于静晨, 张文婷, 姚玉峰


上海交通大学医学院, 上海 200025
收稿日期:2020-04-11;修回日期:2020-08-20;网络出版日期:2020-11-10
基金项目:国家自然科学基金(81772140)
*通信作者:姚玉峰, Tel/Fax:+86-21-64671226; E-mail:yfyao@sjtu.edu.cn.
摘要:细菌蛋白质磷酸化修饰是调控细菌基因表达的一种重要方式,在细菌诸多生命活动中发挥非常关键的作用。本文系统概括了近年来细菌蛋白质磷酸化修饰的种类、双组分调控系统中磷酸化修饰调控信号传导、酪氨酸残基磷酸化修饰以及丝/苏氨酸残基磷酸化修饰等,同时对不同种类细菌蛋白质磷酸化修饰的功能进行综述,这些研究将对人类了解细菌蛋白质翻译后修饰的磷酸化调控及其与控制细菌感染的关系提供参考价值。
关键词:细菌磷酸化双组分调控系统信号传导激酶磷酸酶
Research progress in bacterial protein phosphorylation
Jingchen Yu, Wenting Zhang, Yufeng Yao


School of Medicine, Shanghai Jiao Tong University, Shanghai 200025, China
Received: 11 April 2020; Revised: 20 August 2020; Published online: 10 November 2020
*Corresponding author: Yufeng Yao, Tel/Fax: +86-21-64671226; E-mail:yfyao@sjtu.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (81772140)
Abstract: As a key post-translational modification involved in regulation of gene expression, protein phosphorylation plays critical roles in bacterial physiological processes. Here we summarize the types of phosphorylation modification in bacteria, two-component signal transduction, tyrosine phosphorylation as well as serine/threonine phosphorylation, and discuss the regulatory mechanisms and functions of phosphorylation modifications in bacterial cellular processes in recent years. This review is helpful to understand the phosphorylation regulation of bacterial post-translational modifications and its relationship with the control of bacterial infections in the future.
Keywords: bacterial phosphorylationtwo-component systemsignal transductionkinasephosphatase
蛋白质翻译后修饰(Post-translational modifications,PTMs)在细胞感应外界环境压力和复杂信号传导方面发挥重要作用,其中磷酸化修饰是研究得较为系统的一种修饰方式。与真核生物磷酸化修饰不同的是,细菌的磷酸激酶/磷酸酶家族更具有多样性,且能磷酸化/去磷酸化多种氨基酸残基[1]。
目前为止,细菌蛋白质中能发生磷酸化的氨基酸残基有丝氨酸(Serine,Ser)、苏氨酸(Threonine,Thr)、酪氨酸(Tyrosine,Tyr)、组氨酸(Histidine,His)、精氨酸(Arginine,Arg)、赖氨酸(Lysine,Lys)、天冬氨酸(Aspartic acid,Asp)、半胱氨酸(Cysteine,Cys)。Ser、Thr和Tyr残基的羟基与磷酸基团形成酯键,发生O-磷酸化,热力学上十分稳定[2]。His、Arg、Lys残基的氨基或亚氨基与磷酸基团形成磷酰胺P-N键,此化学键具有酸不稳定性,且相对研究得较少。其中,Arg能否发生磷酸化修饰一直存在争议,直到2009年通过蛋白质质谱鉴定才证明细菌中存在Arg磷酸化修饰[3]。此外,Asp残基侧链的羧基发生酰基磷酸化时形成酸酐和酰基磷酸酯的混合物;His磷酰胺P-N键和Asp酰基磷酸酯键是相对高能化学键,特定Asp基团磷酸化修饰形成的酰基磷酸酯键产生的自由能足够引起蛋白构象改变,对信号传导十分重要[4]。Cys巯基发生磷酸化形成磷硫酯P-S键,由于相对高能,较难检测[5]。
1 双组分调控系统 双组分调控系统(Two-component system,TCS)是细菌和少数真核生物应答环境刺激的主要机制。TCS由感应激酶(Sensor histidine kinase,SHK)和效应调控元件(Response regulator,RR)双组分组成。SHK通常是膜蛋白,感应到胞外或胞质内信号后,在其保守的His位点,利用ATP发生自磷酸化激活,随后将磷酸基团转移到RR的N端接收器(Receiver,REC)保守的Asp位点。SHK由结构上保守的催化核心和输入结构域组成,其催化核心包括结构上非常保守的C端催化基团和ATP结合结构域(Catalytic and ATP-binding,CA)以及保守程度相对较低的蛋白二聚化和His磷酸转移结构域(Dimerization and histidine phosphotransfer,DHp)[6](图 1-A)。此外,SHK也能发挥磷酸酶作用,对磷酸化的RR进行去磷酸化修饰[7]。
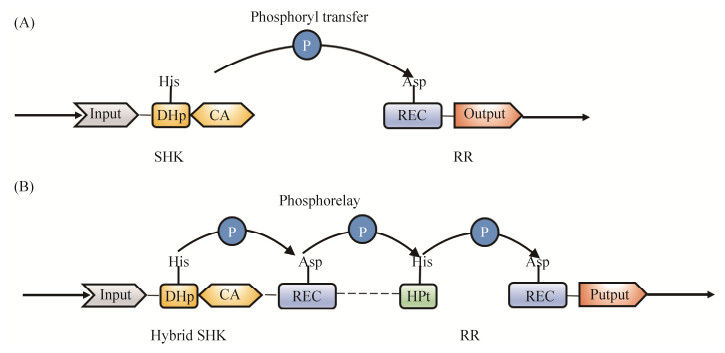 |
| 图 1 经典TCS和多步磷酸化接力传递信号传导系统结构模式图 Figure 1 Schematic diagram of TCS and phosphorelay signal transduction system. A: the prototypical TCS pathway features a phosphoryl transfer between the conserved kinase core (CA) and DHp domains; B: a phosphorelay scheme is utilized by hybrid HKs involving additional REC and HPt domains for multiple phosphotransfer events. |
| 图选项 |
RR通常是DNA结合蛋白,利用其保守Asp位点的磷酸化激活或抑制转录,同时一些RR的效应结构域具有酶活性,可催化磷酸基团转移和自身的去磷酸化。经典的RR接收结构域约由120个氨基酸组成,呈现(α/β)5结构。RR保守Asp的磷酸化通常引起二聚体的形成,并调控其与DNA的结合能力,但很多RR在未磷酸化的条件下也能结合到DNA上,包括双组分调控系统EnvZ/OmpR的调控蛋白OmpR[8]、转录调控蛋白PhoP[9]等。一些RR的N端和C端结构域存在互作,当处于未磷酸化状态时,也可调控不同的下游通路应对环境刺激。例如,SsrB被证明在未磷酸化的状态下仍可激活转录因子csgD,调控细菌生物被膜形成[10]。此外,一些细菌RR的接收结构域缺少保守的Asp位点,此位点通常被谷氨酸替代,如调节蛋白ChxR,其N端接收结构域在未磷酸化状态下的激活机制仍不清楚[11]。
近年来发现Ser/Thr激酶能磷酸化RR蛋白,参与信号传导。枯草芽胞杆菌TCS WalR/K在杆菌肽诱导条件下,Ser/Thr激酶PrkC可特异性磷酸化WalR T101位点,进而抑制(p)ppGpp合成基因sasA的表达,参与抗生素耐受的调控[12]。类似的机制也出现在A型链球菌CovR/S TCS中,Ser/Thr激酶Stk可磷酸化CovR T65位点,此位点的磷酸化可降低D53位点的磷酸化水平,减弱CovR结合到下游cylX启动子的能力,影响全局基因表达以及细菌毒力[13]。
此外,复杂TCS信号传导需要通过多步磷酸化接力传递(Phosphorelay)来完成磷酸基团和能量的转移。其SHK通常具有额外接收结构域和His磷酸转移结构域(Histidine phosphotransfer,HPt),形成多结构域蛋白参与信号传导,传导途径为His-Asp-His-Asp (图 1-B)。此外,多个TCS调控蛋白也可分别携带调控结构域和HPt参与信号传导。在炭疽杆菌中,芽胞形成调控蛋白Spo0F、Spo0B、Spo0A共同参与芽胞形成过程中信号传导[14]。大肠杆菌Rcs信号传导过程中也存在类似复杂的TCS信号传导机制[15]。
2 酪氨酸残基磷酸化与酪氨酸激酶 2.1 细菌酪氨酸残基磷酸化修饰 经典的细菌Tyr激酶(Bacterial tyrosine kinases,BY-激酶)结构由跨膜结构区和胞内催化结构域组成[16]。BY-激酶催化亚基具有结构和功能上保守的基序Walker A和Walker B基序,其最先在ATP/GTP酶中被发现并组成ATP结合位点。一些BY-激酶还有Walker A?基序,位于Walker A和Walker B基序之间。Walker A、Walker A?和Walker B的保守基序分别为GxxxxGK[ST]、[ILVFM](3) DxDxR和[ILVFM](3)DxxP,是细菌BY-激酶特有的保守序列。
BY-激酶酶活中心位于其催化亚基,可发生自磷酸化。该酶发生自磷酸化时以ATP作为磷酸供体,将ATP中的γ位磷酸转移到BY-激酶C端Tyr残基的羟基上[17]。自磷酸化对BY-激酶活性的影响在不同种属细菌中存在差异[18]。BY-激酶发生去磷酸化修饰时,形成八聚体;而发生磷酸化修饰时,八聚体解聚,从而与底物接触发生磷酸化[19]。
2.2 细菌酪氨酸残基去磷酸化修饰 细菌磷酸酶催化Tyr磷酸化蛋白发生去磷酸化修饰,进而促进或抑制磷酸信号的传递。细菌Tyr磷酸酶分为3个家族:Tyr磷酸酶(Protein tyrosine phosphatases,PTPs)和双特异性磷酸酶(Dual-specificity phosphatases,DSPs),该家族的酶除了能去磷酸化Tyr残基,也能去磷酸化Ser/Thr残基;低分子量-Tyr磷酸酶(Low-molecular-weight phosphatases,LMW-PTPs);聚合酶和组氨酸磷酸酶家族(Polymerase and histidinol phosphatases,PHPs),是革兰氏阳性菌的磷酸转移酶家族。PTPs和DSPs、LMW-PTPs在催化结构域处有保守的C(X)5R基序,其中Cys作为亲核基团,进攻底物Tyr残基上的磷酸基团。此外,PTPs和DSPs具有双向性,在某些状况下,能协同同源BY-激酶的活性,在另一状况下,也能拮抗同源BY-激酶的活性[20]。革兰氏阴性菌LMW-PTPs的编码基因与BY-激酶的编码基因位于同一操纵子且位于BY-激酶基因的上游,细菌可通过对自磷酸化激酶去磷酸化修饰从而调控其生物进程,而革兰氏阳性菌LMW-PTPs编码基因与BY-激酶编码基因则位于不同基因区域[21]。PHPs只存在于革兰氏阳性菌中,不仅能使自磷酸化的激酶去磷酸化,同时能对底物进行去磷酸化。表 1展示了部分细菌酪氨酸激酶和磷酸酶的种类和功能。
表 1. 部分细菌酪氨酸激酶和磷酸酶的种类和功能 Table 1. Types and functions of tyrosine kinases and phosphatases
| Bacterium | Tyrosine kinase | Substrate | PTPs DSPs | LMW-PTPs | PHPs | Function | References |
| Escherichia coli | Wzc | UgD | Wzb | Capsular polysaccharide production | [22–23] | ||
| Etk | RpoH RseA | Etp | Antibiotic resistance Heat shock response O-antigen capsule | ||||
| Streptococcus thermophilus | EpsD | EpsE | EpsB | Exopolysaccharide biosynthesis | [24] | ||
| Streptococcus pneumoniae | CpsD | CpsB | Exopolysaccharide biosynthesis | [25] | |||
| Staphylococcus aureus | CapB2 | CapO | PtpA PtpB | CapC1 CapC2 | Exopolysaccharide biosynthesis | [26] | |
| Yersinia pestis | PCas130 FAK | YopH | Phagocytosis inhibition Cytoskeleton rearrangement | [27] | |||
| Salmonella Typhimurium | NSF | SptP | Actin rearrangement Mast cells degranulation | [28] |
表选项
3 丝/苏氨酸残基磷酸化及丝/苏氨酸激酶 3.1 细菌丝/苏氨酸残基磷酸化修饰 Ser/Thr激酶可分为类真核Ser/Thr激酶(Eukaryotic-like serine/threonine kinases,STKs)和非典型激酶等。大部分STKs发现于革兰氏阳性菌,少部分于革兰氏阴性菌中被发现,如致病性大肠杆菌中的Stk[29]。有些细菌具有不止一个STKs,结核分枝杆菌中有11个STKs被鉴定到。研究表明,真核生物、细菌、古菌中STKs在遗传上具有共同进化起源,可能来源于一个共同祖先[30]。
STKs通常为膜蛋白或胞质蛋白,可发生自磷酸化,其激酶结构域分为12个典型的亚结构域,折叠形成双叶的催化核心结构。其中,N端α螺旋和C端活化环发生构象改变,对细菌从非活性状态到活性状态的转变至关重要,也是区别STKs和BY-激酶间底物特异性的因素之一。
STKs通常有额外的亚基调控自身活性,影响其亚细胞定位及与底物的结合等。研究表明,金黄色葡萄球菌单体PknB可形成二聚体或多聚体,利用其胞外段青霉素结合蛋白和Ser/Thr激酶相关结构域(Penicillin-binding and serine/threonine kinase-associated repeats,PASTA)作为膜受体与配体lipid II结合,将信号从细胞外传递到细胞内,其胞内激酶结构域发生自磷酸化激活。激活的STKs PknB不仅可磷酸化细胞分裂蛋白FtsZ,同时可磷酸化TCS RR WalR T101位点,激活WalR下游基因的转录,共同调控细胞壁的合成[31]。图 2展示了金黄色葡萄球菌STKs PknB调控细菌细胞壁合成过程中信号传导。
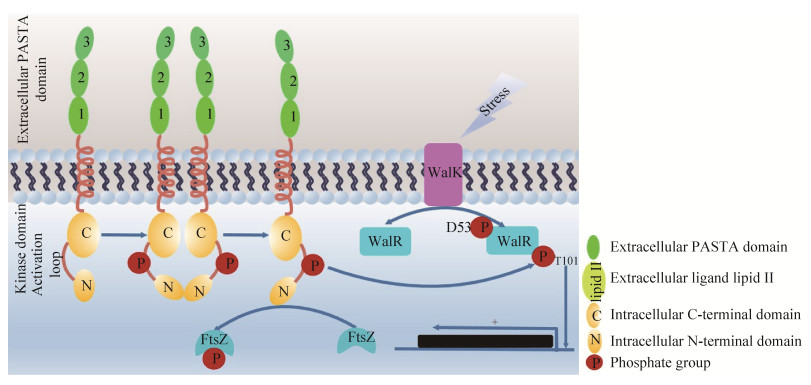 |
| 图 2 金黄色葡萄球菌STKs PknB调控细菌细胞壁合成过程中信号传导 Figure 2 Signal transduction of STKs PknB in regulating Staphylococcus aureus cell wall synthesis. |
| 图选项 |
3.2 细菌丝/苏氨酸残基去磷酸化修饰 细菌不仅需要STKs来应对环境压力,同源Ser/Thr磷酸酶(Serine/threonine phosphatases,STPs)可去磷酸化修饰TCS的调控蛋白、蛋白质翻译及细胞壁合成等过程中的蛋白质参与生命活动的可逆调控。与STKs相比,相对较少的细菌STPs被发现和表征。具有STPs活性的酶通常属于2个不同家族的成员,磷酸化蛋白的磷酸酶(Phosphoprotein phosphatases,PPPs)和金属离子依赖性磷酸酶(Metal-dependent phosphatases,PPMs),绝大多数鉴定的STPs属于PPMs家族[32]。PPMs家族为Mg2+或Mn2+依赖性磷酸酶,其具有由9–11个特征基序组成的保守的催化结构域,其中含有8个“绝对”保守的氨基酸。这些“绝对”保守氨基酸包括基序1和基序2的Asp、基序4的Thr、基序5和基序6甘氨酸(Gly)、基序8的Asp和Gly、基序11的Asp。细菌PPMs家族的STPs与人类PP2C磷酸酶具有结构相似性,在细胞分化、生长、代谢和应对环境压力等方面发挥作用[33]。
4 细菌蛋白磷酸化修饰的功能 4.1 双组分调控系统信号传导功能 生物被膜(Biofilm)的形成是细菌快速适应不利环境的有效方式,TCS信号传导机制在细菌生物被膜的形成中至关重要。例如,在非致病性大肠杆菌中,QseB/C参与调控密度感应系统,从而调控生物被膜的形成[34]。此外,近几年研究表明TCS也参与调控细菌第二信使c-di-GMP的含量、细菌生长趋化、对抗菌药物的耐药性以及毒力等方面[35]。例如结核分枝杆菌的DevR/S[36]、沙门氏菌的PhoP/Q[37]、枯草芽胞杆菌和金黄色葡萄球菌的WalR/K[38]等,都是潜在的抗菌药靶点。
4.2 酪氨酸残基磷酸化修饰功能 细菌BY-激酶可发生特定酪氨酸残基的自磷酸化来调控细菌的生理功能。大多数编码BY-激酶的基因位于多糖合成操纵子上,可调控细菌多糖的产生[17]。同源的LMW-PTPs或PHPs都可调控BY-激酶的自磷酸化状态,影响多糖产生的数量、长度及性状。在特定病原体中,胞外多糖被认为是重要的毒力因子,例如牙龈卟啉单胞菌PHPs的Php1通过调控胞外多糖的产生,参与细菌的定殖和毒力[39]。此外,肺炎链球菌BY-激酶CpsD可通过自磷酸化/去磷酸化修饰从而参与调控细菌荚膜的合成[40]。
BY-激酶不仅可以发生自磷酸化修饰,还能磷酸化修饰细菌底物蛋白来调控细菌的生理过程。第一个被鉴定的细菌BY-激酶的外源底物为UDP-葡萄糖脱氢酶,其磷酸化状态调控细菌多糖前体的产生。此外,Tyr残基磷酸化修饰可能参与细菌毒力的调控,金黄色葡萄球菌LMW-PTPs的PtpA在细菌感染巨噬细胞时可被分泌到巨噬细胞胞内,进而与巨噬细胞骨架相关蛋白coronin-1A发生互作,参与调控细菌感染的发生[26]。相类似的是,在致病性大肠杆菌中存在可磷酸化UDP-葡萄糖脱氢酶的BY-激酶Etk,而在非致病大肠杆菌中则不存在[41]。
4.3 丝/苏氨酸残基磷酸化修饰功能 尽管STKs/STPs不是DNA结合蛋白,但可通过磷酸化/去磷酸化修饰蛋白质来调控基因表达。例如,在结核分枝杆菌中,Ser/Thr磷酸酶PstP对细胞分裂和存活至关重要[42]。无乳链球菌中Ser/Thr激酶Stk1可磷酸化TCS调控蛋白CovR,从而调控与毒力相关的100多种基因的表达,包括β溶血素的生物合成[43]。此外,在肺炎链球菌中,StkP激酶和PhpP磷酸酶可逆磷酸化转录调控因子RitR,调控细胞铁离子的转运[44]。
STKs/STPs不仅能磷酸化/去磷酸化修饰转录调控因子,很多转录翻译过程中的蛋白质也被证实是其底物。研究表明,一些STKs/STPs,例如金黄色葡萄球菌中的Stk1和黄色粘球菌中的Pkn2,可磷酸化修饰细菌类组蛋白HU。Pkn2可磷酸化HUα,阻断其与DNA或其他转录因子的结合,从而调控基因的表达[45-46]。此外,STKs/STPs通过可逆磷酸化DNA和RNA聚合酶从而调控基因表达,包括DNA聚合酶α亚基(PolC)、RNA聚合酶α亚基和β亚基(RpoA和RpoB)、复制起始蛋白DnaA[47]和细菌重组蛋白RecA[48]等。蛋白质翻译过程中翻译延长因子EF-Tu和EF-G也是STKs/STPs的底物,通过可逆磷酸化修饰应对环境变化,调节蛋白质合成[49]。
STKs/STPs不仅能磷酸化/去磷酸化修饰细菌转录翻译过程中的蛋白质,在细胞壁合成、细菌分裂、芽胞形成、细菌代谢过程中也发挥重要作用。结核分枝杆菌的PknA、PknB、PknD、PknE、PknF、PknI、PknK、PknL参与分枝菌酸的生物合成,PknA、PknB、PknH、PknJ参与细胞壁成分阿拉伯糖的生物合成。此外,PknA和PknB可磷酸化细胞分裂蛋白FtsZ和FtsZ-互作蛋白FipA来调控细胞分裂[50]。在枯草芽胞杆菌中,Ser/Thr激酶PrkC参与芽胞形成的初始阶段调控[51]。此外,STKs/STPs在细菌代谢过程中也发挥作用,例如在金黄色葡萄球菌中,Stk/Stp参与调控糖酵解、TCA循环、核酸代谢、毒力因子的合成和分泌等[52]。图 3展示了细菌STKs/STPs调控网络。
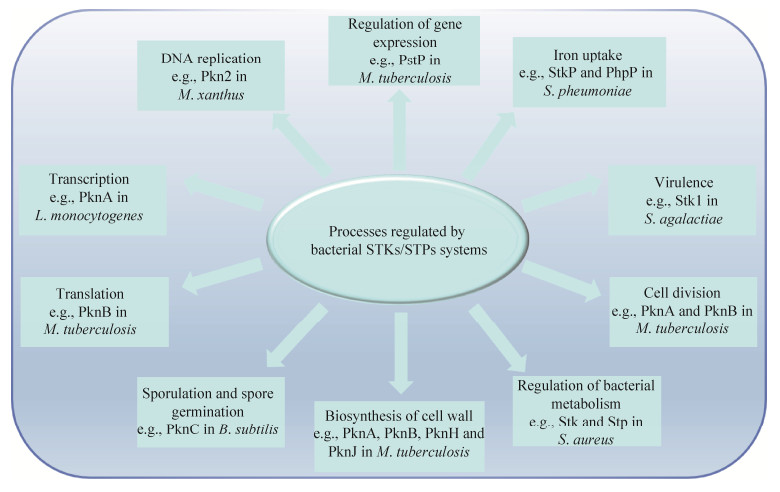 |
| 图 3 细菌STKs/STPs调控网络 Figure 3 Physiological processes regulated by bacterial STKs/STPs. |
| 图选项 |
5 展望 可逆磷酸化修饰是细菌感应外界环境变化和调控其生命活动的关键机制。细菌利用各种各样的调控系统来感应环境变化和调控信号传导,包括TCS、STKs/STPs等。越来越多的研究集中于细菌感应环境信号引发磷酸化信号传导的机制研究,包括TCS、STKs/STPs和翻译过程中某些磷酸化蛋白的互作研究等。这些研究将进一步促进人类对原核调控网络的机制探索。
随着磷酸化修饰质谱学和磷酸化蛋白质组学的发展,越来越多的细菌磷酸化底物被鉴定和表征,表明磷酸化修饰在细菌生理过程中发挥重要的调控作用。此外,定量蛋白质组学研究将高通量质谱与稳定同位素标记技术相结合,极大促进了对细菌激酶-磷酸酶的调控网络的机制研究。其中,近年来发展的利用电子捕获解离技术来片段化细菌肽段,可片段化细菌肽段的同时保留丝/苏氨酸的磷酸化,在细菌磷酸蛋白质组学研究中十分有前景。定量和实时监测技术以及定点磷酸化修饰系统也被用来监测细菌磷酸化依赖的信号传导和调控过程。然而,质谱技术对于细菌蛋白质磷酸化研究仅成功应用于Ser、Thr和Tyr的磷酸化肽段分析。由于His和Asp体内发生磷酸化后的化学不稳定性及质谱富集过程中的快速去磷酸化,使得这一技术的应用仍存在挑战。同时,这些新的研究发现对磷酸蛋白组学的分析提出了新问题,如相近种属细菌的磷酸蛋白质组学重叠性较低、细菌STKs/STPs可调节的胞内蛋白的底物范围是怎样的等[53-54]。
本课题组已有研究发现,鼠伤寒沙门氏菌中重要调控蛋白PhoP中的多个位点存在PTMs调控,其中第201位赖氨酸的乙酰化参与调控PhoP对下游基因启动子的结合[55],而第102位赖氨酸的乙酰化依赖于胞内乙酰磷酸的水平,并影响PhoP磷酸化和其转录活性[56]。课题组进一步根据近年来细菌乙酰化修饰的研究进展,对细菌乙酰化修饰调控病原菌的致病性等进行了总结分析[57]。此外,质谱检测发现,PhoP多个氨基酸位点存在不同类型的PTMs修饰,包括甲基化修饰和磷酸化修饰等(未发表的数据)。研究表明,磷酸化的PhoP处于激活状态,其第52位Asp被预测为接收磷酸基团的关键位点,但目前仍无明确的质谱数据支持这一结论。因此,PhoP是否存在其他有功能的磷酸化修饰位点?PhoP磷酸化修饰在调控细菌生理代谢中发挥着什么样功能?对这些问题的解答,将为沙门氏菌致病机理的研究提供新思路。
References
| [1] | Suskiewicz MJ, Clausen T. Chemical biology interrogates protein arginine phosphorylation. Cell Chemical Biology, 2016, 23(8): 888-890. DOI:10.1016/j.chembiol.2016.08.003 |
| [2] | Hunter T. The genesis of tyrosine phosphorylation. Cold Spring Harbor Perspectives in Biology, 2014, 6(5): a020644. DOI:10.1101/cshperspect.a020644 |
| [3] | Zhou B, Semanjski M, Orlovetskie N, Bhattacharya S, Alon S, Argaman L, Jarrous N, Zhang Y, Macek B, Sinai L, Ben-Yehuda S. Arginine dephosphorylation propels spore germination in bacteria. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(28): 14228-14237. DOI:10.1073/pnas.1817742116 |
| [4] | Mijakovic I, Grangeasse C, Turgay K. Exploring the diversity of protein modifications:special bacterial phosphorylation systems. FEMS Microbiology Reviews, 2016, 40(3): 398-417. DOI:10.1093/femsre/fuw003 |
| [5] | Buchowiecka AK. Puzzling over protein cysteine phosphorylation-assessment of proteomic tools for S-phosphorylation profiling. Analyst, 2014, 139(17): 4118-4123. DOI:10.1039/C4AN00724G |
| [6] | M?glich A. Signal transduction in photoreceptor histidine kinases. Protein Science, 2019, 28(11): 1923-1946. DOI:10.1002/pro.3705 |
| [7] | Hutchings MI, Hong HJ, Buttner MJ. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Molecular Microbiology, 2006, 59(3): 923-935. DOI:10.1111/j.1365-2958.2005.04953.x |
| [8] | Head CG, Tardy A, Kenney LJ. Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. Journal of Molecular Biology, 1998, 281(5): 857-870. DOI:10.1006/jmbi.1998.1985 |
| [9] | Liu W, Hulett FM. Bacillus subtilis phoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. Journal of Bacteriology, 1997, 179(20): 6302-6310. DOI:10.1128/JB.179.20.6302-6310.1997 |
| [10] | Desai SK, Winardhi RS, Periasamy S, Dykas MM, Jie Y, Kenney LJ. The horizontally-acquired response regulator SsrB drives a Salmonella lifestyle switch by relieving biofilm silencing. eLife, 2016, 5: e10747. DOI:10.7554/eLife.10747 |
| [11] | Maule AF, Wright DP, Weiner JJ, Han L, Peterson FC, Volkman BF, Silvaggi NR, Ulijasz AT. The aspartate-less receiver (ALR) domains:distribution, structure and function. PLoS Pathogens, 2015, 11(4): e1004795. DOI:10.1371/journal.ppat.1004795 |
| [12] | Libby EA, Reuveni S, Dworkin J. Multisite phosphorylation drives phenotypic variation in (p)ppGpp synthetase-dependent antibiotic tolerance. Nature Communications, 2019, 10(1): 5133-5142. DOI:10.1038/s41467-019-13127-z |
| [13] | Chiang-Ni C, Kao CY, Hsu CY, Chiu CH. Phosphorylation at the D53 but not the T65 residue of CovR determines the repression of rgg and speB transcription in emm1and emm49-type group A streptococci. Journal of Bacteriology, 2019, 201(4): e00681-18. |
| [14] | Plaut RD, Beaber JW, Zemansky J, Kaur AP, George M, Biswas B, Henry M, Bishop-Lilly KA, Mokashi V, Hannah RM, Pope RK, Read TD, Stibitz S, Calendar R, Sozhamannan S. Genetic evidence for the involvement of the S-layer protein gene sap and the sporulation genes spo0A, spo0B, and spo0F in phage AP50c infection of Bacillus anthracis. Journal of Bacteriology, 2014, 196(6): 1143-154. DOI:10.1128/JB.00739-13 |
| [15] | Sato T, Takano A, Hori N, Izawa T, Eda T, Sato K, Umekawa M, Miyagawa H, Matsumoto K, Muramatsu-Fujishiro A, Matsumoto K, Matsuoka S, Hara H. Role of the inner-membrane histidine kinase RcsC and outer-membrane lipoprotein RcsF in the activation of the Rcs phosphorelay signal transduction system in Escherichia coli. Microbiology, 2017, 163(7): 1071-1080. DOI:10.1099/mic.0.000483 |
| [16] | Grangeasse C, Cozzone AJ, Deutscher J, Mijakovic I. Tyrosine phosphorylation:an emerging regulatory device of bacterial physiology. Trends in Biochemical Sciences, 2007, 32(2): 86-94. DOI:10.1016/j.tibs.2006.12.004 |
| [17] | Whitmore SE, Lamont RJ. Tyrosine phosphorylation and bacterial virulence. International Journal of Oral Science, 2012, 4(1): 1-6. DOI:10.1038/ijos.2012.6 |
| [18] | Elsholz AKW, Wacker SA, Losick R. Self-regulation of exopolysaccharide production in Bacillus subtilis by a tyrosine kinase. Genes and Development, 2014, 28(15): 1710-1720. DOI:10.1101/gad.246397.114 |
| [19] | Olivares-Illana V, Meyer P, Bechet E, Gueguen-Chaignon V, Soulat D, Lazereg-Riquier S, Mijakovic I, Deutscher J, Cozzone AJ, Laprévote O, Morera S, Grangeasse C, Nessler S. Structural basis for the regulation mechanism of the tyrosine kinase CapB from Staphylococcus aureus. PLoS Biology, 2008, 6(6): e143. DOI:10.1371/journal.pbio.0060143 |
| [20] | Zhang ZY. Functional studies of protein tyrosine phosphatases with chemical approaches. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2005, 1754(1/2): 100-107. |
| [21] | Grangeasse C, Doublet P, Vincent C, Vaganay E, Riberty M, Duclos B, Cozzone AJ. Functional characterization of the low-molecular-mass phosphotyrosine-protein phosphatase of Acinetobacter johnsonii. Journal of Molecular Biology, 1998, 278(2): 339-347. DOI:10.1006/jmbi.1998.1650 |
| [22] | Lacour S, Bechet E, Cozzone AJ, Mijakovic I, Grangeasse C. Tyrosine phosphorylation of the UDP-glucose dehydrogenase of Escherichia coli is at the crossroads of colanic acid synthesis and polymyxin resistance. PLoS One, 2008, 3(8): e3053. DOI:10.1371/journal.pone.0003053 |
| [23] | Klein G, Dartigalongue C, Raina S. Phosphorylation-mediated regulation of heat shock response in Escherichia coli. Molecular Microbiology, 2003, 48(1): 269-285. DOI:10.1046/j.1365-2958.2003.03449.x |
| [24] | Minic Z, Marie C, Delorme C, Faurie JM, Mercier G, Ehrlich D, Renault P. Control of EpsE, the phosphoglycosyltransferase initiating exopolysaccharide synthesis in Streptococcus thermophilus, by EpsD tyrosine kinase. Journal of Bacteriology, 2007, 189(4): 1351-1357. DOI:10.1128/JB.01122-06 |
| [25] | Nourikyan J, Kjos M, Mercy C, Cluzel C, Morlot C, Noirot-Gros MF, Guiral S, Lavergne JP, Veening JW, Grangeasse C. Autophosphorylation of the bacterial tyrosine-kinase CpsD connects capsule synthesis with the cell cycle in Streptococcus pneumoniae. PLoS Genetics, 2015, 11(9): e1005518. DOI:10.1371/journal.pgen.1005518 |
| [26] | Gannoun-Zaki L, P?tzold L, Huc-Brandt S, Baronian G, Elhawy MI, Gaupp R, Martin M, Blanc-Potard AB, Letourneur F, Bischoff M, Molle V. PtpA, a secreted tyrosine phosphatase from Staphylococcus aureus, contributes to virulence and interacts with coronin-1A during infection. Journal of Biological Chemistry, 2018, 293(40): 15569-15580. DOI:10.1074/jbc.RA118.003555 |
| [27] | Bahta M, Burke TR. Yersinia pestis and approaches to targeting its outer protein H protein-tyrosine phosphatase (YopH). Current Medicinal Chemistry, 2012, 19(33): 5726-5734. DOI:10.2174/092986712803988866 |
| [28] | Choi HW, Brooking-Dixon R, Neupane S, Lee CJ, Miao EA, Staats HF, Abraham SN. Salmonella typhimurium impedes innate immunity with a mast-cell-suppressing protein tyrosine phosphatase, SptP. Immunity, 2013, 39(6): 1108-1120. DOI:10.1016/j.immuni.2013.11.009 |
| [29] | Li T, Li Z, Chen FH, Liu X, Ning NZ, Huang J, Wang H. Eukaryotic-like kinase expression in enterohemorrhagic Escherichia coli:potential for enhancing host aggressive inflammatory response. The Journal of Infectious Diseases, 2017, 216(9): 1150-1158. DOI:10.1093/infdis/jix160 |
| [30] | Stancik IA, ?estak MS, Ji BY, Axelson-Fisk M, Franjevic D, Jers C, Domazet-Lo?o T, Mijakovic I. Serine/threonine protein kinases from bacteria, archaea and eukarya share a common evolutionary origin deeply rooted in the tree of life. Journal of Molecular Biology, 2018, 430(1): 27-32. DOI:10.1016/j.jmb.2017.11.004 |
| [31] | Hardt P, Engels I, Rausch M, Gajdiss M, Ulm H, Sass P, Ohlsen K, Sahl HG, Bierbaum G, Schneider T, Grein F. The cell wall precursor lipid II acts as a molecular signal for the Ser/Thr kinase PknB of Staphylococcus aureus. International Journal of Medical Microbiology, 2017, 307(1): 1-10. DOI:10.1016/j.ijmm.2016.12.001 |
| [32] | Janczarek M, Vinardell JM, Lipa P, Kara? M. Hanks-type serine/threonine protein kinases and phosphatases in bacteria:roles in signaling and adaptation to various environments. International Journal of Molecular Sciences, 2018, 19(10): e2872. DOI:10.3390/ijms19102872 |
| [33] | Shi YG. Serine/threonine phosphatases:mechanism through structure. Cell, 2009, 139(3): 468-484. DOI:10.1016/j.cell.2009.10.006 |
| [34] | Bearson BL, Bearson SMD, Lee IS, Brunelle BW. The Salmonella enterica serovar typhimurium QseB response regulator negatively regulates bacterial motility and swine colonization in the absence of the QseC sensor kinase. Microbial Pathogenesis, 2010, 48(6): 214-219. DOI:10.1016/j.micpath.2010.03.005 |
| [35] | Xie YP, Shao XL, Zhang YC, Liu JG, Wang TT, Zhang WT, Hua CF, Deng X. Pseudomonas savastanoi two-component system RhpRS switches between virulence and metabolism by tuning phosphorylation state and sensing nutritional conditions. mBio, 2019, 10(2): e02838-18. |
| [36] | Hu QB, Zhang JX, Chen Y, Hu LH, Li WH, He ZG. Cyclic di-GMP co-activates the two-component transcriptional regulator DevR in Mycobacterium smegmatis in response to oxidative stress. Journal of Biological Chemistry, 2019, 294(34): 12729-12742. DOI:10.1074/jbc.RA119.008252 |
| [37] | Tang YT, Gao R, Havranek JJ, Groisman EA, Stock AM, Marshall GR. Inhibition of bacterial virulence:drug-like molecules targeting the Salmonella enterica PhoP response regulator. Chemical Biology & Drug Design, 2012, 79(6): 1007-1017. |
| [38] | Okada A, Igarashi M, Okajima T, Kinoshita N, Umekita M, Sawa R, Inoue K, Watanabe T, Doi A, Martin A, Quinn J, Nishimura Y, Utsumi R. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. The Journal of Antibiotics, 2010, 63(2): 89-94. DOI:10.1038/ja.2009.128 |
| [39] | Jung YJ, Miller DP, Perpich JD, Fitzsimonds ZR, Shen DN, Ohshima J, Lamont RJ. Porphyromonas gingivalis tyrosine phosphatase Php1 promotes community development and pathogenicity. mBio, 2019, 10(5): e02004-19. |
| [40] | Morona JK, Morona R, Paton JC. Attachment of capsular polysaccharide to the cell wall of Streptococcus pneumoniae type 2 is required for invasive disease. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(22): 8505-8510. DOI:10.1073/pnas.0602148103 |
| [41] | Lacour S, Doublet P, Obadia B, Cozzone AJ, Grangeasse C. A novel role for protein-tyrosine kinase Etk from Escherichia coli K-12 related to polymyxin resistance. Research in Microbiology, 2006, 157(7): 637-641. DOI:10.1016/j.resmic.2006.01.003 |
| [42] | Sharma AK, Arora D, Singh LK, Gangwal A, Sajid A, Molle V, Singh Y, Nandicoori VK. Serine/threonine protein phosphatase PstP of Mycobacterium tuberculosis is necessary for accurate cell division and survival of pathogen. Journal of Biological Chemistry, 2016, 291(46): 24215-24230. DOI:10.1074/jbc.M116.754531 |
| [43] | Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, Pellegrini E, Glaser P, Kunst F, Msadek T, Trieu-Cuot P, Poyart C. CovS/CovR of group B streptococcus:a two-component global regulatory system involved in virulence. Molecular Microbiology, 2004, 54(5): 1250-1268. DOI:10.1111/j.1365-2958.2004.04365.x |
| [44] | Osaki M, Arcondéguy T, Bastide A, Touriol C, Prats H, Trombe MC. The StkP/PhpP signaling couple in Streptococcus pneumoniae:cellular organization and physiological characterization. Journal of Bacteriology, 2009, 191(15): 4943-4950. DOI:10.1128/JB.00196-09 |
| [45] | Jin H, Pancholi V. Identification and biochemical characterization of a eukaryotic-type serine/threonine kinase and its cognate phosphatase in Streptococcus pyogenes:their biological functions and substrate identification. Journal of Molecular Biology, 2006, 357(5): 1351-1372. DOI:10.1016/j.jmb.2006.01.020 |
| [46] | Udo H, Lam CK, Mori S, Inouye M, Inouye S. Identification of a substrate for Pkn2, a protein Ser/Thr kinase from Myxococcus xanthus by a novel method for substrate identification. Journal of Molecular Microbiology and Biotechnology, 2000, 2(4): 557-563. |
| [47] | ?ebkowski T, Wolański M, Oldziej S, Fl?rdh K, Zakrzewska-Czerwińska J. AfsK-mediated site-specific phosphorylation regulates DnaA initiator protein activity in Streptomyces coelicolor. Journal of Bacteriology, 2020, 202(3): e00597-19. |
| [48] | Lima A, Durán R, Schujman GE, Marchissio MJ, Portela MM, Obal G, Pritsch O, Pritschcd O, de Mendoza D, Cerve?anskya C. Serine/threonine protein kinase PrkA of the human pathogen Listeria monocytogenes:biochemical characterization and identification of interacting partners through proteomic approaches. Journal of Proteomics, 2011, 74(9): 1720-1734. DOI:10.1016/j.jprot.2011.03.005 |
| [49] | Absalon C, Obuchowski M, Madec E, Delattre D, Holland IB, Seror SJ. CpgA, EF-Tu and the stressosome protein YezB are substrates of the Ser/Thr kinase/phosphatase couple, PrkC/PrpC, in Bacillus subtilis. Microbiology, 2009, 155(Pt 3): 932-943. |
| [50] | Bellinzoni M, Wehenkel AM, Durán R, Alzari PM. Novel mechanistic insights into physiological signaling pathways mediated by mycobacterial Ser/Thr protein kinases. Microbes and Infection, 2019, 21(5/6): 222-229. |
| [51] | Gruszczyński P, Obuchowski M, Ka?mierkiewicz R. Phosphorylation and ATP-binding induced conformational changes in the PrkC, Ser/Thr kinase from B. subtilis. Journal of Computer-Aided Molecular Design, 2010, 24(9): 733-747. DOI:10.1007/s10822-010-9370-4 |
| [52] | Jarick M, Bertsche U, Stahl M, Schultz D, Methling K, Lalk M, Stigloher C, Steger M, Schlosser A, Ohlsen K. The serine/threonine kinase Stk and the phosphatase Stp regulate cell wall synthesis in Staphylococcus aureus. Scientific Reports, 2018, 8(1): 13693-13705. DOI:10.1038/s41598-018-32109-7 |
| [53] | Jers C, Soufi B, Grangeasse C, Deutscher J, Mijakovic I. Phosphoproteomics in bacteria:towards a systemic understanding of bacterial phosphorylation networks. Expert Review of Proteomics, 2008, 5(4): 619-627. DOI:10.1586/14789450.5.4.619 |
| [54] | K?hler JB, Jers C, Senissar M, Shi L, Derouiche A, Mijakovic I. Importance of protein Ser/Thr/Tyr phosphorylation for bacterial pathogenesis. FEBS Letters, 2020, 594(15): 2339-2369. DOI:10.1002/1873-3468.13797 |
| [55] | Ren J, Sang Y, Tan YC, Tao J, Ni JJ, Liu ST, Fan X, Zhao W, Lu J, Wu WJ, Yao YF. Acetylation of lysine 201 inhibits the DNA-binding ability of PhoP to regulate Salmonella virulence. PLoS Pathogens, 2016, 12(3): e1005458. DOI:10.1371/journal.ppat.1005458 |
| [56] | Ren J, Sang Y, Qin R, Su Y, Cui ZL, Mang ZG, Li H, Lu SY, Zhang J, Cheng S, Liu XY, Li JX, Lu J, Wu WJ, Zhao GP, Shao F, Yao YF. Metabolic intermediate acetyl phosphate modulates bacterial virulence via acetylation. Emerging Microbes & Infections, 2019, 8(1): 55-69. |
| [57] | Ren J, Sang Y, Lu J, Yao YF. Protein acetylation and its role in bacterial virulence. Trends in Microbiology, 2017, 25(9): 768-779. DOI:10.1016/j.tim.2017.04.001 |
