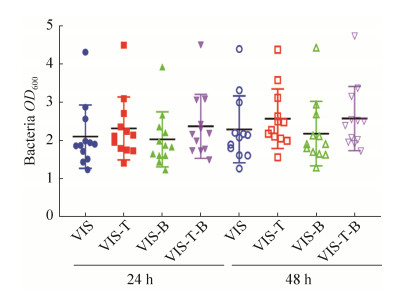 |
| 图 1 银杏双黄酮和银杏内酯B对总细菌含量的影响 Figure 1 Effects of ginkgetin and ginkgolide B on the amount of total bacteria. |
| 图选项 |
2.2 银杏双黄酮和银杏内酯B对人体肠道菌群的α多样性和β多样性的影响 本研究共对60个样品进行了二代测序,初步分析剔除少量非细菌序列后,获得2390377条细菌16S V3–V4区序列,平均每个样品获得近40000条有效序列。从图 2-A可以看出,测序覆盖率均大于99%,说明测序深度已经能满足分析需要。对所有序列进行分析,共鉴定到1171个OTUs。但从每个样品中鉴定到的OTU数目从70到297不等。统计学分析表明,银杏双黄酮和银杏内酯B对人体肠道菌群的α多样性,包括Ace、Chao、Shannon和Simpson等均没有显著影响。PCA、PCoA和NMDS等β多样性分析结果发现,实验样品并没有按照不同处理表现出明显的按组别聚类(结果图没有展示)。H Cluster tree分析也得到类似结果(图 3)。无论是在细菌门水平还是细菌属水平,聚类树分析结果并没有发现明显的规律可循,也没有按照是否添加药物为组进行聚类。说明银杏双黄酮和银杏内酯B单独或混合添加并没有在整体水平对肠道菌群有很大的调控影响。
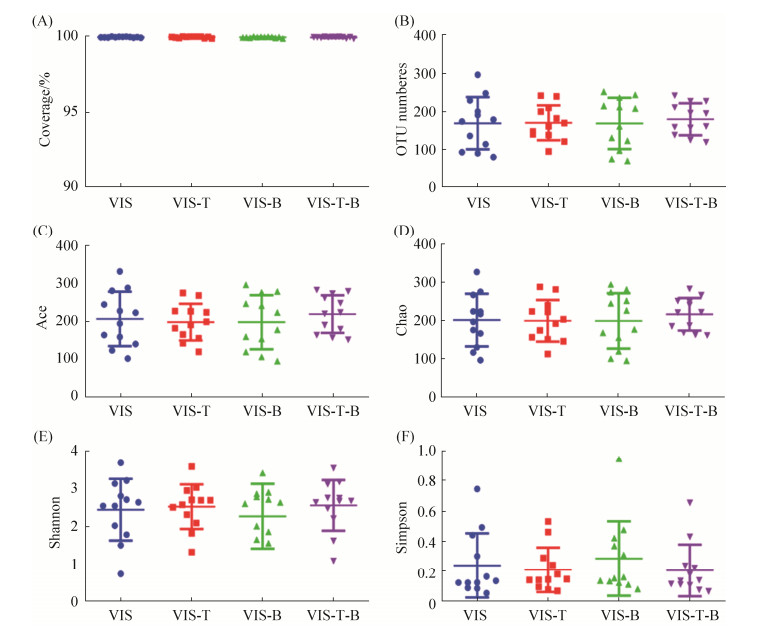 |
| 图 2 银杏双黄酮和银杏内酯B发酵后细菌α多样性分析 Figure 2 α diversity analysis of gut microbiota fermented with ginkgetin and/or ginkgolide B. |
| 图选项 |
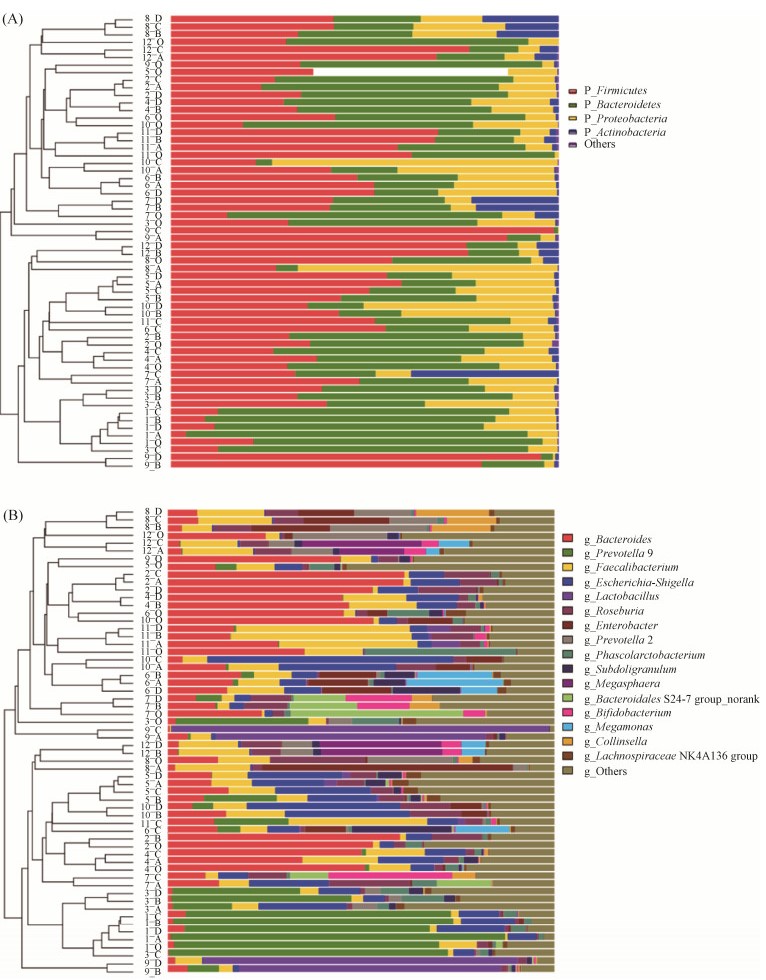 |
| 图 3 银杏双黄酮和/或银杏内酯B体外发酵后细菌组成分析 Figure 3 Bacterial community analysis of gut microbiota fermented with ginkgetin and/or ginkgolide B in vitro. Figures A and B indicated the bacterial composition at the phylum and genus levels, respectively. Numbers 1–12 on the right side of the cluster tree represent the volunteer number; letters A–D represent VIS, VIS-T, VIS-B, AVIS-T-B groups, respectively. |
| 图选项 |
2.3 银杏双黄酮和银杏内酯B对人体肠道细菌组成的影响 在细菌门的水平(图 3-A),在所有检测的12个原始粪便样品和发酵后的48个样品中,大于1%的细菌门共有4个,分别为拟杆菌门、厚壁菌门、放线菌门和变形菌门,分类到这4个细菌门的序列数占了总序列数的99.77%。但以拟杆菌门和厚壁菌门为主,在原始样品中,拟杆菌门和厚壁菌门占了总序列数的89.38%,在发酵样品中占了80.09%。在细菌属的水平(图 3-B),在所有检测样品中的含量大于1%的属有16个,分类到这16个细菌属的序列数占了总序列数的80.57%。其中含量较高的为拟杆菌属、普雷沃菌属、柔嫩梭菌属、大肠杆菌-志贺菌属和乳杆菌属,分类到这5个属的序列数超过了总序列数的50%。
为了研究银杏双黄酮和银杏内酯B对人体肠道菌群在个别菌属水平的影响,我们采用LEfSe进行了进一步的分析。结果发现尽管单独添加银杏双黄酮(VIS-T组)或银杏内酯B(VIS-B组)与不添加药物组(VIS组)相比并没有发现任何存在显著差异的菌群(数据未显示),但复合用药组(VIS-T-B组)与VIS组相比,Coriobacteriaceae科和Cupriavidus属含量明显更高,而Gemella属含量显著更低(图 4-A)。考虑到性别是影响肠道菌群组成的重要因素之一,我们按不同性别对复合用药组(VIS-T-B组)与不添加药物组(VIS组)的差异菌群进行了进一步分析。结果发现在男性志愿者
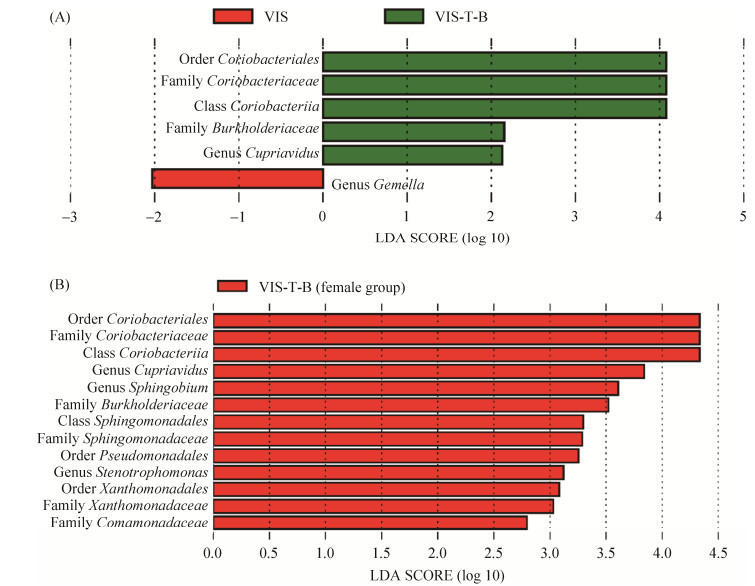 |
| 图 4 LEfSe分析差异显著细菌 Figure 4 Differential abundance of bacterial taxa analyzed by using LEfSe. A: the significantly different bacteria between groups VIS-T-B and VIS; B: the significantly different bacteria among female volunteers between groups VIS-T-B and VIS. |
| 图选项 |
样品中,VIS-T-B组与VIS组之间没有检测到差异显著细菌,但在女性志愿者样品中,不仅检测到Coriobacteriaceae科和Cupriavidus属在VIS-T-B组更高,且Pseudomonadales目、Comamonadaceae科、Stenotrophomonas属和Sphingobium属细菌丰度在VIS-T-B组中显著高于VIS组(图 4-B)。
2.4 银杏双黄酮和银杏内酯B对人体肠道菌群功能基因的影响 由于复合用药组与对照组相比,促进了Coriobacteriaceae科和Cupriavidus属细菌的生长,降低了Gemella属细菌的比例。我们进一步采用PICRUST软件对复合用药在功能基因水平对肠道细菌的影响进行了预测分析。分析结果显示,尽管在COG水平,VIS-T-B组与VIS组之间没有找到差异显著的COG,但在功能基因水平,VIS-T-B组中K00076(7-α-羟甾类脱氢酶[EC: 1.1.1.159])、K12143 (hydrogenase-4 component H)、K07716 (细胞周期传感器组氨酸激酶PleC [EC: 2.7.13.3])和K0020 (环己二烯脱氢酶[EC: 1.3.1.43])所占百分比显著高于VIS组(图 5)。
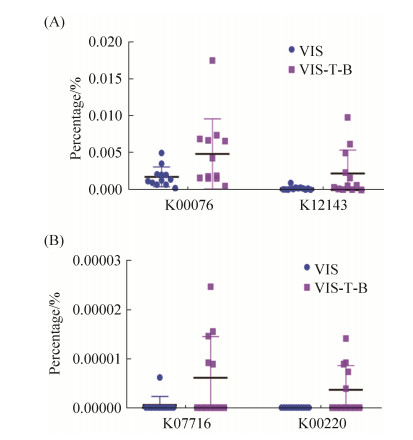 |
| 图 5 PICRUSt预测差异显著功能基因 Figure 5 Significantly different functional genes predicted by PICRUSt software. |
| 图选项 |
2.5 银杏双黄酮和银杏内酯B对人体肠道菌群短链脂肪酸产量的影响 乙酸、丙酸、丁酸、异丁酸、戊酸和异戊酸是肠道菌群产生的常见短链脂肪酸。对发酵上清液进行气相色谱检测发现,银杏双黄酮和银杏内酯B单独或复合添加在发酵后24 h和48 h均没有显著影响这些常见短链脂肪酸的含量(数据未显示)。总短链脂肪酸含量(6种短链脂肪酸的浓度之和)分析发现,各组之间也没有显著差异(图 6)。
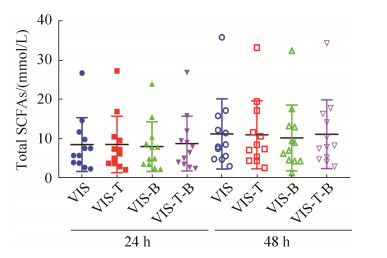 |
| 图 6 银杏双黄酮和/或银杏内酯B体外发酵对短链脂肪酸产量的影响 Figure 6 SCFAs produced by gut microbiota fermented with ginkgetin and/or ginkgolide B in vitro. |
| 图选项 |
2.6 肠道菌群对银杏双黄酮和银杏内酯B的降解修饰分析 根据对照组与实验组的峰面积差异,计算银杏双黄酮和银杏内酯B的降解修饰率。肠道菌群对银杏双黄酮和银杏内酯B的降解修饰率分别为70%和35%左右。发酵后24 h和48 h,两种物质的降解修饰率差异不明显,但在复合添加组中的降解修饰率略低于单一成分添加组(图 7)。
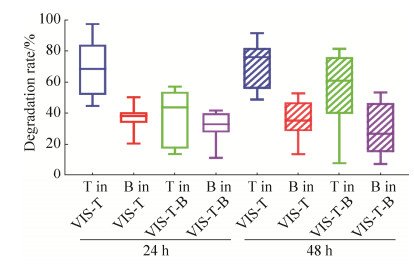 |
| 图 7 肠道菌群对银杏双黄酮和银杏内酯B的降解修饰分析 Figure 7 Degradation or modification of ginkgetin and ginkgolide B by intestinal microbiota. |
| 图选项 |
3 讨论 肠道菌群是目前生命科学研究的热点领域之一。尽管肠道菌群与药物之间的相互作用已经成为新药研发的潜在治疗靶标,很多药物都报道具有调控肠道菌群的功能[40],但并不是每种药物都具有很强的肠道菌群调控功能。如本研究结果表明在发酵培养基中添加16 μg/mL的银杏双黄酮或银杏内酯B对12名志愿者的肠道菌群影响并不显著。但有意思的是,复合添加银杏双黄酮和银杏内酯B虽然对细菌总量和细菌短链脂肪酸的产生没有显著影响,但可以显著增加Coriobacteriaceae科和Cupriavidus属细菌的比例,显著降低Gemella属细菌的丰度。已有研究表明,Coriobacteriaceae科细菌在结肠炎患者[41]和糖尿病小鼠[42]中明显减少,并且与血清和肝脏脂肪含量呈负相关[43]。而Gemella属的含量在患有胆汁淤积性黄疸的婴儿[44]和某些肿瘤组织中[45]较高。因此,我们推测银杏双黄酮与银杏内酯B复合使用可能具有改善健康的作用。进一步按性别分组,分析复合添加银杏双黄酮和银杏内酯B在体外对人体肠道菌群的调控作用。结果发现在男性志愿者中,没有鉴定到丰度出现显著差异的菌属,但在女性志愿者中,复合添加银杏双黄酮和银杏内酯B显著促进Coriobacteriaceae科和Cupriavidus属细菌的生长。这可能是由于男女志愿者体内性激素水平存在差异,引起肠道菌群组成发生变化[46],从而导致其对外界处理的响应不同。Harada等研究发现,性激素水平的不同,可能是引起肠道微生物菌群性别依赖性差异的原因之一,并且可能影响其对饮食和营养的响应[47]。银杏双黄酮和银杏内酯B性别选择性菌群调控作用和潜在的保健功能有待进一步体内实验验证。
临床中草药的使用大多以复方形式存在,每种药方中可能含有多种已知或未知的有效成分。如果单独研究每一种有效成分的功能,然后再将各有效成分的功能进行简单相加,可能会错失很多信息。如本研究发现单独添加银杏双黄酮或银杏内酯B,并没有发现丰度出现显著变化的菌属,但复合添加银杏双黄酮或银杏内酯B后,Coriobacteriaceae科和Cupriavidus属细菌的丰度显著高于VIS对照组。而且在临床上药物配伍增效或配伍禁忌的例子也很多。Yang等发现人参与大黄配伍对急性脑缺血再灌注损伤具有协同保护作用[48]。Cai等发现七叶皂苷、姜黄素和橙皮苷以最佳比例使用时具有协同作用[49]。本研究为进一步研究中药的作用机制,特别是通过改变肠道菌群来发挥作用的中药,具有重要的参考价值。
本研究的另一个发现是人体肠道菌群在体外对银杏双黄酮和银杏内酯B具有降解修饰功能。且复合添加银杏双黄酮和银杏内酯B显著富集了K00076 (7-α-羟类固醇脱氢酶)和K0020 (环己二烯脱氢酶)的比例。这些基因是氧化还原酶,具有作用于CH-OH基团的功能。这些富集的基因可能参与了银杏双黄酮和银杏内酯B的代谢与修饰。因为在银杏内酯B中含有2个CH-OH键,在银杏双黄酮中含有4个C-OH键。已有研究报道肠道微生物可以通过水解、氧化和甲基化修饰等参与药物代谢与修饰[16]。但肠道菌群对银杏双黄酮和银杏内酯B的代谢与修饰还有待进一步研究。
人体肠道菌群的结构与组成复杂多样,受到的影响因素较多,特别是体内实验容易受到饮食、药物与环境等的影响。另外,生物伦理审查和经济成本较高也限制了人体实验的开展。因此,很难评估药物或功能因子与人体肠道菌群的直接相互作用。虽然动物模型尤其是小鼠模型已广泛应用于肠道菌群相关研究,但小鼠与人类的肠道菌群在组成和结构上存在较大差异[50],限制了动物模型的广泛使用。探索人体肠道菌群与药物相互作用的另一种替代方法是使用肠道菌群体外模拟发酵技术[51-52]。已有大量研究表明,体外模拟发酵的菌群与人体粪便菌群具有较高的相似性,是研究药物或功能因子与人体肠道菌群相互作用的较好工具[36, 53]。与人体临床实验相比,肠道菌群体外模拟发酵技术具有操作简便、成本低廉和避免宿主干扰等独特优势[52]。但由于体外模拟发酵实验没有考虑宿主和环境因素等可能会影响肠道菌群与药物之间的相互作用,体外实验结果需要进一步在临床上进行体内实验验证。
References
| [1] | Yang YF, Li Y, Wang JH, Sun K, Tao WY, Wang ZZ, Xiao W, Pan YQ, Zhang SW, Wang YH. Systematic investigation of Ginkgo biloba leaves for treating cardio-cerebrovascular diseases in an animal model. ACS Chemical Biology, 2017, 12(5): 1363-1372. DOI:10.1021/acschembio.6b00762 |
| [2] | Li YP, Zhang LH, Wu HY, Su EZ, Zhao LG, Xiao W. Progress in the study of functional groups and health care efficacy of Ginkgo biloba leaves. Food Research and Development, 2020, 41(15): 182-187. (in Chinese) 李艳萍, 张立虎, 吴红雁, 苏二正, 赵林果, 萧伟. 银杏叶功效群组分及其功效的研究进展. 食品研究与开发, 2020, 41(15): 182-187. DOI:10.12161/j.issn.1005-6521.2020.15.031 |
| [3] | Wang EC, Yang MB. Clinical effect of ginkgolide injection in the adjuvant treatment of acute cerebral infarction. Chinese and Foreign Medical Research, 2020, 18(23): 38-40. (in Chinese) 汪贰成, 杨明波. 银杏内酯注射液辅助治疗急性脑梗死的临床效果. 中外医学研究, 2020, 18(23): 38-40. |
| [4] | Ji HJ, Zhou XH, Wei WL, Wu WY, Yao S. Ginkgo biloba extract as an adjunctive treatment for ischemic stroke: a systematic review and meta-analysis of randomized clinical trials. Medicine, 2020, 99(2): e18568. DOI:10.1097/MD.0000000000018568 |
| [5] | Li JY. Observation on 80 cases of unstable angina pectoris treated with Ginkgo injection. Cardiovascular Disease Electronic Journal of Integrated Traditional Chinese and Western Medicine, 2020, 8(9): 43-44. (in Chinese) 李阶义. 银杏注射液治疗不稳定型心绞痛80例观察. 中西医结合心血管病电子杂志, 2020, 8(9): 43-44. |
| [6] | Tan D, Wu JR, Duan XJ, Cui YY, Liu S, Jing ZW. Efficacy and safety of Ginkgo injections in the treatment of angina pectoris caused by coronary heart disease in China: a network meta-analysis and systematic review. Journal of Traditional Chinese Medicine, 2019, 39(3): 285-296. |
| [7] | Jin L. Effect of Ginkgo biloba capsule on symptom improvement and oxidative stress injury in patients with coronary heart disease and angina pectoris. Journal of Aerospace Medicine, 2020, 31(4): 470-472. (in Chinese) 金玲. 银杏叶胶囊对冠心病心绞痛患者症状改善及氧化应激损伤的影响. 航空航天医学杂志, 2020, 31(4): 470-472. DOI:10.3969/j.issn.2095-1434.2020.04.047 |
| [8] | Wang ZG. Effect of Yinxingdamo injection on renal function and hemorheology in patients with diabetic nephropathy. Henan Medical Research, 2020, 29(16): 3005-3006. (in Chinese) 王志光. 银杏达莫注射液对糖尿病肾病患者肾功能及血液流变学的影响. 河南医学研究, 2020, 29(16): 3005-3006. DOI:10.3969/j.issn.1004-437X.2020.16.062 |
| [9] | Tabrizi R, Nowrouzi-Sohrabi P, Hessami K, Rezaei S, Jalali M, Savardashtaki A, Shahabi S, Kolahi AA, Sahebkar A, Safiri S. Effects of Ginkgo biloba intake on cardiometabolic parameters in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of clinical trials. Phytotherapy Research, 2021, 35(1): 246-255. DOI:10.1002/ptr.6822 |
| [10] | Chen FD, Stappenbeck TS. Microbiome control of innate reactivity. Current Opinion in Immunology, 2019, 56: 107-113. DOI:10.1016/j.coi.2018.12.003 |
| [11] | Visconti A, le Roy CI, Rosa F, Rossi N, Martin TC, Mohney RP, Li WZ, de Rinaldis E, Bell JT, Venter JC, Nelson KE, Spector TD, Falchi M. Interplay between the human gut microbiome and host metabolism. Nature Communications, 2019, 10(1): 4505. DOI:10.1038/s41467-019-12476-z |
| [12] | Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. The New England Journal of Medicine, 2016, 375(24): 2369-2379. DOI:10.1056/NEJMra1600266 |
| [13] | Zhang JH, Zhang JM, Wang R. Gut microbiota modulates drug pharmacokinetics. Drug Metabolism Reviews, 2018, 50(3): 357-368. DOI:10.1080/03602532.2018.1497647 |
| [14] | Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Translational Research, 2017, 179: 204-222. DOI:10.1016/j.trsl.2016.08.002 |
| [15] | Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature, 2019, 570(7762): 462-467. DOI:10.1038/s41586-019-1291-3 |
| [16] | Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science, 2019, 363(6427): eaat9931. DOI:10.1126/science.aat9931 |
| [17] | Walsh J, Griffin BT, Clarke G, Hyland NP. Drug-gut microbiota interactions: implications for neuropharmacology. British Journal of Pharmacology, 2018, 175(24): 4415-4429. DOI:10.1111/bph.14366 |
| [18] | Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia, 2017, 60(6): 943-951. DOI:10.1007/s00125-017-4278-3 |
| [19] | Harris LA, Baffy N. Modulation of the gut microbiota: a focus on treatments for irritable bowel syndrome. Postgraduate Medicine, 2017, 129(8): 872-888. DOI:10.1080/00325481.2017.1383819 |
| [20] | Suk KT, Kim DJ. Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease. Expert Review of Gastroenterology & Hepatology, 2019, 13(3): 193-204. |
| [21] | Uchiyama K, Naito Y, Takagi T. Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharmacology & Therapeutics, 2019, 199: 164-172. |
| [22] | Spanogiannopoulos P, Turnbaugh PJ. Broad collateral damage of drugs against the gut microbiome. Nature Reviews Gastroenterology & Hepatology, 2018, 15(8): 457-458. |
| [23] | Hitchings R, Kelly L. Predicting and understanding the human microbiome's impact on pharmacology. Trends in Pharmacological Sciences, 2019, 40(7): 495-505. DOI:10.1016/j.tips.2019.04.014 |
| [24] | Maier LS, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature, 2018, 555(7698): 623-628. DOI:10.1038/nature25979 |
| [25] | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manner?s-Holm L, St?hlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, B?ckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nature Medicine, 2017, 23(7): 850-858. DOI:10.1038/nm.4345 |
| [26] | Wang XY, Sun GQ, Feng T, Zhang J, Huang X, Wang T, Xie ZQ, Chu XK, Yang J, Wang H, Chang SS, Gong YX, Ruan LF, Zhang GQ, Yan SY, Lian W, Du C, Yang DB, Zhang QL, Lin FF, Liu J, Zhang HY, Ge CR, Xiao SF, Ding J, Geng MY. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Research, 2019, 29(10): 787-803. DOI:10.1038/s41422-019-0216-x |
| [27] | Lam KN, Alexander M, Turnbaugh PJ. Precision medicine goes microscopic: engineering the microbiome to improve drug outcomes. Cell Host & Microbe, 2019, 26(1): 22-34. |
| [28] | Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome, 2018, 6(1): 1-13. DOI:10.1186/s40168-017-0383-2 |
| [29] | Wang J, Feng WW, Tang F, Ao H, Peng C. Gut microbial transformation, a potential improving factor in the therapeutic activities of four groups of natural compounds isolated from herbal medicines. Fitoterapia, 2019, 138: 104293. DOI:10.1016/j.fitote.2019.104293 |
| [30] | Li Y, Yang XY, He YQ, Wang H, Xu WH. Effect of extract of Ginkgo biloba on the intestinal microflora and morphology of intestinal tissues of broilers. Chinese Journal of Veterinary Medicine, 2009, 45(11): 39-41. (in Chinese) 李焰, 杨小燕, 何玉琴, 王华, 许卫华. 银杏叶提取物对肉鸡肠道微生物区系及肠组织形态的影响. 中国兽医杂志, 2009, 45(11): 39-41. DOI:10.3969/j.issn.0529-6005.2009.11.018 |
| [31] | Li Y, Yang XY, Huang QC, He YQ. Effects of Ginkgo biloba extract on growth performance, nutrient utilization and intestinal microflora of broilers. Chinese Journal of Animal Science, 2009, 45(23): 47-49. (in Chinese) 李焰, 杨小燕, 黄其春, 何玉琴. 银杏叶提取物对肉鸡生产性能、营养素利用率和肠道菌群数量的影响. 中国畜牧杂志, 2009, 45(23): 47-49. |
| [32] | Huang QC, Zheng XT, Huang CQ, Chen T, Li HY. Effects of Ginkgo biloba leaves ultrafine powder on growth performance, intestinal microflora and intestinal morphology in weaned piglets. Chinese Journal of Animal Science, 2018, 54(11): 105-109. (in Chinese) 黄其春, 郑新添, 黄翠琴, 陈彤, 李虹仪. 银杏叶超微粉对断奶仔猪生长性能、肠道菌群及其形态的影响. 中国畜牧杂志, 2018, 54(11): 105-109. |
| [33] | Choi MS, Kim JK, Kim DH, Yoo HH. Effects of gut microbiota on the bioavailability of bioactive compounds from Ginkgo leaf extracts. Metabolites, 2019, 9(7): E132. DOI:10.3390/metabo9070132 |
| [34] | Zhang LY, Yang JY, Wang LB. The effect of extract of Ginkgo leaf on lipid metabolism and intestinal normal flora of diabetic rats. Chinese Journal of Microecology, 2004, 16(3): 147-148. (in Chinese) 张磊艺, 杨景云, 王立波. 银杏叶提取物对糖尿病大鼠脂质代谢及肠道正常菌群的影响. 中国微生态学杂志, 2004, 16(3): 147-148. DOI:10.3969/j.issn.1005-376X.2004.03.009 |
| [35] | Chen P, Hei MF, Kong LL, Liu YY, Yang Y, Mu HB, Zhang XY, Zhao ST, Duan JY. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food & Function, 2019, 10(12): 8161-8171. |
| [36] | Yin YS, Fan B, Liu W, Ren RR, Chen HH, Bai SF, Zhu LY, Sun G, Yang YS, Wang X. Investigation into the stability and culturability of Chinese enterotypes. Scientific Reports, 2017, 7(1): 7947. DOI:10.1038/s41598-017-08478-w |
| [37] | Liu GY, Chen HH, Chen JK, Wang X, Gu Q, Yin YS. Effects of bifidobacteria-produced exopolysaccharides on human gut microbiota in vitro. Applied Microbiology and Biotechnology, 2019, 103(4): 1693-1702. DOI:10.1007/s00253-018-9572-6 |
| [38] | Bai SF, Chen HH, Zhu LY, Liu W, Yu HD, Wang X, Yin YS. Comparative study on the in vitro effects of Pseudomonas aeruginosa and seaweed alginates on human gut microbiota. PLoS ONE, 2017, 12(2): e0171576. DOI:10.1371/journal.pone.0171576 |
| [39] | Mesbah MK, Khalifa SI, El-Gindy A, Tawfik KA. HPLC determination of certain flavonoids and terpene lactones in selected Ginkgo biloba L. phytopharmaceuticals. Il Farmaco, 2005, 60(6/7): 583-590. |
| [40] | Savage N. The complex relationship between drugs and the microbiome. Nature, 2020, 577(7792): S10-S11. DOI:10.1038/d41586-020-00196-0 |
| [41] | Millien V, Rosen D, Hou J, Shah R. Proinflammatory sulfur-reducing bacteria are more abundant in colonic biopsies of patients with microscopic colitis compared to healthy controls. Digestive Diseases and Sciences, 2019, 64(2): 432-438. DOI:10.1007/s10620-018-5313-z |
| [42] | Zhang HH, Liu J, Lv YJ, Jiang YL, Pan JX, Zhu YJ, Huang MG, Zhang SK. Changes in intestinal microbiota of type 2 diabetes in mice in response to dietary supplementation with instant tea or matcha. Canadian Journal of Diabetes, 2020, 44(1): 44-52. DOI:10.1016/j.jcjd.2019.04.021 |
| [43] | Guo WL, Chen M, Pan WL, Zhang Q, Xu JX, Lin YC, Li L, Liu B, Bai WD, Zhang YY, Ni L, Rao PF, Lv XC. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (Ⅲ) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. International Journal of Biological Macromolecules, 2020, 145: 1208-1218. DOI:10.1016/j.ijbiomac.2019.09.206 |
| [44] | Wang YZ, Gao XF, Zhang XY, Xiao YM, Huang JD, Yu DB, Li XL, Hu H, Ge T, Li D, Zhang T. Gut microbiota dysbiosis is associated with altered bile acid metabolism in infantile cholestasis. mSystems, 2019, 4(6): e00463-e00419. |
| [45] | Allali I, Delgado S, Marron PI, Astudillo A, Yeh JJ, Ghazal H, Amzazi S, Keku T, Azcarate-Peril MA. Gut microbiome compositional and functional differences between tumor and non-tumor adjacent tissues from cohorts from the US and Spain. Gut Microbes, 2015, 6(3): 161-172. DOI:10.1080/19490976.2015.1039223 |
| [46] | Harada N, Minami Y, Hanada K, Hanaoka R, Kobayashi Y, Izawa T, Sato T, Kato S, Inui H, Yamaji R. Relationship between gut environment, feces-to-food ratio, and androgen deficiency-induced metabolic disorders. Gut Microbes, 2020, 12(1): 1817719. DOI:10.1080/19490976.2020.1817719 |
| [47] | Santos-Marcos JA, Barroso A, Rangel-Zu?iga OA, Perdices-Lopez C, Haro C, Sanchez-Garrido MA, Molina-Abril H, Ohlsson C, Perez-Martinez P, Poutanen M, Lopez-Miranda J, Perez-Jimenez F, Tena-Sempere M, Camargo A. Interplay between gonadal hormones and postnatal overfeeding in defining sex-dependent differences in gut microbiota architecture. Aging, 2020, 12(20): 19979-20000. DOI:10.18632/aging.104140 |
| [48] | Yang WT, Wang Y, Shi YH, Fu H, Xu Z, Xu QQ, Zheng GQ. Herbal compatibility of ginseng and rhubarb exerts synergistic neuroprotection in cerebral ischemia/reperfusion injury of rats. Frontiers in Physiology, 2019, 10: 1174. DOI:10.3389/fphys.2019.01174 |
| [49] | Cai CY, Chen YC, Zhong SP, Zhang YM, Jiang JY, Xu H, Shi GG. Synergistic effect of compounds from a Chinese herb: compatibility and dose optimization of compounds from N-butanol extract of Ipomoea stolonifera. Scientific Reports, 2016, 6: 27014. DOI:10.1038/srep27014 |
| [50] | Xiao L, Feng Q, Liang SS, Sonne SB, Xia ZK, Qiu XM, Li XP, Long H, Zhang JF, Zhang DY, Liu C, Fang ZW, Chou J, Glanville J, Hao Q, Kotowska D, Colding C, Licht TR, Wu DH, Yu J, Sung JJY, Liang QY, Li JH, Jia HJ, Lan Z, Tremaroli V, Dworzynski P, Nielsen HB, B?ckhed F, Doré J, Le Chatelier E, Ehrlich SD, Lin JC, Arumugam M, Wang J, Madsen L, Kristiansen K. A catalog of the mouse gut metagenome. Nature Biotechnology, 2015, 33(10): 1103-1108. |
| [51] | Marzorati M, van de Wiele T. An advanced in vitro technology platform to study the mechanism of action of prebiotics and probiotics in the gastrointestinal tract. Journal of Clinical Gastroenterology, 2016, 50(Suppl 2): S124-S125. |
| [52] | Pham VT, Mohajeri MH. The application of in vitro human intestinal models on the screening and development of pre- and probiotics. Beneficial Microbes, 2018, 9(5): 725-742. DOI:10.3920/BM2017.0164 |
| [53] | Li BY, Chen HH, Cao LY, Hu YF, Chen D, Yin YS. Effects of an Escherichia coli exopolysaccharide on human and mouse gut microbiota in vitro. International Journal of Biological Macromolecules, 2020, 150: 991-999. |
