胡夏佩1, 王惠1, 孔学维1, 佟盼盼2, 田睿3, 刘茂军3, 马勋4, 张海燕5, 张炜1


1. 南京农业大学动物医学院, 江苏 南京 210095;
2. 新疆农业大学动物医学院, 新疆 乌鲁木齐 830052;
3. 江苏省农业科学研究院, 江苏 南京 210014;
4. 新疆石河子大学动物科技学院, 新疆 石河子 832003;
5. 芜湖职业技术学院生物工程学院, 安徽 芜湖 241003
收稿日期:2020-10-10;修回日期:2020-12-18;网络出版日期:2021-05-17
基金项目:国家重点研发计划(2018YFC1602500);国家自然科学基金(U1803109);安徽省高校优秀青年人才支持计划(gxyq2019201);芜湖职业技术学院校级科技团队(wzykjtd202002)
*通信作者:张炜, Tel: +86-25-84395328;Fax: +86-25-84396517;E-mail: vszw@njau.edu.cn.
摘要:产志贺毒素大肠杆菌(Shiga toxin-producing Escherichia coli,STEC)是重要的食源性病原,而STEC往往以正常菌群的形式存在于牛羊等反刍动物肠道。[目的] 本研究对牛羊粪便样品中的STEC分离和鉴定并对分离株进行致病潜力分析。从江苏、云南和河北等地共分离到羊源STEC菌株11株,牛源STEC菌株1株,另新疆农业大学佟盼盼组馈赠牛源菌株10株。[方法] 通过细菌选择培养及特异性基因stx1和stx2的检测进行分离鉴定;并通过Vero细胞毒性试验、溶血活性试验和毒力因子的检测分析STEC分离株的致病潜力。[结果] 分离到羊源分离株11株,分离率17.5%(11/63);分离得到牛源分离株1株,分离率0.7%(1/134);11株羊源分离株中有5株对Vero细胞具有强的毒性,3株有溶血活性;11株牛源分离株中有5株对Vero细胞具有强的毒性,3株有溶血活性。11株羊源STEC分离株eae基因携带率为63.6%(7/11),而11株牛源STEC分离株eae基因携带率仅为9.0%(1/11)。[结论] 结果表明羊源STEC菌株的分离率和致病潜力高于牛源菌株,所以,除牛外,羊作为STEC菌株宿主也应该得到更多的重视。
关键词:产志贺毒素大肠杆菌Vero细胞毒性毒力基因致病潜力溶血活性
Isolation, identification and pathogenic potential analysis of Shiga toxin-producing Escherichia from ruminants
Xiapei Hu1, Hui Wang1, Xuewei Kong1, Panpan Tong2, Rui Tian3, Maojun Liu3, Xun Ma4, Haiyan Zhang5, Wei Zhang1


1. College of Animal Medical, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China;
2. College of Animal Medical, Xinjiang Agricultural University, Urumqi 830052, Xinjiang Uygur Autonomous Region, China;
3. Jiangsu Academy of Agricultural Sciences, Nanjing 210014, Jiangsu Province, China;
4. College of Animal Technology, Xinjiang Shihezi University, Shihezi 832003, Xinjiang Uygur Autonomous Region, China;
5. School of Bioengineering, Wuhu Vocational and Technical College, Wuhu 241003, Anhui Province, China
Received: 10 October 2020; Revised: 18 December 2020; Published online: 17 May 2021
*Corresponding author: Wei Zhang, Tel: +86-25-84395328; Fax: +86-25-84396517; E-mail: vszw@njau.edu.cn.
Foundation item: Supported by the National Key Research and Development Program of China (2018YFC1602500), by the National Natural Science Foundation of China (U1803109), by the Anhui Province University Outstanding Young Talents Support Program (gxyq2019201) and by the School-level Technology Team of Wuhu Vocational and Technical College (wzykjtd202002)
Abstract: Shiga toxin-producing Escherichia coli (STEC) is an important food-borne pathogen, which often exists in the intestines of ruminants, such as cow and sheep, as normal flora. [Objective] In this study, we isolated and identified Shiga toxin-producing Escherichia coli isolates from cow and sheep feces samples, and analyzed their pathogenic potentials Totally 11 Shiga toxin-producing Escherichia coli strains from sheeps and 1 isolate from cattle were isolated from Jiangsu, Yunnan, and Hebei, and another 10 isolates of STEC from cattle were donated by Tong Pan Group of Xinjiang Agricultural University. [Methods] We conducted the separation and identification through bacterial selective culture and detection of specific genes stx1 and stx2. We analyzed the pathogenic potential of the Shiga toxin-producing Escherichia coli isolates by Vero cytotoxicity test, hemolytic activity test, and toxin factor detection. [Results] In this study, the separation and identification result revealed that 11 isolates of sheep origin were isolated with an isolation rate of 17.5% (11/63); 1 isolate from cow origin was isolated with an isolation rate of 0.7% (1/134). The pathogenic potential results showed that, among the 11 sheep-derived isolates, 5 of them had strong toxicity to Vero cells, and 3 had hemolytic activity. Among the 11 cow-derived isolates, 5 had strong toxicity to Vero cells, and 3 had hemolytic activity. The eae gene carrying rate of the 11 sheep-derived Shiga toxin-producing Escherichia coli isolates was 63.6% (7/11), while the eae gene carrying rate of the cow-derived 11 Shiga toxin-producing Escherichia coli isolates was only 9.0% (1/11). [Conclusion] The results indicated that the isolation rate and pathogenic potential of the Shiga toxin-producing Escherichia coli strains derived from sheep were higher than the strains of cow origin. Therefore, sheep, as the host of Shiga toxin-producing Escherichia coli strains, should be paid higher attention than cow.
Keywords: Shiga toxin-producing Escherichia coliVero cytotoxicityvirulence genepathogenicityhemolytic activity
产志贺毒素大肠埃希菌(Shiga toxin-producing Escherichia coli,STEC)是一类携带了通常由前噬菌体编码的一种或两种志贺毒素基因的新发高致病性食源性病原菌,与人类腹泻、出血性结肠炎(hemorrhagic colitic,HC)以及高死亡率的溶血性尿毒综合征(hemolytic uremic syndrome,HUS)等疾病有关[1-2]。根据血清型不同,分为O157 STEC和非O157 STEC,O157:H7是被一致认为是致病力和毒性较强的血清型[3]。但近年来由非O157 STEC引起的散发感染或暴发逐渐增多[4]。所以,各种来源的STEC均应受到重视。
STEC宿主广泛,现在的研究表明反刍动物特别是牛是STEC最主要宿主,主要位于牛的肠道,作为正常菌群的组分之一,并不引起牛感染发病[5]。人类往往通过食用被牛的排泄物直接或间接污染的食物和饮水而感染。STEC感染难以控制的重要原因是其具有极低的感染剂量(小于100个细胞)[6-7]。1982年,STEC O157:H7通过被污染的牛肉在美国首次暴发,引起4人死亡,并且每年在美国引起腹泻疾病超过96000例和3200例住院患者[8-9]。其后在日本等地都有发生,1996年,在日本大阪也曾暴发流行STEC,其中感染人数多达9000余人,9人死亡[10]。我国于1999–2000年在江苏等地暴发了人感染O157:H7血清型STEC大的疫情,报告HUS患者195例,其中死亡177例,血清流行病学调查估计感染人数20326人[11]。现有的研究主要集中在人,对其贮藏宿主的研究相对较少,相对来讲,动物作为传染源更为重要,它往往是动物来源食品污染的根源。而对储藏宿主的研究又主要集中在牛上,1987年,Borczyk等首次发现牛是STEC O157:H7的储藏宿主[12]。姜海清等于2014年江苏某奶牛场分离到STEC菌株,通过BALB/c小鼠模型证明牛源STEC菌株有较强的致病性[13],羊在中国的饲养量也非常大,2016年,羊肉在我国肉类产量中的比重5.4%[14]。世界上多个国家都已对健康绵羊体内STEC的携带情况进行了深入调查,而我国这一方面的研究不多[15],有必要对我国健康绵羊体内STEC的携带情况及基本特征作进一步研究。
STEC主要致病因子有志贺毒素、溶血素、黏附素及其他致病相关因子。志贺毒素(Shiga toxin)包括志贺毒素1 (Stx1)和志贺毒素2 (Stx2),对Vero细胞和Hela细胞有一定的毒性,分别由stx1和stx2编码[16-17]。这两个毒素基因往往是STEC鉴定及毒力分型的重要靶点。并且,不同的stx1和stx2的亚型有着不同的毒力。
本研究对2018–2019年从全国多点采样197份牛羊粪便样品,通过特异性基因stx1和stx2分离鉴定STEC,本实验室分离到11株羊源STEC菌株,1株牛源STEC菌株,10株新疆牛源STEC菌株,并对分离到的STEC菌株进行致病潜力分析,旨在初步了解我国部分地区牛源羊源STEC菌株的流行情况及菌株特征,为进一步针对性地预防人感染STEC的流行和暴发提供参考。
1 材料和方法 1.1 实验材料
1.1.1 病料来源: 63份羊源粪便样品采集自江苏,134份牛源粪便样品采集于2018–2019年江苏、河北、云南等地。样品来源表格见表 1。
表 1. 样品来源表格 Table 1. Sample source form
| Sample collection location | Animal species | Number of samples | Number of isolated strains |
| Jiangsu province | Sheep | 63 | 11 |
| Hebei province | Cow | 10 | 0 |
| Hebei province | Cow | 10 | 0 |
| Inner Mongolia Autonomous Region | Cow | 6 | 0 |
| Jiangsu province | Cow | 30 | 0 |
| Guangdong province | Cow | 6 | 0 |
| Hebei province Shanxi province Shandong province Beijing Guangdong province Yunnan province | Cow Cow Cow Cow Cow Cow | 3 7 10 10 10 32 | 0 0 0 1 0 0 |
表选项
1.1.2 主要试剂: Luria-Bertani (LB)琼脂培养基;O157显色培养基购自青岛海博生物技术有限公司;绵羊血购自南京鼎思生物技术有限公司;引物由南京擎科生物有限公司合成;ddH2O、2×Rapid Taq Master Mix购自南京诺唯赞生物科技有限公司;药敏纸片购自杭州微生物试剂有限公司;Vero细胞为本实验室保存;48孔细胞培养板购自TaKaRa公司。
1.1.3 菌株: O157:H7标准菌株EDL933 (ATCC 43895);O157:H7标准菌株ATCC 43889;O157:H7标准菌株NCTC 12900。所有分离株信息见表 2。
表 2. STEC菌株信息情况表 Table 2. STEC strains information in the experiment
| Strain number | Animal species | Source | Serotype | Strain number | Animal species | Source | Serotype | |
| EC276 | Sheep | Jiangsu | O157:H7 | EC635 | Cow | Xinjiang | O88 | |
| EC278 | Sheep | Jiangsu | O157:H7 | EC626 | Cow | Xinjiang | O179:H8 | |
| EC275 | Sheep | Jiangsu | — | EC631 | Cow | Xinjiang | — | |
| EC277 | Sheep | Jiangsu | — | EC612 | Cow | Beijing | — | |
| EC274 | Sheep | Jiangsu | — | EC632 | Cow | Xinjiang | — | |
| EC279 | Sheep | Jiangsu | — | EC636 | Cow | Xinjiang | — | |
| EC280 | Sheep | Jiangsu | — | EC149 | Cow | Xinjiang | — | |
| EC281 | Sheep | Jiangsu | — | EC150 | Cow | Xinjiang | — | |
| EC282 | Sheep | Jiangsu | — | EC622 | Cow | Xinjiang | — | |
| EC283 | Sheep | Jiangsu | — | EC625 | Cow | Xinjiang | — | |
| EC284 | Sheep | Jiangsu | — | EC623 | Cow | Xinjiang | — |
表选项
1.2 STEC菌株分离及O157:H7分离
1.2.1 STEC的鉴别培养: STEC菌株分离方法参考薛涛等的方法[18]。取适量粪便样品接种于肉汤培养基中,37 ℃增菌培养12 h左右。800 r/min离心2 min去除杂质,取上清作为模板检测STEC特异性基因stx1和stx2,对stx1或stx2阳性的肉汤培养液,划线接种于O157显色培养基,放37 ℃恒温培养箱静置培养18–24 h。
1.2.2 PCR鉴定: 挑取O157显色平板上的紫红色菌落和蓝色菌落各5–10个于LB中,37 ℃、220 r/min振摇培养18–24 h,鉴定stx1和stx2基因确定是否为STEC,再鉴定O157:H7特异性鉴定基因Z3276基因确定是否为O157:H7血清型。PCR阳性样品再送测序进行确认。Mac琼脂平板上连续传两代,单菌落鉴定为阳性的,即为纯化菌株。PCR产物由苏州金唯智公司测序,引物由南京擎科生物有限公司合成。
1.3 志贺毒素基因亚型分析 志贺毒素基因(stx1/stx2)亚型分析采用PCR分型方法[19],引物序列及反应条件见表 3。挑选部分菌株进行stx1/stx2全长扩增与测序,与GenBank数据库中收录的stx1/stx2序列进行BLAST比对,验证PCR分型结果。
表 3. 引物序列 Table 3. Primer sequence
| Identification primer | Primer sequence | Product size/bp | Annealing temperature/℃ | References |
| stx1 | GAGCGAAATAATTTATATGTG | 504 | [18] | |
| TTGATGATGGCAATTCAGTAT | ||||
| stx2 | CCATGACAACGGACAGCAGTT | 627 | [18] | |
| CCTGTCAACTGAGCACTTTGC | ||||
| z3276 | CGCGGATCCTTTAGTAAAAGTGTC | 1035 | [18] | |
| GGCAAGCTTAATCGTATTTCACGTT | ||||
| Stx gene subtype | ||||
| stx1a | CTCTTTCCAGGACAACAGCGGTT | 478 | 64 | [19] |
| GGAAACTCATCAGATGCCATTCTGG | ||||
| stx1c | CCTTTCCTGGTACAACTGCGGTT | 252 | 64 | [19] |
| CAAGTGTTGTACGAAATCCCCTCTGA | ||||
| stx1d | CAGTTAATGCGATTGCTAAGGAGTTTACC | 203 | 64 | [19] |
| stx2a ? ? stx2b? ? stx2c ? stx2d ? stx2e ? stx2f | CAGTTAATGCGATTGCTAAGGAGTTTACC GCGATACTGRGBACTGTGGCC CCGKCAACCTTCACTGTAAATGTG GCCACCTTCACTGTGAATGTG AAATATGAAGAAGATATTTGTAGCGGC CAGCAAATCCTGAACCTGACG GAAAGTCACAGTTTTTATATACAACGGGTA CCGGCCACYTTTACTGTGAATGTA AAARTCACAGTCTTTATATACAACGGGTG TTYCCGGCCACTTTTACTGTG GCCTGATGCACAGGTACTGGAC CGGAGTATCGGGGAGAGGC CTTCCTGACACCTTCACAGTAAAGGT TGGGCGTCATTCACTGGTTG TAATGGCCGCCCTGTCTCC | 349 ? ? 251 ? 177 ? 179 ? ? 411 ? 424 | 64 ? ? 64 ? ? 64 ? 69 ? 64 ? 64 | [19] ? ? [19] ? [19] ? ? [19] ? [19] ? ? [19] |
| stx2g | CACCGGGTAGTTATATTTCTGTGGATATC GATGGCAATTCAGAATAACCGCT | 573 | 60 | [19] |
| Virulence gene | ||||
| eae | TCAATGCAGTTCCGTTATCAGTT | 482 | 57 | [20] |
| GTAAAGTCCGTTACCCCAACCTG | ||||
| rfbE | AATTGAAGATTGCGCTGAAGCCTTTG | 507 | 55 | [20] |
| GAGTACATTGGCATCGTGTGGACAG | ||||
| efa1 | GAGACTGCCAGAGAAAG | 479 | 51 | [20] |
| GGTATTGTTGCATGTTCAG | ||||
| saa ? tox B ? ehxA ? ast A ? subA ? katP ? paa | CGTGATGAACAGGCTATTGC ATGGACATGCCTGTGGCAAC ATACCTACCTGCTCTGGATTGA TTCTTACCTGATCTGATGCAGC GGTGCAGCAGAAAAAGTTGTAG TCTCGCCTGATAGTGTTTGGTA CCATCAACACAGTATATCCGA GGTCGCGAGTGACGGCTTTGT TATGGCTTCCCTCATTGCC TATAGCTGTTGCTTCTGACG CTTCCTGTTCTGATTCTTCTGG AACTTATTTCTCGCATCATCC ATGAGGAAACATAATGGCAGG TCTGGTCAGGTCGTCAATAC | 119 ? 602 ? 1551 ? 111 ? 556 ? 2125 ? 350 | 52 ? 55 ? 57 ? 55 ? 60 ? 56 ? 60 | [20] ? [20] ? [20] ? [20] ? [20] ? [20] ? [20] |
表选项
1.4 毒力基因及黏附相关基因检测 采用PCR方法对所有22株STEC菌株进行黏附相关基因ihaefaI saa paa eibG、毒力相关基因astAsubA katP toxB和溶血相关基因ehxAhly检测。引物及其反应条件详见表 3[20]。
1.5 细胞模型和溶血性评价STEC菌株毒力 通过对Vero细胞的细胞毒性检测和溶血活性的检测共同评定STEC菌株的毒力大小。产志贺毒素大肠杆菌能够产生一种毒素使Vero细胞发生病变,这种毒素也称为Vero细胞毒素,Vero细胞毒性实验可判定STEC志贺毒素生物活性的强弱[21],也是判断产志贺毒素大肠杆菌志贺毒素活性的一种金标准[22]。对Vero细胞的细胞毒性检测:挑单菌接种于LB培养基中,培养12 h,12000 r/min离心8 min,取上清液以0.22 μm滤器过滤除菌。所获得的无菌上清液将用于Vero细胞毒性测定。Vero细胞培养于含有10%胎牛血清的细胞营养液DMEM中,铺于48孔细胞板中,放置37 ℃,5% CO2条件培养箱中培养。待细胞铺满,用PBS洗3次,用DMEM培养液以1:4稀释度稀释细菌上清液,加入细胞孔中,标准株O157:H7EDL933为阳性对照,DH5α为阴性对照。18、36、48 h用倒置显微镜观察收集结果,并根据出现细胞病变时间与细胞病变程度评出4、3、2和1分。每株菌做3次重复实验。溶血活性实验:根据溶血环大小,将溶血活性分为3、2和1分。每株菌做3次重复实验。
2 结果和分析 2.1 样品中的细菌分离鉴定结果 63份羊粪便样品分离到11株羊源STEC菌株,分离率17.5%;其中2株为O157:H7血清型。128份牛粪便样品分离到1株牛源STEC菌株。O157显色培养基的结果见图 1,部分PCR鉴定结果见图 2和图 3。
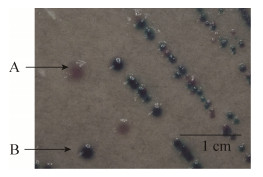 |
| 图 1 用O157显色培养基分离STEC菌株图 Figure 1 The image of STEC strain isolated with O157 chromogenic medium. A: suspected as O157: H7; B: non-O157 STEC. |
| 图选项 |
 |
| 图 2 部分菌株的stx1基因鉴定结果 Figure 2 Identification results of stx1 gene of some strains. M: DNA standard DL2000 PLUS; cane 1: EC276; cane 2: EC278; cane 3: control. |
| 图选项 |
 |
| 图 3 Z3276基因鉴定结果 Figure 3 Z3276 gene identification results. M: DNA standard DL2000; cane 1: ATCC 43895; cane 2: ATCC 43889; cane 3: EC276; cene 4: EC278; cane 5: control. |
| 图选项 |
2.2 志贺毒素基因亚型结果 11株羊源STEC分离株:11株都携带stx1基因,其中stx1a亚型1株,stx1c亚型2株,stx1d亚型2株,其余为不确定型(ONT);9株携带stx2基因,其中stx2a亚型1株,stx2b亚型1株,stx2c亚型3株,其余为不确定型(ONT)。11株牛源STEC分离株:8株携带stx1基因,其中stx1a亚型4株,stx1d亚型2株,其余为不确定型(ONT);7株携带stx2基因,其中stx2a亚型4株,其余为不确定型(ONT)。
2.3 毒力基因及黏附相关基因检测 11株羊源STEC分离株eae基因携带率为63.6% (7/11),而11株牛源STEC分离株eae基因携带率仅为9.0% (1/11)。11株羊源STEC分离株:其他的黏附基因saa、paa、iha、efa1和eibG携带率分别为0 (0/11)、9.0% (1/11)、45.5% (5/11)、36.4% (4/11)和0% (0/11)。毒力相关基因astA、subA、katP和toxB携带率分别为9.0% (1/ 11)、0 (0/ 11)、45.5% (5/11)和27.3% (3/11)。溶血基因ehxA和hly携带率分别为63.6% (7/11)和54.5% (6/11)。11株牛源STEC分离株:其他的黏附基因saa、paa、iha、efa1和eibG携带率分别为0 (0/11)、0 (0/11)、45.5% (5/11)、0 (0/11)和0 (0/11)。毒力相关基因astA、subA、katP和toxB携带率分别为9.0% (1/11)、18.2% (2/11)、0 (0/11)和0 (0/11)。溶血基因ehxA和hly携带率分别为81.8% (9/11)和45.5% (5/11)。
2.4 STEC菌株Vero细胞毒性和溶血活性强弱评定 STEC菌株的Vero细胞毒性检测结果见图 4,阳性对照(ATCC 43985)的Vero细胞形态变圆皱缩,之后逐渐死亡,阴性对照(DH5α) Vero细胞形态正常。部分STEC分离株出现不同程度的变圆皱缩和死亡现象,且出现细胞死亡时间不同,据此得出评分结果,见表 4。羊源STEC分离株中有Vero细胞毒性的有EC276、EC278、EC281、EC282、EC283、EC281。牛源STEC分离株中有Vero细胞毒性的有EC626、EC635、EC623、EC622、EC636。溶血活性检测结果见图 5。结果可见,部分STEC菌株有溶血环,且溶血环大小不一,部分菌株不溶血,根据溶血环大小进行3、2、1和0的评分。羊源STEC分离株中有溶血活性的有EC278、EC275、EC281;牛源STEC分离株中有溶血活性的有EC623、EC622、EC635。综合评分4分以上认为是致病潜力较强的菌株,致病潜力较强的菌株有EC278、EC282、EC635。
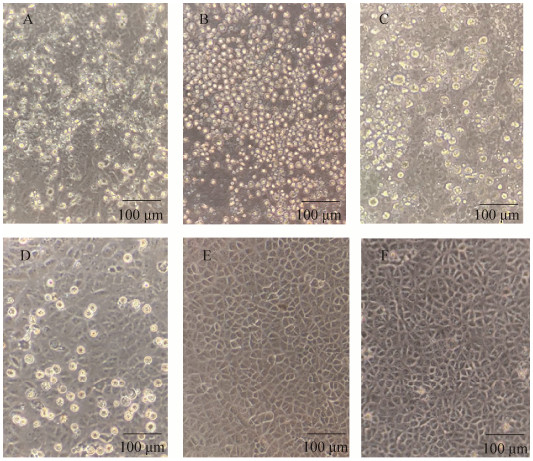 |
| 图 4 Vero细胞接种细菌滤液后36 h病变图 Figure 4 The cytopathic effect of Vero cells after inoculating bacterial filtrate and normal cells. A: ATCC 43985; B: EC282; C: EC283; D: EC276; E: DH5α; F: control. |
| 图选项 |
表 4. STEC溶血活性和vero细胞毒作用评分 Table 4. STEC hemolytic activity and vero cytotoxicity score
| Original | Hemolytic activity | Cytotoxicity | ||||||
| number | First repeat | Repeat two | Repeat three | 16 h | 32 h | 48 h | ||
| ATCC 43895 | 4 | 4 | 4 | 0 | 0 | 0 | ||
| EC276 | 0 | 0 | 0 | 2 | 2 | 3 | ||
| EC278 | 1 | 1 | 1 | 3 | 3 | 4 | ||
| EC275 | 1 | 1 | 1 | 0 | 0 | 0 | ||
| EC277 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC274 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC279 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC280 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC281 | 2 | 2 | 2 | 1 | 1 | 1 | ||
| EC282 | 0 | 0 | 0 | 4 | 4 | 4 | ||
| EC283 | 0 | 0 | 0 | 3 | 3 | 3 | ||
| EC284 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC612 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC625 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| EC635 | 1 | 2 | 1 | 3 | 3 | 4 | ||
| EC626 | 0 | 0 | 0 | 3 | 3 | 4 | ||
| EC148 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC149 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC631 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC623 | 1 | 2 | 1 | 2 | 3 | 4 | ||
| EC622 | 1 | 1 | 1 | 2 | 2 | 4 | ||
| EC632 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EC636 | 0 | 0 | 0 | 1 | 1 | 1 | ||
| DH5α | 0 | 0 | 0 | 0 | 0 | 0 | ||
表选项
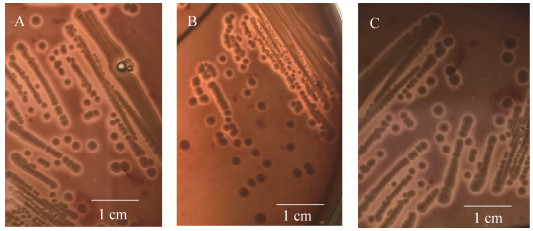 |
| 图 5 细菌划线接种血平板12 h溶血图片 Figure 5 The hemolytic effect of blood plate after inoculating bacterial. A: EC281; B: EC278; C: EC275. |
| 图选项 |
3 讨论 本研究从197份牛羊粪便样品中分离到12株STEC菌株,另有10株牛源分离株来自新疆,并通过Vero细胞毒性试验、溶血活性试验和毒力因子的检测分析STEC分离株的致病潜力大小。
薛涛等在江苏部分地区牛场分离STEC菌株,696份粪便样品分离到77株STEC,分离率为11.1%[18]。本实验于2018–2019年江苏、河北、云南等多地采集134份牛源粪便样品,仅分离到1株STEC菌株,分离率0.7%。有研究者对江苏健康绵羊STEC菌株进行研究,550份绵羊粪便样品分离到109株STEC菌株,分离率为19.8%[19]。本研究在江苏句容采集63份健康绵羊粪便样品分离到11株STEC菌株,分离率为17.5%。以上这些研究提示,在江苏地区,羊源STEC的分离率高于牛源分离率。2020年有研究报道在新疆阿克苏地区某羊养殖场中STEC分离率达到(9/21) 42.86%,分离率较高[22]。根据以往研究,牛被公认为STEC主要宿主,对STEC菌株的分离多集中于牛源。但近年来关于羊源STEC菌株分离率较高,提示绵羊作为STEC的储藏宿主,对其的研究应引起重视。
西班牙、德国、澳大利亚多项对HUS病人的调查,证明eae基因的携带与引起人类HUS疾病的STEC菌株关系密切,而eae基因在羊源STEC中很少出现,只有约5%[23-24],11株羊源STEC分离株eae基因携带率为63.6% (7/11)。2019年Ferhat等的研究报道,在阿尔及尔市的363个绵羊样本,eae基因的阳性率18.9%[25]。Zaheri等的研究中报道72个绵羊山羊粪便样本中eae基因的阳性率为0[26]。郑晓风等报道新疆阿克苏地区羊源STEC菌株中eae基因的阳性率4.7%[27]。张凌等报道新疆地区羔羊源STEC菌株中eae基因的阳性率0[28]。刘英玉等报道新疆库尔勒地区羊源产志贺毒素大肠杆菌中eae基因的阳性率5.5%[29]。本实验中STEC分离株中eae基因的阳性率也远高于之前研究报道的分离率,也高于近几年国内外羊源STEC菌株eae基因的阳性率。提示这些羊源STEC菌株一旦传播给人,可能引起严重的疾病。应通过定期检测动物粪便样本,及时治疗,及时应对,避免传播到人类。
stx1+stx2基因型占的比例最多(81.8%),stx1、stx2基因型分别占18.2%、0%。与郑晓风等新疆地区羊STEC菌株相比,stx1+stx2基因型比例提高将近一倍[27]。与刘英玉等新疆库尔勒地区羊源STEC stx1+stx2基因型比例35.5%相比,显著提高[29]。stx1和stx2基因的共表达可增强STEC的毒力[30],也提示了本批羊源STEC菌株致病潜力较大。
通过stx1/stx2基因及亚型的检测,共检测出stx1a、stx1c、stx1d、stx2a、stx2b、stx2c等亚型。研究报道,stx2a、stx2d和stx2c等stx2亚型菌株具有较高的毒力[24]。11株羊源STEC分离株中stx2a亚型1株、stx2c亚型3株,11株牛源STEC分离株中stx2a亚型4株。提示本实验室的分离株具有较大的致病潜力,且牛源羊源携带高毒力亚型的比率相等。提示羊源菌株的致病潜力不亚于牛源菌株。
本实验分离到的11株羊源STEC菌株,其中2株经O157:H7特异性鉴定基因Z3276基因鉴定为O157:H7血清型。O157:H7血清型STEC菌株一直以来危害最为严重,毒性最强。对Vero细胞的毒性结果显示,11株羊源分离株中有5株(EC276、EC278、EC281、EC282、EC283)、牛源分离株中也有5株(EC626、EC635、EC623、EC622、EC636)对Vero细胞有一定的毒性。能使Vero细胞变圆皱缩,之后逐渐死亡,且程度不同。溶血实验结果显示,11株羊源STEC菌株中有3株(EC275、EC278、EC281)、牛源STEC分离株中也有3株(EC623、EC622、EC635)有溶血活性。在对Vero细胞的细胞毒性检测和溶血活性等方面来看,牛源羊源菌株的致病潜力相当。STEC菌株引起人发病,并不引起牛羊等动物发病。只有小鼠作为模型模拟人的发病,但小鼠并非理想的动物模型,由于小鼠肠道内大量繁殖革兰氏阳性厌氧菌[25],STEC并不能在小鼠肠道内大量繁殖,当接种STEC于SPF级或者清洁级小鼠体内时,只能在粪便中短时间检测到,几天之内,细菌数量迅速减少[25-26]。小鼠的肠道感染情况与人类的肠道感染情况相差较大,所以小鼠模型不能准确反应对人类的致病性,未做动物实验。
综上所述,从分离率、毒力基因、亚型分析、对Vero细胞的细胞毒性检测和溶血活性等方面综合来看,提示本研究从健康绵羊分离的STEC菌株致病潜力较大。以往忽略对健康动物在食品安全中的威胁,现如今应重视绵羊作为宿主在STEC流行中的影响。其他反刍动物如鹿等的STEC研究也可以开展。
References
| [1] | Grisaru S, Xie JL, Samuel S, Hartling L, Tarr PI, Schnadower D, Freedman SB, for the Alberta Provincial Pediatric Enteric Infection Team. Associations between hydration status, intravenous fluid administration, and outcomes of patients infected with shiga toxin-producing Escherichia coli. JAMA Pediatrics, 2017, 171(1): 68. DOI:10.1001/jamapediatrics.2016.2952 |
| [2] | Purwar S, Roy S, Metgud S. Non-O157:H7 shiga toxin producing diarrhoeagenic Escherichia coli (STEC) in southern India: a tinderbox for starting epidemic. Journal of Clinical and Diagnostic Research, 2016, 10(10): DC11-DC15. |
| [3] | Doorduyn Y, de Jager CM, van der Zwaluw WK, Friesema IHM, Heuvelink AE, de Boer E, Wannet WJB, van Duynhoven YTHP. Shiga toxin-producing Escherichia coli (STEC) O157 outbreak, The Netherlands, September-October 2005. Euro Surveillance, 2006, 11(7): 182-185. |
| [4] | Coombes BK, Wickham ME, Mascarenhas M, Gruenheid S, Finlay BB, Karmali MA. Molecular analysis as an aid to assess the public health risk of non-O157 Shiga toxin-producing Escherichia coli strains. Applied and Environmental Microbiology, 2008, 74(7): 2153-2160. DOI:10.1128/AEM.02566-07 |
| [5] | Gyles CL. Shiga toxin-producing Escherichia coli: an overview. Journal of Animal Science, 2007, 85(suppl_13): E45-E62. DOI:10.2527/jas.2006-508 |
| [6] | Hussein HS, Sakuma T. Invited review: prevalence of shiga toxin-producing Escherichia coli in dairy cattle and their products. Journal of Dairy Science, 2005, 88(2): 450-465. DOI:10.3168/jds.S0022-0302(05)72706-5 |
| [7] | Varma JK, Greene KD, Reller ME, DeLong SM, Trottier J, Nowicki SF, DiOrio M, Koch EM, Bannerman TL, York ST, Lambert-Fair MA, Wells JG, Mead PS. An outbreak of Escherichia coli O157 infection following exposure to a contaminated building. JAMA, 2003, 290(20): 2709-2712. DOI:10.1001/jama.290.20.2709 |
| [8] | Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerging Infectious Diseases, 2005, 11(4): 603-609. DOI:10.3201/eid1104.040739 |
| [9] | Friesema I, Sigmundsdottir G, van der Zwaluw K, Heuvelink A, Schimmer B, de Jager C, Rump B, Briem H, Hardardottir H, Atladottir A, Gudmundsdottir E, van Pelt W. An international outbreak of Shiga toxin-producing Escherichia coli O157 infection due to lettuce, September-October 2007. Eurosurveillance, 2008, 13(50): 19065. |
| [10] | Watanabe H, Wada A, Inagaki Y, Itoh K, Tamura K. Outbreaks of enterohaemorrhagic Escherichia coli O157:H7 infection by two different genotype strains in Japan, 1996. Lancet, 1996, 348(9030): 831-832. DOI:10.1016/S0140-6736(05)65257-9 |
| [11] | Xiong YW, Wang P, Lan RT, Ye CY, Wang H, Ren J, Jing HQ, Wang YT, Zhou ZM, Bai XM, Cui ZG, Luo X, Zhao AL, Wang Y, Zhang SM, Sun H, Wang L, Xu JG. A novel Escherichia coli O157:H7 clone causing a major hemolytic uremic syndrome outbreak in China. PLoS ONE, 2012, 7(4): e36144. DOI:10.1371/journal.pone.0036144 |
| [12] | Borczyk AA, Karmali MA, Lior H, Duncan LM. Bovine reservoir for verotoxin-producing Escherichia coli O157:H7. Lancet, 1987, 1(8524): 98. |
| [13] | 姜海清. 江苏某奶牛场产志贺毒素大肠杆菌的分子流行病学与遗传相关性分析研究. 扬州大学硕士学位论文, 2015. |
| [14] | 赵印, 杜立新, 刘强德. 2017年中国羊产业发展报告与发展预测. 第十五届(2018)中国羊业发展大会论文集. 兰考, 2018: 8-17. |
| [15] | 周琼. 江苏部分地区牛群肠出血性大肠杆菌分离株分子流行病学及EHEC O157: H7鞭毛蛋白单克隆抗体的研制. 扬州大学硕士学位论文, 2008. |
| [16] | Liu F, Song DZ, Li J, Yu XH. Research progress on shiga toxin-produing Escherichia coli epidemiology and virulence factors. China Animal Husbandry & Veterinary Medicine, 2014, 41(1): 187-191. (in Chinese) 刘飞, 宋定州, 李键, 于学辉. 产志贺毒素大肠杆菌的流行病学及致病因子的研究进展. 中国畜牧兽医, 2014, 41(1): 187-191. |
| [17] | Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clinical Microbiology Reviews, 1998, 11(3): 450-479. |
| [18] | Xue T, Gu CC, Gao S, Jiao XA, Zhou Q, Zhang WJ, Liu XF. Identification of shiga toxin-producing Escherichia coli from healthy cattle in one farm of Jiangsu province and their pathogenicity on mice. Chinese Journal of Animal and Veterinary Sciences, 2011, 42(6): 830-837. (in Chinese) 薛涛, 顾丛丛, 高崧, 焦新安, 周琼, 张文俊, 刘秀梵. 江苏某奶牛场健康奶牛体内产志贺毒素大肠杆菌的分离鉴定及其对小鼠致病性的研究. 畜牧兽医学报, 2011, 42(6): 830-837. |
| [19] | Bettelheim KA, Beutin L. Rapid laboratory identification and characterization of verocytotoxigenic (Shiga toxin producing) Escherichia coli (VTEC/STEC). Journal of Applied Microbiology, 2003, 95(2): 205-217. DOI:10.1046/j.1365-2672.2003.02031.x |
| [20] | Gu CC, Xue T, Xu TT, Gao S, Jiao XA, Liu XF. Epidemiological investigation of Shiga toxin-producing Escherichia coli isolates originated from healthy sheep in one farm of Jiangsu province and their pathogenicity. Acta Microbiologica Sinica, 2011, 51(5): 676-683. (in Chinese) 顾丛丛, 薛涛, 徐婷婷, 高崧, 焦新安, 刘秀梵. 江苏某地健康绵羊群产志贺毒素大肠杆菌体内分离株的分子流行病学及致病力. 微生物学报, 2011, 51(5): 676-683. |
| [21] | Konowalchuk J, Speirs JI, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infection and Immunity, 1977, 18(3): 775-779. DOI:10.1128/iai.18.3.775-779.1977 |
| [22] | Vaz TMI, Irino K, Kato MAMF, Dias AMG, Gomes TAT, Medeiros MIC, Rocha MMM, Guth BEC. Virulence properties and characteristics of shiga toxin-producing Escherichia coli in Sao Paulo, Brazil, from 1976 through 1999. Journal of Clinical Microbiology, 2004, 42(2): 903-905. DOI:10.1128/JCM.42.2.903-905.2004 |
| [23] | Werber D, Beutin L, Pichner R, Stark K, Fruth A. Shiga toxin-producingEscherichia coliSerogroups in food and patients, Germany. Emerging Infectious Diseases, 2008, 14(11): 1803-1806. DOI:10.3201/eid1411.080361 |
| [24] | Dong HJ, Lee S, Kim W, An JU, Kim J, Kim D, Cho S. Prevalence, virulence potential, and pulsed-field gel electrophoresis profiling of Shiga toxin-producing Escherichia coli strains from cattle. Gut Pathogens, 2017, 9: 22. DOI:10.1186/s13099-017-0169-x |
| [25] | Fujii J, Kita T, Yoshida S, Takeda T, Kobayashi H, Tanaka N, Ohsato K, Mizuguchi Y. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H-in mitomycin-treated mice. Infection and Immunity, 1994, 62(8): 3447-3453. DOI:10.1128/iai.62.8.3447-3453.1994 |
| [26] | Sun Y, Liu J, Feng SZ, Gao F, Guo XJ, Chang GQ. Mouse model for infectious disease caused by enterohemorrhagic Escherichia coli O157:H7. Laboratory Animal Science and Administration, 2003, 20(4): 6-9. (in Chinese) 孙洋, 刘军, 冯书章, 高丰, 郭学军, 常国权. 肠出血性大肠杆菌O157:H7感染小鼠动物模型的初步建立. 实验动物科学与管理, 2003, 20(4): 6-9. |
| [27] | Zheng XF, Zhang Y, Liu YY, Zhu MY, Zheng XQ, Lu W, Jiang JD, Zhu MH. Detection and analysis of shiga toxin-producing Escherichia coli isolates from cattle and sheep sources in some regions of Xinjiang, China. Chinese Journal of Animal and Veterinary Sciences, 2020, 51(10): 2518-2527. (in Chinese) 郑晓风, 张妍, 刘英玉, 朱明月, 郑晓琴, 卢玮, 蒋金豆, 朱梦含. 新疆部分地区牛羊源产志贺毒素大肠埃希菌菌株检测与分析. 畜牧兽医学报, 2020, 51(10): 2518-2527. |
| [28] | Zhang L, Tong PP, Zhang Y, Ma XY, Zhang MM, Liu LY, Yao G, Chen TJY, Su ZQ. Analysis of drug resistance, virulence genes and serotypes of STEC in a large-scale sheep farm in Xinjiang. Xinjiang Agricultural Sciences, 2020, 57(10): 1921-1930. (in Chinese) 张凌, 佟盼盼, 张毅, 马晓玉, 张萌萌, 刘璐瑶, 姚刚, 陈童锦悦, 苏战强. 羔羊STEC的耐药性、毒力基因和血清型分析. 新疆农业科学, 2020, 57(10): 1921-1930. |
| [29] | Liu YY, Zhu MY, Su XY, Xu L, Liu JF, Zheng XF, Zhang Y, Zheng XQ. The origin of sheep in Xinjiang Korla region Shiga toxin-producing Escherichia coli and its drug resistance analysis. Journal of Agricultural Science and Technology, 2020, 22(5): 122-128. (in Chinese) 刘英玉, 朱明月, 苏晓月, 胥兰, 刘俊飞, 郑晓风, 张妍, 郑晓琴. 新疆库尔勒地区羊源产志贺毒素大肠杆菌及其耐药性分析. 中国农业科技导报, 2020, 22(5): 122-128. |
| [30] | Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H. Escherichia coli harboring shiga toxin 2 gene variants: frequency and association with clinical symptoms. The Journal of Infectious Diseases, 2002, 185(1): 74-84. |
