焦宇洲, 于江旭, 高东阳, 王颢然, 蔡旭旺


华中农业大学动物医学院, 农业微生物学国家重点实验室, 湖北武汉 430070
收稿日期:2020-05-27;修回日期:2020-08-20;网络出版日期:2020-08-24
基金项目:国家重点研发计划(2016YFD0501607);中央高校基本科研业务费专项基金(2020BC208)
*通信作者:蔡旭旺, Tel/Fax: +86-27-87282608;E-mail: caixuwang@mail.hzau.edu.cn.
摘要:球虫病给养禽业带来巨大经济损失,人们对绿色健康食品的迫切需求使球虫病的防控面临新的挑战。伴随世界"禁抗"进程的不断推进,家禽养殖业亟需一种安全有效的新型抗球虫方法。益生菌可竞争性排斥病原菌定殖以防止球虫病继发感染,可刺激宿主抗菌肽、黏蛋白和紧密连接蛋白的分泌以抵御球虫入侵,还可激活免疫反应以增强机体抗球虫感染的能力。本文从调控肠道微生物群、改善肠黏膜屏障和调节免疫系统功能等方面综述了益生菌在预防和控制家禽球虫病中的作用,以期为高效抗球虫益生菌制剂的研发提供参考。
关键词:球虫病益生菌肠道屏障
Advances in prevention of chicken coccidiosis by probiotics regulating intestinal barrier
Yuzhou Jiao, Jiangxu Yu, Dongyang Gao, Haoran Wang, Xuwang Cai


State Key Laboratory of Agricultural Microbiology, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, Hubei Province, China
Received: 27 May 2020; Revised: 20 August 2020; Published online: 24 August 2020
*Corresponding author: Xuwang Cai, Tel/Fax: +86-27-87282608; E-mail: caixuwang@mail.hzau.edu.cn.
Foundation item: Supported by the National Key Research and Development Program of China (2016YFD0501607) and by the Fundamental Research Funds for the Central Universities (2020BC208)
Abstract: Coccidiosis brings great economic loss to the poultry industry. The urgent need of green and healthy feed makes the prevention and control of coccidiosis face new challenges. In recent years, a safe and effective new anticoccidial method is needed by the poultry farming, owing to increasing bans of anticoccidial drugs. Recent researches have demonstrated probiotics can prevent coccidiosis-induced secondary infection by competitively excluding colonization of pathogens bacteria and stimulate the secretion of host antibacterial peptides, mucins and tight junction proteins to resist coccidiosis. In addition, it can enhance the anti-coccidial ability by activating the immune response. This review summarizes what is currently known on mechanisms about how probiotics prevent and control coccidiosis by modulating the gut microbiota, ameliorating mucus barrier, affecting function of immune system, to provide a reference for the development of probiotic products to control coccidiosis.
Keywords: coccidiosisprobioticsintestinal barrier
鸡球虫病由顶复门(Apicomplexa)、孢子虫纲(Sporozoa)、真球虫目(Eucoccidiorida)、艾美耳科(Eimeriidae)、艾美耳属(Eimeria)的真核单细胞原生动物感染引起。世界公认危害家禽业的球虫种类有7种,其中柔嫩艾美耳球虫(Eimeria tenella)、巨型艾美耳球虫(Eimeria maxima)、毒害艾美耳球虫(Eimeria necatrix)对肉鸡危害最为严重[1],可以在肠道的不同部位定殖,引起出血性肠炎、营养不良和雏鸡高死亡率[2]。随着集约化养禽业的不断发展,鸡球虫病已经成为危害养禽业最严重的疾病之一。据调查报道,鸡球虫病对全世界养禽业造成的经济损失每年可达30亿美元[3]。由于活疫苗存在致病风险、减毒活疫苗生产效率低[4]以及种间交叉保护差等问题[5],目前对球虫病的控制在很大程度上仍然依赖于抗球虫药物[6–7]。但随着耐药性问题愈发严重,以及消费者越来越重视食品中的药物残留及其对环境的污染,使养殖业不得不减少抗球虫药物的使用[5],从而促进了抗球虫药物替代方法的应用和发展[8–11]。
世界粮农组织和世界卫生组织将益生菌定义为:通过摄取适当的量对食用者的身体健康能发挥有益作用的活菌。益生菌在宿主体内通过调控肠道微生态平衡、改善肠道形态结构、促进饲料摄入和消化、增强宿主免疫功能等方式促进宿主的健康[12]。球虫感染主要引起肠道损伤,因此益生菌可通过增强宿主肠道微生物屏障、肠黏膜屏障和免疫屏障的功能来抵抗球虫病带来的损害[13],本文综述了益生菌预防球虫病研究取得的最新进展(图 1)。
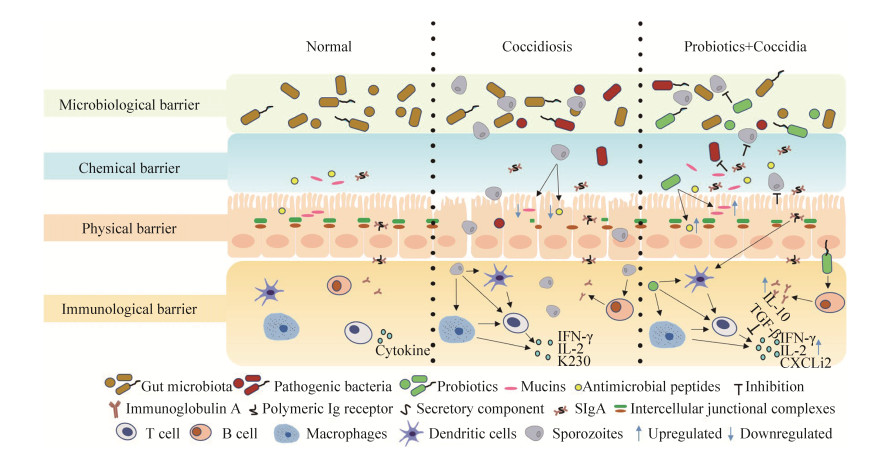 |
| 图 1 益生菌在肉鸡肠道屏障内发挥抗球虫作用的机制 Figure 1 The anti-coccidial mechanisms of probiotics in the intestinal barrier of broilers. |
| 图选项 |
1 益生菌对肠道微生物的调控 肠道微生物对于肠道营养吸收、代谢和免疫都具有重要作用。球虫感染会引起肠道菌群构成发生剧烈变化,Hume等[14]用变性梯度凝胶电泳法检测肉鸡被球虫感染后十二指肠、回肠和盲肠的肠道菌群构成,发现感染前后其菌群相似性仅为36.7%、55.4%和36.2%;柔嫩艾美耳球虫感染会破坏盲肠微生物群的完整性,感染后用16S rRNA基因测序技术对鸡盲肠菌群组成进行分析,结果显示肠道共生菌Lactobacillus、Faecalibacterium、Ruminococcaceae UCG–013、Romboutsia和Shuttleworthia相对丰度下降,而条件致病性肠球菌和链球菌定殖增多[15]。这种球虫感染引起的菌群变化很可能增加产气荚膜梭菌[16]、肠炎沙门菌[17]和空肠弯曲菌[18]等致病菌在肠道的定殖,增加继发感染的风险。例如,坏死性肠炎是由产气荚膜梭菌引起的鸡肠道疾病,单独使用产气荚膜梭菌对肉鸡进行感染并未对肉鸡肠道微生物群造成显著的干扰,但预先感染艾美耳球虫可引起肠道微生物群发生改变,极易促进产气荚膜梭菌的定殖导致肠道损伤[19–20]。
鸡胃肠道中的共生菌群不仅可以帮助宿主吸收营养,而且在竞争性排斥病原菌定殖、刺激肠道局部免疫反应方面也发挥着重要作用[21]。如盲肠拟杆菌分泌的酶能将大分子营养物质分解成宿主容易吸收的小分子,添加枯草芽孢杆菌能增加艾美耳球虫感染肉鸡肠道中拟杆菌的数量(益生菌组66.63%,感染对照组26.18%)[22],同时还可以减少肠道沙门菌、产气荚膜梭菌等病原菌定殖(P < 0.05)[23–24],并促进乳酸菌等肠道有益微生物的生长[25]。我们在前期研究中也发现,添加益生菌不仅改善了球虫感染引起的肠道拟杆菌属定殖减少,增加了乳酸杆菌数量,而且减少了弯曲杆菌的定殖。因此,添加益生菌可增加肠道有益菌数量,促进肠道营养物质消化吸收、提高肉鸡生长性能,同时减少病原菌的定殖,维护肠道健康。
2 益生菌改善肠黏膜屏障功能 肠黏膜屏障包括化学屏障和物理屏障[26],不仅可以帮助宿主吸收肠道内营养物质,还能保护肠黏膜免受共生微生物和外来致病微生物的侵害,对于维持肠道健康具有重要意义。
2.1 肠黏膜化学屏障 肠黏膜化学屏障主要指肠上皮细胞上层黏液层中的黏蛋白(mucin,MUC)、抗菌肽(antimicrobial peptides,AMPs)、再生胰岛衍生蛋白3 (regenerating islet-derived protein 3,Reg3)家族的蛋白和溶菌酶等,在抵御肠道细菌侵入上皮细胞时发挥重要作用[27]。黏蛋白由杯状细胞分泌,其中MUC2是主要黏液蛋白,对维持黏液层结构和防止微生物入侵具有至关重要的作用。艾美耳球虫感染可以显著降低空肠杯状细胞的数量(P < 0.05)[28]、下调MUC2基因的表达[28–30],导致黏液层中黏蛋白的含量降低,破坏了肠黏膜屏障的完整性,这可能是导致球虫穿过黏液层定殖上皮细胞的重要原因之一。而枯草芽孢杆菌可以显著提高MUC2 mRNA的转录水平(P < 0.05),乳酸菌能显著增加杯状细胞数量和绒毛长度(P < 0.05)[31],增强肠道屏障功能,从而发挥抗球虫作用。益生菌不仅可以调控黏蛋白的表达,还可以刺激宿主分泌具有抗菌和免疫调节特性的抗菌肽。抗菌肽能破坏病原菌外膜的完整性直接杀灭病原菌,也能诱导黏蛋白和紧密连接蛋白的表达来增强肠黏膜物理屏障功能[32]。在早熟艾美耳球虫感染后第3天,十二指肠和回肠中肝表达抗菌肽2 (LEAP2)的含量分别降至对照组的27%和56%,阳离子氨基酸转运蛋白1 (CAT1)、兴奋性氨基酸转运蛋白3 (EAAT3)、L型氨基酸转运蛋白1 (LAT1)、寡肽转运体1 (PepT1)和锌离子转运体1 (ZnT1)等的表达量也下降[33],在堆型艾美耳球虫感染中也有相似发现[34]。在艾美耳球虫感染后防御素的表达量在不同品系肉鸡中具有差异性[35],乳酸杆菌和细菌混合物VSL#3 (8种革兰氏阳性菌)能通过诱导核因子NF-κB途径和激活蛋白AP-1以及丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)上调β-防御素2来增强肠道屏障功能[36]。从这些研究结果可以看出,艾美耳球虫侵入宿主后会下调宿主抗菌肽的表达,削弱宿主对病原的抵抗力,而益生菌可以刺激宿主产生黏蛋白和抗菌肽等活性分子增强黏液层的保护功能,可能是其增强机体抗球虫感染能力的作用方式之一。
2.2 肠黏膜物理屏障 物理屏障的主要功能是使肠道菌群和肠上皮细胞空间分离,主要包括覆盖肠黏膜的黏液层、肠上皮细胞微绒毛上的糖萼和肠上皮细胞间的连接[27]。肠上皮单层柱状细胞通过细胞间连接来调控细胞旁通透性,从而加强肠黏膜物理屏障的功能。细胞间连接包括桥粒(desmosomes)、黏着连接(adherens junctions)、间隙连接(gap junctions)和紧密连接(tight junctions)。其中,紧密连接是调节物理屏障的关键[37],由闭锁蛋白(occludin,OCLN)、闭合蛋白(claudins,CLDNs)、连接黏附分子(junction adhesion molecules,JAMs)和闭合小环蛋白(zonula occludens,ZOs)等组成。混合艾美耳球虫和产气荚膜梭菌感染导致空肠中紧密连接蛋白CLDN1、OCLN、JAM2、ZO1的表达明显下调[30, 38],通过抑制紧密连接蛋白的表达改变肠道上皮细胞层的通透性,使其更易定殖及侵入上皮细胞。
研究证实,饲料中添加益生菌可调控肠道紧密连接蛋白的表达,通过保护和改善肠黏膜的屏障功能维护肠道健康。与基础饲粮喂养组相比,添加枯草芽孢杆菌能够提高肠道紧密连接蛋白JAM2(P=0.0004)、ZO1(P=0.0001)和OCLN (P=0.0008)的表达水平(9.37×10–2、8.60×10–2和1.08×10–1)[39],显著增加肉鸡空肠中JAM2蛋白的含量,其中枯草芽孢杆菌747还显著增加OCLN蛋白在空肠中的含量(P < 0.05)[40]。地衣芽孢杆菌和解淀粉芽孢杆菌可以增加盲肠、十二指肠和空肠中JAM2蛋白的含量(P < 0.05),提高感染肉鸡的生长性能(P < 0.01)、减少肠道损伤和卵囊脱落(P < 0.05)[41]。上述结果表明,球虫感染会抑制紧密连接蛋白的表达,使上皮细胞通透性增加,益生菌可提高紧密连接蛋白的表达水平,从而维持上皮细胞正常生理功能,防止球虫定殖及侵入上皮细胞。
3 益生菌改善肠道免疫功能 艾美耳球虫生命周期复杂,主要包括外源性阶段(卵囊孢子化)和裂殖生殖(无性生殖)、配子生殖(有性生殖)的内源性阶段[42]。艾美耳球虫孢子化卵囊被摄入后,在肠道进行增殖分化引起了宿主肠道的免疫应答。益生菌可以通过激活巨噬细胞、刺激分泌性抗体产生、调节T细胞免疫功能来参与抗球虫免疫。
3.1 调控巨噬细胞活性 机体可以通过肠道上皮细胞表面的模式识别受体(PRRs)识别病原微生物表面的病原相关分子模式(PAMPs),诱导免疫细胞增殖(激活巨噬细胞、树突状细胞、白细胞和自然杀伤细胞)和细胞因子分泌,通过激活机体免疫反应来抵抗球虫感染。幼雏的免疫系统在孵化后的最初几周尚未发育成熟,艾美耳球虫最容易侵入其体内引起感染和损伤,因此,促进幼雏肠道免疫系统的发育对鸡球虫病的预防具有重要意义。巨噬细胞在识别和提呈抗原、分泌细胞因子等方面具有重要作用[43],益生菌能促进巨噬细胞活化成熟,并释放具有免疫调节作用的一氧化氮(nitric oxide,NO)作为促炎因子参与抗球虫免疫反应[44]。例如,饲料添加枯草芽孢杆菌可诱导NO产生,调控巨噬细胞的免疫功能[45],添加鼠李糖乳杆菌可使巨噬细胞产生的NO增加,增强宿主免疫功能[46],充分显示益生菌在增强机体先天性免疫中发挥重要作用。
3.2 刺激分泌型抗体的产生 免疫球蛋白A(immunoglobulin A,IgA)在黏膜免疫中具有重要地位,浆细胞分泌的IgA与上皮细胞分泌成分(secretory component,SC)连接形成分泌型免疫球蛋白A(sIgA),在抵御细菌、病毒和寄生虫等多种病原感染方面发挥着重要作用[47]。sIgA除了直接作用于病原体的毒力因子和发挥免疫排斥作用外,还可以促进黏膜表面的抗炎反应,如树突状细胞识别sIgA可以抑制IL-12的分泌,诱导辅助性T细胞2(Th2)和调节性T细胞(Treg)参与免疫反应[48],在肠道免疫反应中发挥重要作用。球虫感染时肠道sIgA的表达增至350.4% (P < 0.05)[49],因此sIgA可能参与了抗球虫免疫反应。在我们前期工作中,益生菌能够增加球虫感染时肠道中sIgA的含量(P < 0.05),Bai等[50]也发现,在肉鸡饲料中添加枯草芽孢杆菌fmbj能够显著提高空肠和回肠sIgA的浓度(P < 0.05)。以上结果表明球虫入侵会引起宿主免疫反应,但益生菌可以刺激免疫细胞产生IgA抗体参与免疫,并且IgA可以穿过肠上皮细胞形成sIgA进入肠腔,增强肠道黏液屏障功能,从而抵抗球虫入侵。
3.3 诱导T细胞免疫 T细胞免疫是鸡抗球虫感染的关键,促炎性细胞因子IFN-γ分泌量增加能刺激T细胞的增殖。IFN-γ是一种比较常见的细胞免疫标志物,与抗球虫感染的保护性免疫应答相关[51–52],能抑制寄生虫发育、提高自由基产量、激活抗体依赖性细胞介导的细胞毒性效应、促进穿孔素和蛋白酶释放[52],而且用重组IFN-γ处理鸡细胞可以抑制柔嫩艾美耳球虫在细胞内的发育[53],这些结果均表明,IFN-γ在宿主抗艾美耳球虫的免疫反应中具有重要作用。除IFN-γ外,在艾美耳球虫感染时肠道中细胞因子IL-1β、IL-2、IL-6、IL-15、IL-16、IL-17、TGF-β (转化生长因子)和CC趋化因子K230、MIP-1β水平均升高,可能参与抗球虫免疫反应[54–59]。给感染球虫的雏鸡饲喂枯草芽孢杆菌,可以提高幼雏特异性抗体水平,并且通过调控鸡肠道中细胞因子IL-1β、IFN-γ和趋化因子CXCLi2的表达来调控肠道免疫,减轻球虫感染带来的损害[60]。IL-2主要由活化的T细胞和NK细胞产生,在堆型艾美耳球虫原发性和继发性感染后,随着γδT细胞增多,脾脏和肠道中的IL-2 mRNA转录水平显著提高(P < 0.05)[61]。乳酸杆菌类饲料产品(Primalac?)能增加鸡肠道IFN-γ和IL-2的表达,使粪便中卵囊数相比于对照组减少了14% (P < 0.05)[62]。在我们前期研究中也发现,益生菌添加组肉鸡IFN-γ、IL-2含量也显著高于对照组(P < 0.05)。益生菌不仅能够增强机体免疫反应,还可以刺激抑炎因子IL-10和TGF-β产生,对体内免疫反应进行调控,防止过度炎症给机体带来损害[41]。上述结果表明,益生菌能够促进幼雏免疫系统的发育,在球虫感染早期可增强免疫反应抵抗球虫感染,并可通过调控细胞因子调节机体免疫反应,避免产生过度炎症造成机体损伤,在预防鸡球虫感染中发挥重要作用。
4 总结和展望 本文综述了益生菌用于预防家禽球虫病的最新研究进展。现有研究证实益生菌对鸡球虫病具有明显的预防效果,但益生菌抗球虫的确切机制尚未深入阐明,严重阻碍了益生菌抗球虫产品的应用和推广。笔者综合益生菌在调控肠道微生物群、改善肠黏膜屏障和影响免疫系统等方面的研究进展,提出了新的、拟解决以及有待进一步深入探讨的科学问题:(1) 益生菌调控肠道菌群抵御球虫感染的机制。肠道菌群是机体重要的组成部分,益生菌和球虫均可以改变肠道菌群的构成,明确益生菌、球虫和宿主间相互作用机制对于调控肠道菌群健康稳定,改善家禽生长性能和预防球虫病具有重要意义。明确肠道菌群及其代谢产物调控家禽生理的分子机制,将为益生菌应用于家禽球虫病防控提供新的解决策略。(2) 肠道屏障活性分子的解析。通过转录组学和蛋白组学等生物技术分析肠道屏障中黏蛋白、紧密连接蛋白、防御素和固有层细胞因子等的动态表达,揭示益生菌在肠道屏障的作用靶标,解析益生菌发挥抗球虫作用的分子机制,可为高效益生菌分离株的筛选和应用提供科学依据。(3) 益生菌抗球虫有效成分的解析。宏基因组研究的发展为揭示益生菌,特别是非可培养益生菌的有效作用分子提供了新的技术手段,运用高通量测序技术结合代谢组学解析益生菌在球虫感染肉鸡肠道中的作用及其效应成分,可为抗球虫益生菌产品的升级换代奠定理论基础。(4) 高效抗球虫益生菌制剂的研发。筛选高效安全的抗球虫益生菌分离株,揭示益生菌不同菌种、不同菌株间的相互作用机制,优化高效益生菌配伍组方设计,精准制备和添加益生菌特异性抗球虫效应分子,可研制更加高效安全的抗球虫微生态制剂。综上所述,在禁抗政策下益生菌在家禽疾病防控中的作用愈发重要,只有充分了解益生菌在球虫感染机体时发挥有益作用的分子机制,才能进一步促进益生菌抗球虫产品的应用和发展,开发出能够替代抗生素用于预防和控制球虫病的高效益生菌制剂。
References
| [1] | Quiroz-Casta?eda RE, Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. BioMed Research International, 2015, 2015: 430610. |
| [2] | Chapman HD. Milestones in avian coccidiosis research: a review. Poultry Science, 2014, 93(3): 501-511. DOI:10.3382/ps.2013-03634 |
| [3] | Noack S, Chapman HD, Selzer PM. Anticoccidial drugs of the livestock industry. Parasitology Research, 2019, 118(7): 2009-2026. DOI:10.1007/s00436-019-06343-5 |
| [4] | Fatoba AJ, Adeleke MA. Transgenic Eimeria parasite: a potential control strategy for chicken coccidiosis. Acta Tropica, 2020, 205: 105417. DOI:10.1016/j.actatropica.2020.105417 |
| [5] | Lillehoj HS, Lillehoj EP. Avian coccidiosis: a review of acquired intestinal immunity and vaccination strategies. Avian Diseases, 2000, 44(2): 408-425. DOI:10.2307/1592556 |
| [6] | Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clinical Microbiology Reviews, 2002, 15(1): 58-65. DOI:10.1128/CMR.15.1.58-65.2002 |
| [7] | Williams RB. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathology, 2002, 31(4): 317-353. DOI:10.1080/03079450220148988 |
| [8] | Blake DP. Eimeria genomics: where are we now and where are we going?. Veterinary Parasitology, 2015, 212(1/2): 68-74. |
| [9] | Bozkurt M, Aysul N, Kü?ükyilmaz K, Aypak S, Ege G, ?atli AU, Ak?it H, ??ven F, Seyrek K, Cinar M. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poultry Science, 2014, 93(2): 389-399. DOI:10.3382/ps.2013-03368 |
| [10] | Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Review of Vaccines, 2006, 5(1): 143-163. DOI:10.1586/14760584.5.1.143 |
| [11] | Lee KW, Lillehoj HS, Jang SI, Lee SH. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Research in Veterinary Science, 2014, 97(2): 304-308. DOI:10.1016/j.rvsc.2014.07.021 |
| [12] | Kabir SML. The role of probiotics in the poultry industry. International Journal of Molecular Sciences, 2009, 10(8): 3531-3546. DOI:10.3390/ijms10083531 |
| [13] | Ducatelle R, Goossens E, De Meyer F, Eeckhaut V, Antonissen G, Haesebrouck F, Van Immerseel F. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Veterinary Research, 2018, 49: 43. DOI:10.1186/s13567-018-0538-6 |
| [14] | Hume ME, Clemente-Hernández S, Oviedo-Rondón EO. Effects of feed additives and mixed Eimeria species infection on intestinal microbial ecology of broilers. Poultry Science, 2006, 85(12): 2106-2111. DOI:10.1093/ps/85.12.2106 |
| [15] | Chen HL, Zhao XY, Zhao GX, Huang HB, Li HR, Shi CW, Yang WT, Jiang YL, Wang JZ, Ye LP, Zhao Q, Wang CF, Yang GL. Dissection of the cecal microbial community in chickens after Eimeria tenella infection. Parasites & Vectors, 2020, 13(1): 56. |
| [16] | Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, Gaskins HR. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Veterinary Immunology and Immunopathology, 2008, 122(1/2): 104-115. |
| [17] | Qin ZR, Fukata T, Baba E, Arakawa A. Effect of Eimeria tenella infection on Salmonella enteritidis infection in chickens. Poultry Science, 1995, 74(1): 1-7. DOI:10.3382/ps.0740001 |
| [18] | Macdonald SE, van Diemen PM, Martineau H, Stevens MP, Tomley FM, Stabler RA, Blake DP. Impact of Eimeria tenella coinfection on Campylobacter jejuni colonization of the chicken. Infection and Immunity, 2019, 87(2): e00772-18. |
| [19] | Stanley D, Wu SB, Rodgers N, Swick RA, Moore RJ. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One, 2014, 9(8): e104739. DOI:10.1371/journal.pone.0104739 |
| [20] | Wu SB, Stanley D, Rodgers N, Swick RA, Moore RJ. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Veterinary Microbiology, 2014, 169(3/4): 188-197. |
| [21] | Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infection and Immunity, 2011, 79(7): 2755-2763. DOI:10.1128/IAI.01375-10 |
| [22] | Wang X, Farnell YZ, Kiess AS, Peebles ED, Wamsley KGS, Zhai W. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poultry Science, 2019, 98(9): 3839-3849. DOI:10.3382/ps/pez096 |
| [23] | Jayaraman S, Thangavel G, Kurian H, Mani R, Mukkalil R, Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poultry Science, 2013, 92(2): 370-374. DOI:10.3382/ps.2012-02528 |
| [24] | Park JH, Kim IH. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poultry Science, 2014, 93(8): 2054-2059. DOI:10.3382/ps.2013-03818 |
| [25] | Jeong JS, Kim IH. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poultry Science, 2014, 93(12): 3097-3103. DOI:10.3382/ps.2014-04086 |
| [26] | Okumura R, Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflammation and Regeneration, 2018, 38: 5. DOI:10.1186/s41232-018-0063-z |
| [27] | Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Experimental & Molecular Medicine, 2017, 49(5): e338. |
| [28] | de Souza Khatlab A, Vesco APD, Neto ARO, Almeida FLA, Gasparino E. Dietary supplementation with free methionine or methionine dipeptide improves environment intestinal of broilers challenged with Eimeria spp.. Journal of Animal Science, 2019, 97(12): 4746-4760. DOI:10.1093/jas/skz339 |
| [29] | Jiang ZY, Applegate TJ, Lossie AC. Cloning, annotation and developmental expression of the chicken intestinal MUC2 gene. PLoS One, 2013, 8(1): e53781. DOI:10.1371/journal.pone.0053781 |
| [30] | Stefanello C, Rosa DP, Dalmoro YK, Segatto AL, Vieira MS, Moraes ML, Santin E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Frontiers in Veterinary Science, 2019, 6: 491. |
| [31] | Aliakbarpour HR, Chamani M, Rahimi G, Sadeghi AA, Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australasian Journal of Animal Sciences, 2012, 25(9): 1285-1293. DOI:10.5713/ajas.2012.12110 |
| [32] | Robinson K, Deng Z, Hou YQ, Zhang GL. Regulation of the intestinal barrier function by host defense peptides. Frontiers in Veterinary Science, 2015, 2: 57. |
| [33] | Yin H, Sumners LH, Dalloul RA, Miska KB, Fetterer RH, Jenkins MC, Zhu Q, Wong EA. Changes in expression of an antimicrobial peptide, digestive enzymes, and nutrient transporters in the intestine of E. praecox-infected chickens. Poultry Science, 2015, 94(7): 1521-1526. DOI:10.3382/ps/pev133 |
| [34] | Su S, Miska KB, Fetterer RH, Jenkins MC, Wong EA. Expression of digestive enzymes and nutrient transporters in Eimeria acervulina-challenged layers and broilers. Poultry Science, 2014, 93(5): 1217-1226. DOI:10.3382/ps.2013-03807 |
| [35] | Su S, Miska KB, Fetterer RH, Jenkins MC, Lamont SJ, Wong EA. Differential expression of intestinal nutrient transporters and host defense peptides in Eimeria maxima-infected Fayoumi and Ross chickens. Poultry Science, 2018, 97(12): 4392-4400. DOI:10.3382/ps/pey286 |
| [36] | Schlee M, Harder J, K?ten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte β-defensin 2. Clinical and Experimental Immunology, 2008, 151(3): 528-535. DOI:10.1111/j.1365-2249.2007.03587.x |
| [37] | Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annual Review of Cell and Developmental Biology, 2006, 22: 207-235. DOI:10.1146/annurev.cellbio.22.010305.104219 |
| [38] | Lee YS, Lee SH, Gadde UD, Oh ST, Lee SJ, Lillehoj HS. Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens. Poultry Science, 2018, 97(6): 1899-1908. DOI:10.3382/ps/pey031 |
| [39] | Gadde U, Oh ST, Lee YS, Davis E, Zimmerman N, Rehberger T, Lillehoj HS. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics and Antimicrobial Proteins, 2017, 9(4): 397-405. DOI:10.1007/s12602-017-9275-9 |
| [40] | Park I, Lee Y, Goo D, Zimmerman NP, Smith AH, Rehberger T, Lillehoj HS. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poultry Science, 2020, 99(2): 725-733. DOI:10.1016/j.psj.2019.12.002 |
| [41] | Chaudhari AA, Lee Y, Lillehoj HS. Beneficial effects of dietary supplementation of Bacillus strains on growth performance and gut health in chickens with mixed coccidiosis infection. Veterinary Parasitology, 2020, 277: 109009. DOI:10.1016/j.vetpar.2019.109009 |
| [42] | Blake DP, Tomley FM. Securing poultry production from the ever-present Eimeria challenge. Trends in Parasitology, 2014, 30(1): 12-19. DOI:10.1016/j.pt.2013.10.003 |
| [43] | Kim WH, Chaudhari AA, Lillehoj HS. Involvement of T cell immunity in avian coccidiosis. Frontiers in Immunology, 2019, 10: 2732. DOI:10.3389/fimmu.2019.02732 |
| [44] | Lillehoj HS, Li GX. Nitric oxide production by macrophages stimulated with coccidia sporozoites, lipopolysaccharide, or interferon-γ, and its dynamic changes in SC and TK strains of chickens infected with Eimeria tenella. Avian Diseases, 2004, 48(2): 244-253. DOI:10.1637/7054 |
| [45] | Lee KW, Li GX, Lillehoj HS, Lee SH, Jang SI, Babu US, Lillehoj EP, Neumann AP, Siragusa GR. Bacillus subtilis-based direct-fed microbials augment macrophage function in broiler chickens. Research in Veterinary Science, 2011, 91(3): e87-e91. DOI:10.1016/j.rvsc.2011.01.018 |
| [46] | Korhonen R, Korpela R, Saxelin M, M?ki M, Kankaanranta H, Moilanen E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation, 2001, 25(4): 223-232. DOI:10.1023/A:1010971703271 |
| [47] | Tian EJ, Zhou BH, Wang XY, Zhao J, Deng W, Wang HW. Effect of diclazuril on intestinal morphology and sIgA expression in chicken infected with Eimeria tenella. Parasitology Research, 2014, 113(11): 4057-4064. DOI:10.1007/s00436-014-4074-7 |
| [48] | Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Vos WM, Mercenier A, Nauta A, Garcia-Rodenas CL. Homeostasis of the gut barrier and potential biomarkers. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2017, 312(3): G171-G193. DOI:10.1152/ajpgi.00048.2015 |
| [49] | Zhou BH, Liu LL, Liu J, Yuan FW, Tian EJ, Wang HW. Effect of diclazuril on the bursa of fabricius morphology and sIgA expression in chickens infected with Eimeria tenella. The Korean Journal of Parasitology, 2015, 53(6): 675-682. DOI:10.3347/kjp.2015.53.6.675 |
| [50] | Bai KW, Feng CC, Jiang LY, Zhang LG, Zhang JF, Zhang LL, Wang T. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poultry Science, 2018, 97(7): 2312-2321. DOI:10.3382/ps/pey116 |
| [51] | Lee SH, Lillehoj HS, Lillehoj EP, Cho SM, Park DW, Hong YH, Chun HK, Park HJ. Immunomodulatory properties of dietary plum on coccidiosis. Comparative Immunology, Microbiology and Infectious Diseases, 2008, 31(5): 389-402. DOI:10.1016/j.cimid.2007.06.005 |
| [52] | Yun CH, Lillehoj HS, Choi KD. Eimeria tenella infection induces local gamma interferon production and intestinal lymphocyte subpopulation changes. Infection and Immunity, 2000, 68(3): 1282-1288. DOI:10.1128/IAI.68.3.1282-1288.2000 |
| [53] | Lillehoj HS, Choi KD. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Diseases, 1998, 42(2): 307-314. DOI:10.2307/1592481 |
| [54] | Dalloul RA, Lillehoj HS. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Diseases, 2005, 49(1): 1-8. DOI:10.1637/7306-11150R |
| [55] | Lillehoj HS, Min W, Dalloul RA. Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poultry Science, 2004, 83(4): 611-623. DOI:10.1093/ps/83.4.611 |
| [56] | Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Developmental & Comparative Immunology, 2000, 24(2/3): 303-324. |
| [57] | Min W, Lillehoj HS, Kim S, Zhu JJ, Beard H, Alkharouf N, Matthews BF. Profiling local gene expression changes associated with Eimeria maxima and Eimeria acervulina using cDNA microarray. Applied Microbiology and Biotechnology, 2003, 62(4): 392-399. DOI:10.1007/s00253-003-1303-x |
| [58] | Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Veterinary Immunology and Immunopathology, 2006, 114(3/4): 209-223. |
| [59] | Min W, Lillehoj HS. Isolation and characterization of chicken interleukin-17 cDNA. Journal of Interferon & Cytokine Research, 2002, 22(11): 1123-1128. |
| [60] | Lee KW, Lillehoj HS, Jang SI, Lee SH, Bautista DA, Siragusa GR. Effect of Bacillus subtilis-based direct-fed microbials on immune status in broiler chickens raised on fresh or used litter. Asian-Australasian Journal of Animal Sciences, 2013, 26(11): 1592-1597. DOI:10.5713/ajas.2013.13178 |
| [61] | Choi KD, Lillehoj HS. Role of chicken IL-2 on γδ T-cells and Eimeria acervulina-induced changes in intestinal IL-2 mRNA expression and γδ T-cells. Veterinary Immunology and Immunopathology, 2000, 73(3/4): 309-321. |
| [62] | Dalloul RA, Lillehoj HS, Tamim NM, Shellem TA, Doerr JA. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comparative Immunology, Microbiology and Infectious Diseases, 2005, 28(5/6): 351-361. |
