陈玉莹1,3, 张志好1,3, 刘勇勤1,2,3


1. 中国科学院青藏高原环境变化与地表过程重点实验室, 北京 100101;
2. 中国科学院青藏高原地球科学卓越创新中心, 北京 100101;
3. 中国科学院大学, 北京 100049
收稿日期:2020-04-04;修回日期:2020-05-25;网络出版日期:2020-05-29
基金项目:国家自然科学基金(91851207);第二次青藏高原综合科学考察研究(2019QZKK0503);中国科学院战略性先导科技专项(A类)(XDA20050101)
作者简介:刘勇勤, 博士, 中国科学院青藏高原研究所研究员。2007年毕业于中国科学院青藏高原研究所。先后主持了国家自然科学基金面上项目、国家****基金项目、科技部科技基础性工作专项、"水圈微生物驱动地球元素循环的机制"重大研究计划重点支持项目, 已在Environmental Microbiology、Journal of Glaciology、FEMS Microbiology Ecology等专业期刊发表科研论文60余篇。主要研究方向为青藏高原冰川、湖泊微生物及其对气候变化的响应.
*通信作者:刘勇勤, E-mail:yqliu@itpcas.ac.cn.
摘要:冰川占地球陆地表面的11%,储存了约104 Pg有机碳。随着冰川消融有机碳被释放至下游生态系统中,刺激海洋、湖泊和径流的初级生产力进而影响其生态系统。微生物参与的固碳过程决定了冰川有机碳储量及向下游输出碳量。研究冰川固碳微生物群落构成及其生态功能,可为估算冰川碳积累量和保护下游生态系统提供数据基础。本文综述了冰川碳储量和释放量、冰川生态系统主要固碳途径、固碳微生物群落组成、固碳速率以及影响固碳速率的环境因素。最后基于研究现状展望了冰川生态系统固碳微生物的未来研究和发展方向。
关键词:冰川固碳微生物碳储量固碳途径影响因素
Research progress of carbon fixation microorganisms in glaciers
Yuying Chen1,3, Zhihao Zhang1,3, Yongqin Liu1,2,3


1. Key Laboratory of Tibetan Environment Changes and Land Surface Processes, Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing 100101, China;
2. CAS Center for Excellence in Tibetan Plateau Earth Sciences, Chinese Academy of Sciences, Beijing 100101, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China
Received: 4 April 2020; Revised: 25 May 2020; Published online: 29 May 2020
*Corresponding author: Yongqin Liu, E-mail: yqliu@itpcas.ac.cn.
Foundation item: Supported by the National Natural Science Foundation of China (91851207), by the Second Tibetan Plateau Scientific Expedition and Research (STEP) Program (2019QZKK0503), by the Strategic Priority Research Program (A) of the Chinese Academy of Sciences (XDA20050101)
Abstract: Glaciers occupy roughly 11% of the Earth's land surface and possess about 104 Pg organic carbon. As glaciers melt, organic carbon is released into downstream ecosystems, stimulating the primary productivity and affecting the ecosystem of oceans, lakes, and runoff. Microbial carbon-fixing processes determine organic carbon storage in glaciers and carbon output from glaciers to downstream ecosystems. Investigations on community composition and function of carbon-fixing microbes in glaciers can provide a data basis for estimating the carbon accumulation in glaciers and protecting their downstream ecosystems. This review summarized the carbon storage and release of glaciers, carbon fixing pathways, the community composition of carbon-fixing microorganisms in glacial ecosystems, carbon fixation rate, and influencing environmental factors, followed by prospects on future research directions of carbon-fixing microorganisms in glacial ecosystems.
Keywords: glacierscarbon fixating microorganismscarbon storagecarbon fixation pathwaysinfluencing factors
冰川是由雪或其他固态降水通过压缩、重结晶、融化再冻结等方式积累演化形成的处于流动状态的冰体,占全球淡水资源总量的70%。根据其规模不同,通常将冰川分为山地冰川和冰盖[1]。尽管冰川持续低温、缺乏营养并遭受强辐射,但仍生存有丰富且活跃的微生物,是一个独特的生态系统。冰川生态系统包含多种类型的生物栖息地(图 1),如冰川表面雪、表面冰、冰尘穴、冰川径流、冰川内部冰和冰下沉积物等。冰川表面环境(雪、冰、冰尘穴等)能直接接受到太阳辐射,在夏季形成液态水,是冰川生态系统中生物活动的热点地区[2]。
冰川碳循环是全球碳循环的重要组成部分。据估计冰川储藏有机碳超过104 Pg[3]。近半世纪以来,在全球变暖的大背景下,冰川消融速率加快[5],部分有机碳随冰川融水释放到下游,对下游生态系统产生重要影响[3-4]。
冰川微生物广泛参与到冰川生态系统的生物地球化学循环过程,是冰川碳循环过程的主要驱动者(图 1)。微生物介导的碳循环包括对无机碳的
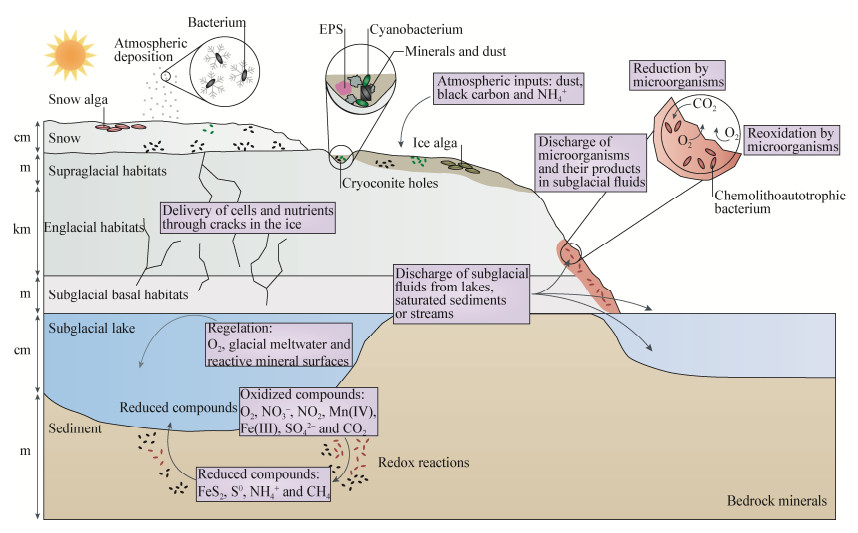 |
| 图 1 冰川生态系统及微生物介导的碳循环示意图[16] Figure 1 A diagram of the glacier ecosystem and the main processes of carbon cycle mediated by microorganisms[16]. |
| 图选项 |
固定过程和通过呼吸和发酵对有机物的分解过程[5-9]。微生物固碳过程指的是微生物吸收无机碳并将其转化为生物量的过程,这一过程是自然界无机碳转化为有机碳并进入生物圈的重要步骤,参与这一过程的微生物被称作固碳微生物[10-11]。研究微生物的固碳过程是我们全面了解冰川生态系统碳循环的重要途径。
气候、环境往往对冰川理化性质有极大的影响,进而影响冰川表面微生物群落。有研究表明冰川环境的温度、pH、营养物浓度等是影响群落结构的重要环境因子[12-15]。固碳微生物作为冰川生物群落的重要组成部分,其群落和功能也受到冰川理化性质的影响,这些影响可能造成冰川碳固定通量的变化,进而影响冰川整体碳收支。因此,研究冰川碳固定速率及其对环境因子的响应,有助于我们更好地理解冰川碳循环的过程。
本文从冰川碳储量和释放量、冰川微生物固碳途径、固碳微生物群落组成、固碳速率及其与环境因子之间的关系进行了简要总结。在此基础上,提出冰川生态系统固碳微生物研究的未来展望。
1 冰川生态系统碳储量和释放量 冰川生态系统的碳储量大小是评估其在全球碳循环中作用的主要因素。近些年来的估算研究认为,冰川潜藏大量有机碳,对全球碳循环具有直接或间接的影响[17]。
冰下沉积物是冰川碳储量最高的部分。南极冰盖下沉积物储量最高,颗粒性有机碳(POC)储量为6000–21000 Pg,比北半球永久冻土中POC储量(1672 Pg)高1–2个数量级[17]。北极格陵兰冰盖下沉积物潜在储量较小,POC储量为0.5–27.0 Pg。这主要是由于北极冰盖下沉积物层比南极冰盖下沉积物层厚度薄[18]。
冰川冰是另一个重要的有机碳存储库。南极冰盖具有巨大的总体积(2450万km2,占世界陆地冰量的90%),冰川冰中总有机碳(POC+DOC)中93%储存在南极冰盖,5%储存在格陵兰冰盖,2%储存在山地冰川[3]。Wadham估算得出南极冰盖冰中的总有机碳储量为5.45 Pg,而北极冰盖冰中总有机碳储量为0.29 Pg[17]。全球冰中POC储量为1.39 Pg,91%储存在南极冰盖,5%在格陵兰冰盖,4%储存于山地冰川[3]。
冰川消融导致冰川中存储的有机碳流失,据估计全球冰川每年碳流失约4250 Pg C/yr[19-21]。根据全球5个大洲超过300个样点的冰川DOC浓度数据,Hood估计全球冰川径流将释放约1.04±0.18 Tg C/yr DOC到淡水和海洋生态系统。冰川融水释放有机碳量的全球分布模式与冰川中有机碳储量分布模式不同,冰川融水释放的DOC主要来自于山地冰川(0.58±0.07 Tg C/yr),占全部释放量的56%,南极冰盖占23% (0.24±0.16 Tg C/yr),格陵兰冰盖占21% (0.22±0.04 Tg C/yr)[3]。山地冰川较高的有机碳释放量, 可能是由于有机碳以溶解态随冰川径流释放,这一过程主要与冰川质量损失有关。近些年来的研究表明,山地冰川消融速率比南、北极冰盖更高[22]。Wadham估计全球冰盖融水和冰断裂中的总有机碳释放量大约为每年6 Tg,其中南极冰盖通过融水释放总有机碳约0.5 Tg C/yr,通过冰断裂输出的总有机碳约为4 Tg C/yr[17],而北极冰盖释放的总有机碳为1.5 Tg C/yr。
冰川消融向下游生态系统排放大量冰川融水,导致海平面上升,同时也会把储存在冰川内部的有机碳排放到下游生态系统中。碳是生命活动生长和繁殖所必需的营养元素,释放后可作为营养物质被下游微生物利用。有机碳随冰川径流迁移到下游水生环境中,影响其生物生长和水域中的初级生产力。研究发现冰川融水中25%–95%的DOC可以被异养微生物短期内代谢并能刺激细菌活性[23-24]。含有丰富营养物质的冰川融水的输入,使南大西洋初级生产力提高了30%[17]。这凸显了冰川径流对下游生态系统的潜在影响。另外,有机碳通过冰川融水迅速转移至下游水生生态系统中,不存在土壤中潜在的生物清除途径[25],对下游河流和湖泊的影响更为直接。冰川的不断消融也会导致冰下沉积物的暴露,使得大量碳以不同形式释放,加剧对全球碳循环的影响。这一影响在未来气候变暖中可能变得越来越重要。模型计算表明,从现在到2050年,随着冰川质量损失的增加,冰川径流碳释放总量约为48 Tg[17],DOC释放量约为15.34 Tg,其中很大一部分(63%)来源于山地冰川的释放。
因此,冰川不仅在水循环中起着重要的作用,在碳循环中也扮演着重要的角色[26],其所固定的有机碳储量以及释放量是生态系统可持续发展的基础,需要进行系统化研究。
2 冰川固碳微生物研究进展 2.1 微生物固碳途径和关键基因 目前已知的微生物固碳途径主要有7条:卡尔文循环、还原型三羧酸循环、还原乙酰辅酶A途径、3-羟基丙酸双循环、3-羟基丙酸/4-羟基丁酸循环、二羧酸/4-羟基丁酸循环和反向甘氨酸裂解途径(表 1)[10-11, 27]。
表 1. 微生物固定CO2的途径的关键酶、功能基因、主要类群及发现环境[12] Table 1. Key enzymes, functional genes, dominant taxanomy, discovered environment of different microbial carbon fixation pathways[12]
| Carbon fixation pathways | Enzymes | Function genes | Dominant taxanomy | Discovered environment |
| Reductive pentose phosphate cycle (Calvin cycle) | Ribulose-1, 5-bisphosphate carboxylase/oxygenase | cbbL(rbcL) cbbM | Plants, algae, Cyanobacteria, most aerobic or facultative aerobic Eubacteria | Aquatic ecosystems, terrestrial ecosystems, extreme environments |
| Reductive tricarboxylic acid cycle | Pyruvate:Ferredoxin oxidoreductase 2-Oxoglutarate:Ferredoxin oxidoreductase Citrate lyase | porA/nifJ oorA aclB | Chlorobiales, Aquificales, Epsilonproteobacteria, Deltaproteobacteria, Alphaproteobacteria (Magnetococcus), Nitrospirae | Dark ocean[33], freshwater lakes[34], groundwaters[35], soils[36], cold seeps[37] |
| Reductive acetyl-CoA pathway (Wood- Ljungdahl pathway) | CO dehydrogenase Acetyl-Co A synthase | acsA(cooS) acsB | Methanogenic and sulfate reducing Euryarchaeota, acetogenic Firmicutes, some Spirochaetes, many Deltaproteobacteria, Annamox bacteria of Planctomycetes | Marine sediments[38], deep-sea hydrothermal vent[39], microbial mat in Antarctica[40], semi-arid desert[41] |
| 3-Hydroxypropionate bicycle | Malonyl-Co A reductase Propionyl-Co A synthase Malyl-CoA/β-methylmalyl- CoA/citramalyl-CoA lyase | Pcc/Acc | Chloroflexaceae | Microbial mat in Antarctica[40], semi-arid desert[41] |
| 3-Hydroxypropionate/ 4-Hydroxybutylate cycle | Acetyl-CoA/propionyl-CoA carboxylase 4-Hydroxybutyryl- CoA dehydratase Malonyl-CoA reductase | Aerobic Sulfolobales (Crenarchaeota), possibly marine and soil ammonia oxidizing Crenarchaeota (Thaumarchaeota) | Oligotrophic lake[45], hot springs[46-47], dark ocean, agricultural soils[48] | |
| Dicarboxylate/ 4-Hydroxybutyrate cycle | Phosphoenolpyruvate carboxylase Pyruvate synthase 4-Hydroxybutyryl- CoA dehydratase | Anaerobic Thermoproteales, Desulfurococcales (Crenarchaeota) | Ocean[50], hot springs[47] | |
| Reverse glycine cleavage pathways | Proteobacteria (Candidatus Phosphitivorax) | Wasterwater treatment sludge[51] |
表选项
卡尔文循环(Reductive pentose phosphate cycle,CBB循环)是光能自养微生物固定CO2的途径,整个循环分成三个阶段:(1)羧化反应;(2)还原反应;(3) CO2受体再生。限制卡尔文循环速率的关键酶为核酮糖1, 5-二磷酸羧化酶/加氧酶(Ribulose-1, 5-bisphosphate carboxylase/oxygenase, RubisCO),它是催化卡尔文循环第一步CO2固定的关键酶(表 1),参与卡尔文循环的RubisCO酶主要存在两种形式,Ⅰ型RubisCO和Ⅱ型RubisCO酶。Ⅰ型RubisCO酶是由cbbL基因编码的(其他命名法中或指真核生物时也被称作rbcL基因)。Ⅰ型RubisCO又包括IA型、IB型、IC型和ID型RubisCO酶。cbbL基因在类绿色自养菌群(包括植物、绿藻、蓝藻和一些变形杆菌)、类红色自养菌群(包括非绿色藻类和一些变形杆菌)和自养真核生物中被发现。该途径目前被广泛发现于水生和陆地生态系统,在一些极端环境中,如南极土壤[28]、深海热液口[29]、冰川表面[30-31]等中也被发现。
还原型三羧酸循环(Reductive tricarboxylic acid cycle,rTCA循环)是另一种重要的碳固定途径,由Evans在1966年提出的存在于少数光合紫色细菌和绿硫细菌中自养固定CO2的途径。在还原型三羧酸循环中丙酮酸︰铁氧化还原蛋白氧化还原酶(Pyruvate:Ferredoxin oxidoreductase)、2-氧戊二酸:铁氧还原蛋白氧化还原酶(2-Oxoglutarate: Ferredoxin oxidoreductase)和柠檬酸裂解酶(Citrate lyase)是3个最关键的酶,编码基因分别为porCDAB/nifJ、oorDABC和aclAB(表 2)。由于该循环中的关键酶对氧气非常敏感,该途径最初被发现于厌氧和微氧环境中,如深海热液喷口和热泉。但最近的研究发现由于酶的适应性使其在有氧条件下保持稳定,使得该途径可以在有氧环境中发挥作用[32]。目前此途径也在生存于真光层下海水的亚硝酸盐氧化菌(Nitrospinae)中被发现,Pachiadaki等通过单细胞基因组学和群落宏基因组学技术,揭示了Nitrospinae是全球海洋中丰度最高且分布广泛的亚硝酸盐氧化细菌,其单扩增组装基因组包含有三磷酸腺苷柠檬酸裂解酶和其它能指示参与还原型三羧酸循环(rTCA)的碳固定基因,表明此途径在有氧条件下也能发挥作用[33]。在淡水湖泊中发现的硝化螺旋菌(Nitrospira)也通过该途径同化CO2[34],其他生境如地下水[35]、土壤[36]、冷泉[37]等环境中也发现了该固碳途径。
表 2. 冰川固碳微生物固碳途径及主要类群 Table 2. Carbon fixation pathways and dominant microorganisms in glaciers
| Study region | Glacier name | Study type | Study method | Main carbon fixation pathway | Dominant phylum | References |
| Antarctica | Signy Island | Cryoconite | PCR | CBB | Proteobacteria, Heterokonta, Haptophyceae | [62] |
| McMurdo Dry Valleys | Glacial melt water | Functional genes sequencing | CBB | [31] | ||
| Robertson Glacier | Subglacial sediment | Metagenome sequencing | WL | [63] | ||
| Arctic | Midtre Love’nbreen | Cryoconite | PCR | CBB | Actinobacteria, Cyanobacteria, Haptophyta, Heterokonta | [62] |
| Matanuska | Glacier ice | Metagenome sequencing | CBB | [30] | ||
| Canada | Robertson | Cryoconite | PCR | CBB | Proteobacteria | [53] |
| Borup Fiord Pass | Glacial melt water | Metagenome sequencing | rTCA | [56] | ||
| Borup Fiord Pass | Cryoconite | Metagenome sequencing | rTCA | [57] | ||
| Alps | Froni | Cryoconite | Metagenome sequencing | CBB | Cyanobacteria, Proteobacteria | [52] |
| Baltoro | Cryoconite | Metagenome sequencing | CBB | Cyanobacteria, Proteobacteria | [52] | |
| Tibetan Plateau | Satrundi | Glacial melt water | Genome sequencing | CBB | [54] | |
| Qiangyong | Glacial melt water | qPCR | CBB | Proteobacteria, Cyanobacteria, Heterokonta, Cryptophyta, Haptophyta | [55] |
表选项
还原乙酰辅酶A途径(Reductive acetyl-CoA pathway,WL循环)是近年来在可利用氢的化能自养厌氧菌(如产乙酸菌、硫酸盐还原菌和产甲烷菌等)中发现的CO2固定途径,该类细菌不存在卡尔文循环,而利用乙酰辅酶A途径来进行CO2还原。还原乙酰辅酶A途径有两个分支,一个分支固定CO2后通过一系列反应生成甲基化合物;另一条分支固定CO2以后生成羰基化合物。一氧化碳脱氢酶(CODH)和乙酰辅酶A合成酶(ACS)是还原乙酰辅酶A途径中的关键酶,编码这两种酶的功能基因分别为acsA和acsB (表 1)。这条途径仅以2分子的CO2就能合成乙酸,要比通过复杂的卡尔文循环更经济快捷。由于该固碳过程依赖CO脱氢酶(CO dehydrogenase)、乙酰辅酶A合成酶(Acetyl-Co A synthase)、甲酰四氢叶酸合成酶(Formyltetrahydrofolate synthetase)及ATP酶(ATPases)等一系列对氧气十分敏感的酶,因此该途径需要在严格厌氧条件下进行。目前该途径在海洋沉积物[38]、深海热液口[39]、南极微生物席[40]、半干旱沙漠[41]等环境中被发现。
3-羟基丙酸双循环途径(3-Hydroxypropionate bicycle,3HP循环)于1989年由Holo在绿色非硫细菌绿屈挠菌科(Chloroflexus aurantiacus)中首次发现[42],但一直到2009年才被Zarzycki和Fuchs等完全确立。与卡尔文循环直接固定CO2不同,3HP途径固定以HCO3–形式存在的无机碳。该途径包含两个循环过程,第一个循环将2分子的碳酸氢盐转化为乙醛酸盐,第二个循环乙醛酸盐和丙酰辅酶A歧化生成丙酮酸和乙酰辅酶A完成固碳过程。该途径的关键酶包括丙二酰辅酶A还原酶(Malonyl-Co A reductase)、丙酰辅酶A合成酶(Propionyl-Co A synthase)和苹果酰辅酶A/β-甲基苹果酰辅酶A/柠苹酰辅酶A裂解酶(Malyl-CoA/β-methylmalyl-CoA/citramalyl-CoA lyase)[43]。目前已有研究主要针对丙酰辅酶A/乙酰辅酶A羧化酶(Propionyl-CoA/acetyl-CoA carboxylase)的功能基因Pcc/Acc开展。该途径目前被发现于南极微生物席[40]、半干旱沙漠[41]等环境。
3-羟基丙酸/4-羟基丁酸酯(3-Hydroxypropionate/ 4-Hydroxybutylate cycle,3HP/4HB循环)被认为是最节能的好氧碳固定途径[34],非常适合在营养受限的环境中进行[44]。该途径与3-羟基丙酸双循环的固碳酶相同,也是以HCO3–为底物,但该途径涉及的酶与3HP在系统发育方面不相关。目前发现该途径存在于硫化叶菌目(Sulfolobales)、勤奋金属球菌(Metallosphaera sedula)等泉古菌门以及一些奇古菌门中。目前已经发现的3HP/4HB途径存在于寡营养湖泊[45]、温泉[46]、热泉[47]、海洋[33]、农业土壤[48]中。
二羧酸/4-羟基丁酸循环途径(Dicarboxylate/4- Hydroxybutyrate cycle,DC/4HB)是近些年来新发现的厌氧固碳途径,最早由Huber等发现于嗜热古菌(Ignicoccus hospitalis)中,其能量来源于氢气和单质硫[49]。该途径以CO2和HCO3–为底物,其关键酶包括磷酸烯醇丙酮酸羧化酶(Phosphoenolpyruvate carboxylase)、丙酮酸合成酶(Pyruvate synthase)和4-羟丁酰辅酶A脱氢酶(4-Hydroxybutyryl-CoA dehydratase)。该途径目前被发现于海洋[50]和热泉[47]中。
反向甘氨酸裂解途径是最近提出的第7种固碳途径。Figueroa等在变形杆菌(Candidatus Phosphitivorax)的基因组中发现磷氧化和CO2还原的基因,而缺少其他任何已知的天然固碳途径,据此提出了通过脱氧磷脂氧化将CO2还原,然后通过还原性甘氨酸进行碳固定的途径[51]。最近的研究证明该途径可以作为一种天然自养途径发挥作用,尤其在缺乏替代终端电子受体的环境中。该途径目前被发现于废水处理污泥中[51]。
总体来说,卡尔文循环广泛存在于有氧环境中,在光的参与下进行固碳。3-羟基丙酸双循环和3-羟基丙酸/4-羟基丁酸循环也是好氧的固碳途径,主要分布于有氧或微氧的环境中。由于还原型三羧酸循环酶的适应性,该途径不仅仅局限于厌氧环境,也被发现于真光层以下海水或淡水湖泊的有氧水团中。还原乙酰辅酶A途径和二羧酸/ 4-羟基丁酸循环途径目前主要存在于厌氧环境中,如深海沉积物或深海热液口等。反向甘氨酸裂解途径目前被发现较少(表 1)。由于酶的适应性等,不同途径可能由于条件的改变进行改变。因此,了解冰川环境中的固碳途径对揭示微生物对环境适应有重要意义。
2.2 冰川微生物主要固碳途径 目前关于冰川固碳微生物的研究主要涉及南极、北极、北美加拿大落基山区、欧洲阿尔卑斯地区和青藏高原地区,其中对北极地区的研究最多,而对山地冰川尤其是青藏高原的高海拔冰川研究较少。目前在冰川生态系统中发现三种主要固碳途径:卡尔文循环、还原型三羧酸循环和还原乙酰辅酶A途径[30-31, 52-55]。其中卡尔文循环在冰川生态系统内存在范围较广,而另外两种途径较少,且仅在冰下沉积物和富硫的冰川表面中被发现[7, 56-57]。
卡尔文循环是南、北极冰川表面栖息地(如冰川冰、冰尘、表面径流等)的主要固碳途径(表 2)。例如:Kayani等对北极Matanuska冰川的冰川冰进行了宏基因组测序,通过基因组装技术获得了9个基因草图,且在组装的基因草图中找到了RubisCO酶编码基因,证明了卡尔文循环固碳途径的存在[30];Cameron使用PCR扩增技术,用cbbLR、cbbLG和rbcL特定引物,从北极Midtre Love’nbreen和南极Signy Island冰川冰尘中均检测到了Ⅰ型RubisCO编码基因。Barretto等从南极McMurdo Dry Valleys的冰川融水中,检测到IA、IB、IC和ID四种类型的RubisCO基因[31]。
卡尔文循环也是山地冰川表面环境的主要固碳途径。Franzetti等在阿尔卑斯地区山地冰川Forni和Baltoro冰川冰尘中,发现了编码RubisCO酶的关键基因rbcLS基因,且该基因相对覆盖度较高[52]。Boyd等从加拿大Robertson冰川表面沉积物中通过PCR技术检测到cbbL基因的存在,且类红色自养类群丰度高于类绿色自养类群[53]。青藏高原的冰川表面径流同样表现出以卡尔文循环为主。Kumar等从喜马拉雅Satrundi冰川(4500m)的冰川径流中分离出的细菌Glaciimonas sp. PCH181 (Proteobacteria)基因组中发现该物种使用卡尔文循环固定CO2[54]。Kong等使用定量PCR技术对青藏高原枪勇冰川径流水样进行了检测,发现了RubisCO酶的存在,且form IC和ID类型的RubisCO酶相对丰度高于IA/B类型[55]。不同的冰川表面的不同生境均以卡尔文循环为主可能是由于冰川表面是一个寡营养的氧化环境,因此高丰度的真核藻类和含叶绿素的原核细菌在冰川表面进行光合作用以维持生长。
另外两种碳固定途径在一些厌氧和富硫的特殊的冰川环境如冰下沉积物、富含硫的冰川冰尘和冰川径流中被发现。如Wright等通过宏基因组测序从加拿大Borup Fiord Pass冰川表面富含硫的温泉中发现了rTCA的关键基因acl基因,但没找到RubisCO相关基因,表明该环境中碳固定主要通过rTCA循环进行[56]。Trivedi从同一冰川表面含硫的沉积冰尘和表面融水样品中获得了31个组装的基因草图,在17个中发现rTCA循环的相关基因,表明rTCA循环在该环境中广泛分布[57]。
由于还原乙酰辅酶A途径需要严格厌氧的条件,所以往往在冰下沉积物这种厌氧环境中被发现。如Boyd等从南极Robertson Glacier冰川的冰下沉积物中发现WL途径是主要的CO2固定方式[7]。
本课题组对青藏高原3条冰川冰尘的样品进行了宏基因组测序分析,结果显示每条冰川中均检测到了CBB循环、rTCA循环和WL循环三种主要碳固定途径的关键基因,及尚未被报道的3-羟基丙酸双循环关键基因的存在(未发表数据)。考虑到青藏高原与其他地区冰川相比具有更强的辐射和较高的温度等特征,是否可能形成一些特殊固碳途径需要更多深入的研究。
2.3 冰川固碳微生物类群构成 通过对固碳微生物关键基因的系统发育分析,发现参与冰川碳固定的微生物包括原核细菌和真核藻类。细菌主要包括Actinobacteria、Cyanobacteria和Proteobacteria,而真核藻类主要包括Haptophyta、Heterokonta和Cryptophyceae (表 2)。
目前关于冰川固碳微生物的研究主要集中于北极、南极和阿尔卑斯地区,而对青藏高原冰川的研究较少。本课题组通过16S rRNA基因测序对青藏高原冰川雪、冰尘、冰中细菌群落的研究发现,青藏高原冰川微生物群落由Proteobacteria、Cyanobacteria、Bacteroidetes、Actinobacteria和Chloroflexi等为代表的优势菌组成[14, 58-61]。并且通过对南极、北极、青藏高原冰川雪、冰、冰尘的测序数据进行收集、整理和分析,发现青藏高原3种生境中Proteobacteria、Cyanobacteria和Actinobacteria的平均相对丰度分别占总群落的47%、15%和15%,3个门的总占比约为77%;而北极地区冰川Proteobacteria相对丰度约20%,Cyanobacteria相对丰度约30%,Actinobacteria相对丰度约12%;南极地区冰川Proteobacteria相对丰度约28%,Cyanobacteria相对丰度约14%,Actinobacteria相对丰度约7%。因此青藏高原冰川Proteobacteria和Actinobacteria相对丰度比南、北极地区高,而Cyanobacteria相对丰度低于北极,但3个门的总占比高于南、北极冰川(未发表数据)。
南极地区冰川生态系统固碳微生物中真核藻类较为丰富,而北极和其他山地冰川则表现为原核细菌更为丰富。如Cameron等通过对cbbLR/G form I类基因的扩增,发现北极Midtre Love’nbreen冰川微生物主要属于Actinobacteria (Mycobacterium)、Cyanobacteria (Oscillatoriales Leptolyngbya)和Cyanobacteria (Nostocsequence),而南极Signy Island冰川微生物主要属于Betaproteobacteria (Burkholderiales)。用rbcL基因扩增冰尘微生物群落中的真核微生物,发现北极和南极真核固碳微生物也不同:来自北极的Midtre Love’nbreen冰川的固碳微生物大多数(82.5%)属于Heterokonta (Botrydiopsis constricta),而南极Signy Island群落大多属于Heterokonta (Stramenopiles和Haptophyceae)[62]。Varin等对高北极冰盖Ward Hunt Ice Shelf (WHI)和Markham Ice Shelf (MIS),以及南极冰盖McMurdo Ice Shelf (MCM)上的微生物席(Mat)取样并进行宏基因组测序,发现尽管南北极的固碳微生物群落组成具有相似性,主要属于Proteobacteria和藻类,然而不同微生物种群的相对比例差异明显:南极有更丰富的蓝藻基因,而北极固碳细菌Actinobacteria和Alphaproteobacteria丰度较高[63]。
Franzetti等对阿尔卑斯地区的Forni和Baltoro冰川上rbcL基因的分类学研究发现,其主要属于Cyanobacteria和Proteobacteria,且Proteobacteria相对丰度高于Cyanobacteria[52]。Kong等对青藏高原枪勇冰川径流的T-RFLP分析发现,其中IC类型相对丰度最高,主要属于Alphaproteobacteria、Betaproteobacteria和Gammaproteobacteria,且Gammaproteobacteria的相对丰度最高。相对低丰度的ID类型序列主要属于真核微生物,包括Heterokonta (Stramenopiles)、Cryptophyta、Haptophyta (Haptophyceae)和Cyanobacteria (以Stramenopiles和Oscillatoriales为主)[55]。Boyd等从加拿大Robertson冰川表面沉积物测序结果中发现类绿色cbbL基因主要属于Betaproteobacteria (Sideroxydans lithotrophicus ES-1和Sulfuricella denitrificans skB26),而类红色自养菌主要属于Betaproteobacteria (Methylibium petroleiphilum PM1和Nitrosospira multiformis ATCC 25196)[53]。
2.4 冰川微生物固碳速率及其影响因素 净生态系统生产力(Net ecosystem productivity,NEP)是衡量自养(CO2固定成有机碳)和异养(有机碳代谢成CO2)之间平衡的指标,是初级生产力(Primary productivity)与群落呼吸(Community Respiration)的差值。当NEP大于0时,表明群落生产力高于群落呼吸,生态系统为净自养;而当NEP小于0时,生态系统为净异养。通过对冰川表面冰尘穴初级生产力和呼吸速率的汇总发现,大多数冰尘穴初级生产力高于群落呼吸,表现为净自养(表 3,表 4)。
表 3. 冰川表面冰尘穴上覆水中初级生产力(PP)、群落呼吸(R)和净生态系统生产力(NEP) Table 3. Primary productivity (PP), community respiration (R), and net ecosystem productivity (NEP) in cryoconite hole water on the surface of glaciers
| Study region | Glacier name | Latitude | Longitude | Primary productivity/ [μg C/(L·d)] | Community respiration/ [μg C/(L·d)] | Net ecosystem productivity/ [μg C/(L·d)] | Autotrophy/ Heterotrophy | References |
| Arctic | Austre Br? ggerbreen | 78°53′N | 12°04′E | 72.9±29.8 | 12.12 | Autotrophy | [72] | |
| [8] | ||||||||
| Midre Love’nbreen Glacier | 78°31′N | 12°20′E | (8.16–253.44) | [9] | ||||
| 15.12±0.03 | [9] | |||||||
| 590.40±0.75 | [9] | |||||||
| 748.80±2.10 | [9] | |||||||
| 3768±4 | [9] | |||||||
| 515.13 | 517.74 | 22.9 | Autotrophy | [72] | ||||
| 36.00±18.76 (26.4–62.4) | [73] | |||||||
| 1564±23 (1212–2572) | [71] | |||||||
| Svalbard | 78°55′N | 11°56′E | 7.4 (2.6–12.2) | 85.6 (25.1–146.0) | –78.2 (–22.5–134.0) | Autotrophy | [45] | |
| Vestre Br? ggerbreen | 79°00′N | 12°00′E | 521.97 | 636.45 | 49 | Autotrophy | [72] | |
| Froya Glacier | 74°24′N | 20°50′W | 53.5±59.7 (7.97–183) | [70] | ||||
| nr Kangerlussuaq | 66.97°N | 49.27°W | –10±35 | 14±160 | 4±10 | Autotrophy | [75] | |
| Werenskioldbreen | 77°04′N | 15°15′E | (7.92–46.08) | (0.29–5.76) | Autotrophy | [45] | ||
| 84.6±2.2 | 10.56±0.17 | Autotrophy | [45] | |||||
| 103.2 | [45] | |||||||
| Alps | Stubacher Sonnblickkees | 47°13′N | 12°60′E | 86.7±17.9 | [8] |
表选项
表 4. 冰川表面冰尘穴沉积物中初级生产力(PP)、群落呼吸(R)和净生态系统生产力(NEP) Table 4. Primary productivity (PP), community respiration (R), and net ecosystem productivity (NEP) in cryoconite hole sediment on the surface of glaciers
| Study region | Glacier name | Latitude | Longitude | Primary productivity /[μg C/(L·d)] | Community respiration /[μg C/(L·d)] | Net ecosystem productivity /[μg C/(L·d)] | Autotrophy/ Heterotrophy | References |
| Arctic | Austre Brggerbreen | 78°53′N | 12°04′E | 48.0±35.9 (11.2–125.0) | 15.3±5.0 (6.2–28.6) | Autotrophy | [8, 72] | |
| Longyearbeen | 78°10′N | 15°30′E | 17.2±9.7 (2.09–25.70) | 19.2±5.5 (11.0–25.1) | –2.0±6.4 | Heterotrophy | [78] | |
| Longyearbyen | 78°10′N | 15°30′E | 23.0 (4.2–35.8) | –0.6 (–7.1–2.8) | Heterotrophy | [78] | ||
| Midtre Love’nbreen | 78°31′N | 12°20′E | 17.3 | 0.6 | Autotrophy | [74] | ||
| 28.1±0.2 (21.8–34.6) | [73] | |||||||
| 353.0±248.0 (72.2–756.0) | 28.20±4.37 (21.9–34.6) | Autotrophy | [8] | |||||
| GrIS Ice sheet zone | 67°00′N | 50°00′W | 0.12±0.02 | Autotrophy | [71] | |||
| 1.9±0.9 | Autotrophy | [76] | ||||||
| –0.2±0.4 | Autotrophy | [71] | ||||||
| 4.1±3.0 | Autotrophy | [76] | ||||||
| 10.2±10.4 | Autotrophy | [76] | ||||||
| 2.8±1.1 | Autotrophy | [72] | ||||||
| 18.7±10.1 (4.7–28.7) | 20.9±8.2 (12.2–29.6) | Heterotrophy | Unpublished | |||||
| 22.4 (1.3–40.8) | [79] | |||||||
| 15.6 (0.1–28.7) | –1.8 (–10–1.3) | Heterotrophy | [71] | |||||
| Werenskioldbreen | 77°04′N | 15°15′E | (0.07–1.80) | [79] | ||||
| Vestre Brggerbreen | 79°00′N | 12°00′E | 208±106 (101–368) | 34.30±2.18 (32.0–37.9) | Autotrophy | [8] | ||
| Froya Glacier | 74°24′N | 20°50′W | 115.0±56.3 (35.5–205.0) | [8] | ||||
| nr Kangerlussuaq | 66°34′N | 49°27′W | 24.5 | 18.4 | 6.11 | Autotrophy | [76] | |
| Svalbard | 78°55′N | 11°56′E | 17.3 (6.5–32.2) | 20.1 (4.3–37.1) | –1.3 (–11.9–8.0) | Heterotrophy | [74] | |
| 18.7±10.3 | 18.7±9.1 | –0.12±4.10 | Heterotrophy | [72] | ||||
| Antarctica | East Antarctica ice sheet | 70°50′S | 77°04′E | 2.4 (0.7–9.3) | [78] | |||
| Antarctic Blue Ice | 79°46′S | 83°19′W | 2.2 | 1.99 | 0.23 | Autotrophy | [80] | |
| Canada Glacier | 77°37′S | 162°57′E | 1.4 | 2.2 | Heterotrophy | [77] | ||
| 0.39±0.23 | [81] | |||||||
| 0.45±0.37 | [81] | |||||||
| 0.19±0.17 | [81] | |||||||
| 0.42±0.29 | [81] | |||||||
| Vestfold Hills | 68°35′S | 77°58′E | 2.1±1.5 (0.21–4.82) | 1.86±1.51 (0.40–4.54) | Autotrophy | Unpublished | ||
| Alps | Rotmoosferner | 46°50′N | 11°03′E | 24.6±21.4 (6–67) | [70] | |||
| Stubacher Sonnblickkees | 47°13′N | 12°60′E | 147.0±78.3 (2.83–2059.00) | 42.1±7.91 (29.7–44.8) | Autotrophy | [8, 70] |
表选项
汇总结果发现冰尘穴沉积物的初级生产力高于其上覆水,呼吸速率低于其上覆水(表 3,表 4)。例如Anesio对南、北极及阿尔卑斯冰川表面冰尘穴中微生物初级生产力及呼吸速率的研究表明,冰尘穴沉积物的初级生产力范围为11.2–2059 μg C/(g·d),群落呼吸速率为6.23–44.8 μg C/(g·d),而上覆水的初级生产力范围为5.38–234 μg C/(g·d),群落呼吸速率为72.9–86.7 μg C/(g·d)[8]。冰尘穴上覆水的微生物活动速率(初级生产力和呼吸)与寡营养水生生态系统相似[64],而沉积物的微生物活动速率与温带土壤相似[65-66]。因此冰尘穴沉积物是冰川表面碳积累的主要生境。
此外,北极和阿尔卑斯地区的微生物活动速率高于南极地区,这可能与冰川表面辐射、温度、pH和营养条件有关。大部分研究表明,强太阳辐射和高温更有利于微生物的固碳作用。如Cook等利用滤光片改变进入北极冰尘穴的太阳辐射强度,结果发现强太阳辐射条件下冰尘穴微生物群落从最初的净异养状态向净自养转变的时间短于弱太阳辐射条件[6]。Stibal等通过研究北极冰尘穴中溶解性无机碳(DIC)的摄取率,也发现高温和强入射辐射更加有利于微生物活动,DIC吸收效率在18 ℃时显著高于3 ℃。同时微生物群落暴露在高辐照度下,能在较短时间内出现高DIC吸收峰[67]。这些研究表明冰尘穴中的微生物活动速率可能受到太阳辐射和温度的限制。
除了太阳辐射和温度外,pH值也是影响碳固定速率的一个重要因素。研究发现,冰尘穴中初始pH值与细胞内DIC固定速率显著相关,并呈上升趋势,DIC最高吸收速率发生在pH值为7时,而在pH值为4和10时的固碳速率都非常低[67]。其他因素,如有机质含量、营养物质(如磷和氮)的有效性、病毒裂解等也可能是影响固碳速率重要因素[67-68]。
相较于北极和阿尔卑斯地区,南极冰川具有更低的气温和更少的营养输入,并具有特殊的封闭式冰尘穴系统。南极冰尘穴由于常年低温,表面常年被冰覆盖而与大气隔离。在封闭系统中,由于pH值增加二氧化碳分压显著降低,会抑制光合作用的速率[69]。因此相较于北极和阿尔卑斯地区,南极地区冰川冰尘穴的初级生产力相对较低。然而即使在南极这种特殊环境中,大部分冰尘穴也表现出净自养状态,因此冰川表面生态系统中碳固定量可以维持相当比例的原位微生物活动。
通过细菌生产力、呼吸速率和冰尘覆盖面积,可以对冰川表面冰尘微生物碳固定量进行估计。Anesio根据大尺度的野外实验获得的冰尘穴中初级生产力和呼吸速率结果,使用冰尘穴中群落初级生产力值[7.35 μg C/(g·h)]、群落呼吸速率值[0.95 μg C/(g·h)]和冰尘的覆盖面积,估算出除南极外的冰尘微生物年净固碳量大约为64 Gg[70]。随后,Hodson等综合考虑固碳速率的时空变化,对北极格陵兰地区冰川生产力和呼吸速率进行了测量,估算出北极格陵兰冰盖每年净固碳量大约为10–100 Gg[71]。因此冰川生态系统是CO2的主要汇,微生物活动,尤其是冰尘微生物,对有机碳的积累可能加速冰川消融,因而对全球碳循环具有重要影响。
3 总结和研究展望 综上所述,近些年国内外****对冰川固碳微生物进行了宏观和微观的研究,在主要固碳途径、固碳微生物类群、固碳速率及其影响因素方面取得了进展。固碳机制的运行涉及体内能量的积累、对外界环境因子变化的响应、体内各代谢途径能量的分配,以及通过食物链和食物网向生态系统能量输送的各个反应,因此微生物可能主动选择更高效的固碳途径,从而提高固碳效率并节省体内积累的能量。固碳途径并非一成不变,可根据环境的变化作出相应调控,并在一定程度上具有很高的关联性。全球气候变化下,环境改变可能会对冰川微生物的固碳途径产生不同程度的影响。冰川有巨大的碳储量和释放量,因此对冰川固碳途径、固碳微生物组成、固碳速率的综合研究有利于我们准确预测冰川向下游生态系统输送有机碳量等关键参数。此外,对冰川微生物的研究可能对揭示微生物在极端环境条件下的生存机制具有重要科学意义。目前对冰川固碳微生物的研究还处于基础阶段,仍有一些问题有待深入的研究与探讨。
卡尔文循环、还原乙酰辅酶A途径及还原型三羧酸循环被发现是冰川表面主要的固碳途径,目前对这三条固碳途径的研究较为丰富和成熟。而其他固碳途径,如3-羟基丙酸双循环、3-羟基丙酸/4-羟基丁酸循环、二羧酸/4-羟基丁酸循环和反向甘氨酸裂解途径尚未在冰川中发现。由于这些途径发现时间较晚,酶和相关基因研究并不完善,可能导致在研究过程被其他途径掩盖。此外由于参与固碳的微生物的类群复杂,存在大量未知类群,对冰川环境下固碳微生物活性、固碳微生物功能类群、及其对环境因子的响应关系等问题尚不明确。
目前关于固碳微生物的分子生物学研究主要集中在南、北极和阿尔卑斯地区。关于青藏高原冰川固碳微生物的研究还较少。青藏高原拥有两极外最大的冰川面积(冰川面积49873 km2,冰储量约4561 km3),素有“亚洲水塔”之称。这些冰川融水维系着十几个国家10多亿人的生存[82],也向下游生态系统输送了大量的有机碳。随着全球气候变暖,青藏高原大多数冰川在近几十年间经历了从快速退缩到加速退缩的过程[22]。与两极地区的冰川相比,青藏高原的冰川具有更强的辐射、更高的温度和更快的冰川消融速率,这种特殊的环境可能生存有更活跃和多样的微生物,从而表现出不同的碳固定途径。由于冰川表面直接接收太阳辐射,主要为氧化状态,因此主要以光能自养途径为主。冰川内部和冰下沉积物环境为厌氧状态,同时由于冰川移动等冰川物理过程产生还原小分子(如氢气、硫化氢等),可能有利于厌氧和微氧固碳微生物的化能自养,可能沿冰川剖面,冰川表面卡尔文循环效率较高,而冰川和冰下沉积物表现为厌氧的还原乙酰辅酶A途径循环固碳效率高,固碳微生物由光能自养型微生物向化能自养型微生物过渡。但这些过程与机理还有待进一步地深入研究。
开展青藏高原冰川固碳微生物的研究,对于准确估算冰川有机碳积累量及预测冰川向下游生态系统输送有机碳量等关键参数具有至关重要的作用,因而在青藏高原展开冰川固碳微生物具有巨大的研究前景。研究可围绕以下几个方面展开:青藏高原特殊的地理位置和环境条件下的冰川固碳途径和固碳微生物特征,青藏高原冰川微生物净碳固定效率和碳积累量,影响青藏高原碳固定效率和固碳微生物的气候环境条件。
References
| [1] | 秦大河, 姚檀栋, 丁永建. 冰冻圈科学概论. 北京: 科学出版社, 2017. |
| [2] | Edwards A, Douglas B, Anesio AM, Rassner SM, Irvine-Fynn TDL, Sattler B, Griffith GW. A distinctive fungal community inhabiting cryoconite holes on glaciers in Svalbard. Fungal Ecology, 2013, 6(2): 168-176. |
| [3] | Hood E, Battin TJ, Fellman J, O'Neel S, Spencer RGM. Storage and release of organic carbon from glaciers and ice sheets. Nature Geoscience, 2015, 8(2): 91-96. DOI:10.1038/ngeo2331 |
| [4] | Wynn PM, Hodson AJ, Heaton THE, Chenery SR. Nitrate production beneath a High Arctic glacier, Svalbard. Chemical Geology, 2007, 244(1/2): 88-102. |
| [5] | Smith HJ, Foster RA, McKnight DM, Lisle JT, Littmann S, Kuypers MMM, Foreman CM. Microbial formation of labile organic carbon in Antarctic glacial environments. Nature Geoscience, 2017, 10(5): 356-359. DOI:10.1038/ngeo2925 |
| [6] | Cook J, Edwards A, Takeuchi N, Irvine-Fynn T. Cryoconite:the dark biological secret of the cryosphere. Progress in Physical Geography:Earth and Environment, 2016, 40(1): 66-111. DOI:10.1177/0309133315616574 |
| [7] | Boyd ES, Skidmore M, Mitchell AC, Bakermans C, Peters JW. Methanogenesis in subglacial sediments. Environmental Microbiology Reports, 2010, 2(5): 685-692. DOI:10.1111/j.1758-2229.2010.00162.x |
| [8] | Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B. High microbial activity on glaciers:importance to the global carbon cycle. Global Change Biology, 2009, 15(4): 955-960. DOI:10.1111/j.1365-2486.2008.01758.x |
| [9] | S? wstr? m C, Mumford P, Marshall W, Hodson A, Laybourn-Parry J. The microbial communities and primary productivity of cryoconite holes in an Arctic glacier (Svalbard 79°N). Polar Biology, 2002, 25(8): 591-596. DOI:10.1007/s00300-002-0388-5 |
| [10] | Liu YY, Wang S, Li SZ, Deng Y. Advances in molecular ecology on microbial functional genes of carbon cycle. Microbiology China, 2017, 44(7): 1676-1689. (in Chinese) 刘洋荧, 王尚, 厉舒祯, 邓晔. 基于功能基因的微生物碳循环分子生态学研究进展. 微生物学通报, 2017, 44(7): 1676-1689. |
| [11] | Yuan HZ, Qin HL, Liu SL, Nie SA, Wei WX, Wu JS. Advances in research of molecular ecology of carbon fixation microorganism. Scientia Agricultura Sinica, 2011, 44(14): 2951-2958. (in Chinese) 袁红朝, 秦红灵, 刘守龙, 聂三安, 魏文学, 吴金水. 固碳微生物分子生态学研究. 中国农业科学, 2011, 44(14): 2951-2958. |
| [12] | Zarsky JD, Stibal M, Hodson A, Sattler B, Schostag M, Hansen LH, Jacobsen CS, Psenner R. Large cryoconite aggregates on a Svalbard glacier support a diverse microbial community including ammonia-oxidizing archaea. Environmental Research Letters, 2013, 8(3): 035044. DOI:10.1088/1748-9326/8/3/035044 |
| [13] | Ambrosini R, Musitelli F, Navarra F, Tagliaferri I, Gandolfi I, Bestetti G, Mayer C, Minora U, Azzoni RS, Diolaiuti G, Smiraglia C, Franzetti A. Diversity and assembling processes of bacterial communities in cryoconite holes of a Karakoram glacier. Microbial Ecology, 2017, 73(4): 827-837. DOI:10.1007/s00248-016-0914-6 |
| [14] | Liu YQ, Vick-Majors TJ, Priscu JC, Yao TD, Kang SC, Liu KS, Cong ZY, Xiong JB, Li Y. Biogeography of cryoconite bacterial communities on glaciers of the Tibetan Plateau. FEMS Microbiology Ecology, 2017, 93(6): fix072. |
| [15] | Lutz S, McCutcheon J, McQuaid JB, Benning LG. The diversity of ice algal communities on the Greenland Ice Sheet as revealed by oligotyping. Microbial Genomics, 2018, 4(3): e000159. |
| [16] | Edwards A, Irvine-Fynn T, Mitchell AC, Rassner SME. A germ theory for glacial systems?. Wiley Interdisciplinary Reviews:Water, 2014, 1(4): 331-340. DOI:10.1002/wat2.1029 |
| [17] | Wadham JL, Hawkings JR, Tarasov L, Gregoire LJ, Spencer RGM, Gutjahr M, Ridgwell A, Kohfeld KE. Ice sheets matter for the global carbon cycle. Nature Communications, 2019, 10(1): 3567. DOI:10.1038/s41467-019-11394-4 |
| [18] | Hodson A, Anesio AM, Tranter M, Fountain A, Osborn M, Priscu J, Laybourn-Parry J, Sattler B. Glacial ecosystems. Ecological Monographs, 2008, 78(1): 41-67. DOI:10.1890/07-0187.1 |
| [19] | Bamber J, van den Broeke M, Ettema J, Lenaerts J, Rignot E. Recent large increases in freshwater fluxes from Greenland into the North Atlantic. Geophysical Research Letters, 2012, 39(19): L19501. |
| [20] | Bliss A, Hock R, Radi? V. Global response of glacier runoff to twenty-first century climate change. Journal of Geophysical Research:Earth Surface, 2014, 119(4): 717-730. DOI:10.1002/2013JF002931 |
| [21] | Rignot E, Bamber JL, van den Broeke MR, Davis C, Li YH, van de Berg WJ, van Meijgaard E. Recent Antarctic ice mass loss from radar interferometry and regional climate modelling. Nature Geoscience, 2008, 1(2): 106-110. DOI:10.1038/ngeo102 |
| [22] | Yao TD, Xue YK, Chen DL, Chen FH, Thompson L, Cui P, Koike T, Lau WKM, Lettenmaier D, Mosbrugger V, Zhang RG, Xu BQ, Dozier J, Gillespie T, Gu Y, Kang SC, Piao SL, Sugimoto S, Ueno K, Wang L, Wang WC, Zhang F, Sheng YW, Guo WD, Ailikun, Yang XX, Ma YM, Shen SSP, Su ZB, Chen F, Liang SL, Liu YM, Singh VP, Yang K, Yang DQ, Zhao XQ, Qian Y, Zhang Y, Li Q. Recent third pole's rapid warming accompanies cryospheric melt and water cycle intensification and interactions between monsoon and environment:multidisciplinary approach with observations, modeling, and analysis. Bulletin of the American Meteorological Society, 2019, 100(3): 423-444. DOI:10.1175/BAMS-D-17-0057.1 |
| [23] | Hood E, Fellman J, Spencer RGM, Hernes PJ, Edwards R, D'Amore D, Scott D. Glaciers as a source of ancient and labile organic matter to the marine environment. Nature, 2009, 462(7276): 1044-1047. DOI:10.1038/nature08580 |
| [24] | Lawson EC, Wadham JL, Tranter M, Stibal M, Lis GP, Butler CEH, Laybourn-Parry J, Nienow P, Chandler D, Dewsbury P. Greenland Ice Sheet exports labile organic carbon to the Arctic oceans. Biogeosciences Discussions, 2013, 10(12): 19311-19345. DOI:10.5194/bgd-10-19311-2013 |
| [25] | Striegl RG, Aiken GR, Dornblaser MM, Raymond PA, Wickland KP. A decrease in discharge-normalized DOC export by the Yukon River during summer through autumn. Geophysical Research Letters, 2005, 32(21): L21413. DOI:10.1029/2005GL024413 |
| [26] | Anesio AM, Laybourn-Parry J. Glaciers and ice sheets as a biome. Trends in Ecology & Evolution, 2012, 27(4): 219-225. |
| [27] | Fuchs G. Alternative pathways of carbon dioxide fixation:insights into the early evolution of life?. Annual Review of Microbiology, 2001, 65: 631-658. |
| [28] | Tahon G, Tytgat B, Stragier P, Willems A. Analysis of cbbL, nifH, and pufLM in soils from the S? r Rondane Mountains, Antarctica, reveals a large diversity of autotrophic and phototrophic bacteria. Microbial Ecology, 2016, 71(1): 131-149. DOI:10.1007/s00248-015-0704-6 |
| [29] | Elsaied HE, Kimura H, Naganuma T. Composition of archaeal, bacterial, and eukaryal RuBisCO genotypes in three Western Pacific arc hydrothermal vent systems. Extremophiles, 2007, 11(1): 191-202. DOI:10.1007/s00792-006-0025-2 |
| [30] | Kayani MUR, Doyle SM, Sangwan N, Wang GQ, Gilbert JA, Christner BC, Zhu TF. Metagenomic analysis of basal ice from an Alaskan glacier. Microbiome, 2018, 6(1): 123. DOI:10.1186/s40168-018-0505-5 |
| [31] | Barretto KM, Kalmbach AJ, de la Torre JR, Falcón LI, Carpenter EJ. Understanding microalgal species composition and contributions in Antarctic glacial melt water through rbcL high throughput sequencing. American Geophysical Union, 2016: MM44A-0475. |
| [32] | Berg IA. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Applied and Environmental Microbiology, 2011, 77(6): 1925-1936. DOI:10.1128/AEM.02473-10 |
| [33] | Pachiadaki MG, Sintes E, Bergauer K, Brown JM, Record NR, Swan BK, Mathyer ME, Hallam SJ, Lopez-Garcia P, Takaki Y, Nunoura T, Woyke T, Herndl GJ, Stepanauskas R. Major role of nitrite-oxidizing bacteria in dark ocean carbon fixation. Science, 2017, 358(6366): 1046-1051. DOI:10.1126/science.aan8260 |
| [34] | Alfreider A, Grimus V, Luger M, Ekblad A, Salcher MM, Summerer M. Autotrophic carbon fixation strategies used by nitrifying prokaryotes in freshwater lakes. FEMS Microbiology Ecology, 2018, 94(10): fiy163. |
| [35] | Alfreider A, Vogt C. Genetic evidence for bacterial chemolithoautotrophy based on the reductive tricarboxylic acid cycle in groundwater systems. Microbes and Environments, 2012, 27(2): 209-214. DOI:10.1264/jsme2.ME11274 |
| [36] | van Groenigen KJ, Forristal D, Jones M, Smyth N, Schwartz E, Hungate B, Dijkstra P. Using metabolic tracer techniques to assess the impact of tillage and straw management on microbial carbon use efficiency in soil. Soil Biology and Biochemistry, 2013, 66: 139-145. DOI:10.1016/j.soilbio.2013.07.002 |
| [37] | Assié A, Leisch N, Meier DV, Gruber-vodicka H, Tegetmeyer HE, Meyerdierks A, Kleiner M, Hinzke T, Joye S, Saxton M, Dubilier N, Petersen JM. Horizontal acquisition of a patchwork Calvin cycle by symbiotic and free-living Campylobacterota (formerly Epsilonproteobacteria). The ISME Journal, 2020, 14(1): 104-122. DOI:10.1038/s41396-019-0508-7 |
| [38] | Wasmund K, Schreiber L, Lloyd KG, Petersen DG, Schramm A, Stepanauskas R, J? rgensen BB, Adrian L. Genome sequencing of a single cell of the widely distributed marine subsurface Dehalococcoidia, phylum Chloroflexi. The ISME Journal, 2014, 8(2): 383-397. DOI:10.1038/ismej.2013.143 |
| [39] | Fortunato CS, Huber JA. Coupled RNA-SIP and metatranscriptomics of active chemolithoautotrophic communities at a deep-sea hydrothermal vent. The ISME Journal, 2016, 10(8): 1925-1938. DOI:10.1038/ismej.2015.258 |
| [40] | Lezcano Má, Moreno-Paz M, Carrizo D, Prieto-Ballesteros O, Fernández-Martínez Má, Sánchez-García L, Blanco Y, Puente-Sánchez F, de Diego-Castilla G, Garcia-Villadangos M, Fairén AG, Parro V. Biomarker profiling of microbial mats in the geothermal band of cerro caliente, deception island (Antarctica):life at the edge of heat and cold. Astrobiology, 2019, 19(12): 1490-1504. DOI:10.1089/ast.2018.2004 |
| [41] | Liu Z, Sun YF, Zhang YQ, Feng W, Lai ZG, Fa KY, Qin SG. Metagenomic and 13C tracing evidence for autotrophic atmospheric carbon absorption in a semiarid desert. Soil Biology and Biochemistry, 2018, 125: 156-166. DOI:10.1016/j.soilbio.2018.07.012 |
| [42] | Holo H. Chloroflexus aurantiacus secretes 3-hydroxypropionate, a possible intermediate in the assimilation of CO2 and acetate. Archives of Microbiology, 1989, 151(3): 252-256. DOI:10.1007/BF00413138 |
| [43] | Zarzycki J, Brecht V, Müller M, Fuchs G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(50): 21317-21322. DOI:10.1073/pnas.0908356106 |
| [44] | K? nneke M, Schubert DM, Brown PC, Hügler M, Standfest S, Schwander T, von Borzyskowski LS, Erb TJ, Stahl DA, Berg IA. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(22): 8239-8244. DOI:10.1073/pnas.1402028111 |
| [45] | Stibal M, Tranter M, Benning LG, ?ehák J. Microbial primary production on an Arctic glacier is insignificant in comparison with allochthonous organic carbon input. Environmental Microbiology, 2008, 10(8): 2172-2178. DOI:10.1111/j.1462-2920.2008.01620.x |
| [46] | Klatt CG, Bryant DA, Ward DM. Comparative genomics provides evidence for the 3-hydroxypropionate autotrophic pathway in filamentous anoxygenic phototrophic bacteria and in hot spring microbial mats. Environmental Microbiology, 2007, 9(8): 2067-2078. DOI:10.1111/j.1462-2920.2007.01323.x |
| [47] | Jennings RDM, Moran JJ, Jay ZJ, Beam JP, Whitmore LM, Kozubal MA, Kreuzer HW, Inskeep WP. Integration of metagenomic and stable carbon isotope evidence reveals the extent and mechanisms of carbon dioxide fixation in high-temperature microbial communities. Frontiers in Microbiology, 2017, 8: 88. |
| [48] | Offre P, Nicol GW, Prosser JI. Community profiling and quantification of putative autotrophic thaumarchaeal communities in environmental samples. Environmental Microbiology Reports, 2011, 3(2): 245-253. DOI:10.1111/j.1758-2229.2010.00217.x |
| [49] | Huber H, Gallenberger M, Jahn U, Eylert E, Berg IA, Kockelkorn D, Eisenreich W, Fuchs G. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(22): 7851-7856. DOI:10.1073/pnas.0801043105 |
| [50] | Lannes R, Olsson-Francis K, Lopez P, Bapteste E. Carbon fixation by marine ultrasmall prokaryotes. Genome Biology and Evolution, 2019, 11(4): 1166-1177. DOI:10.1093/gbe/evz050 |
| [51] | Figueroa IA, Barnum TP, Somasekhar PY, Carlstr? m CI, Engelbrektson AL, Coates JD. Metagenomics-guided analysis of microbial chemolithoautotrophic phosphite oxidation yields evidence of a seventh natural CO2 fixation pathway. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(1): E92-E101. DOI:10.1073/pnas.1715549114 |
| [52] | Franzetti A, Tagliaferri I, Gandolfi I, Bestetti G, Minora U, Mayer C, Azzoni RS, Diolaiuti G, Smiraglia C, Ambrosini R. Light-dependent microbial metabolisms drive carbon fluxes on glacier surfaces. The ISME Journal, 2016, 10(12): 2984-2988. DOI:10.1038/ismej.2016.72 |
| [53] | Boyd ES, Hamilton TL, Havig JR, Skidmore ML, Shock EL. Chemolithotrophic primary production in a subglacial ecosystem. Applied and Environmental Microbiology, 2014, 80(19): 6146-6153. DOI:10.1128/AEM.01956-14 |
| [54] | Kumar V, Thakur V, Ambika, Kumar V, Kumar R, Singh D. Genomic insights revealed physiological diversity and industrial potential for Glaciimonas sp. PCH181 isolated from Satrundi glacier in Pangi-Chamba Himalaya. Genomics, 2020, 112(1): 637-646. DOI:10.1016/j.ygeno.2019.04.016 |
| [55] | Kong WD, Liu JB, Ji MK, Yue LY, Kang SC, Morgan-Kiss RM. Autotrophic microbial community succession from glacier terminus to downstream waters on the Tibetan Plateau. FEMS Microbiology Ecology, 2019, 95(6): fiz074. DOI:10.1093/femsec/fiz074 |
| [56] | Wright KE, Williamson C, Grasby SE, Spear JR, Templeton AS. Metagenomic evidence for sulfur lithotrophy by Epsilonproteobacteria as the major energy source for primary productivity in a sub-aerial arctic glacial deposit, Borup Fiord Pass. Frontiers in Microbiology, 2013, 4: 63. |
| [57] | Trivedi CB, Stamps BW, Lau GE, Grasby SE, Templeton A, Spear JR. Metagenomic insights into microbial metabolisms of a sulfur-influenced glacial ecosystem. bioRxiv, 2020. |
| [58] | Liu YQ, Priscu JC, Yao TD, Vick-Majors TJ, Michaud AB, Sheng L. Culturable bacteria isolated from seven high-altitude ice cores on the Tibetan Plateau. Journal of Glaciology, 2019, 65(249): 29-38. DOI:10.1017/jog.2018.86 |
| [59] | Liu YQ, Priscu JC, Yao TD, Vick-Majors TJ, Xu BQ, Jiao NZ, Santibá? ez P, Huang SJ, Wang NL, Greenwood MC, Michaud AB, Kang SC, Wang JJ, Gao Q, Yang YF. Bacterial responses to environmental change on the Tibetan Plateau over the past half century. Environmental Microbiology, 2016, 18(6): 1930-1941. DOI:10.1111/1462-2920.13115 |
| [60] | Liu YQ, Yao TD, Jiao NZ, Kang SC, Xu BQ, Zeng YH, Huang SJ, Liu XB. Bacterial diversity in the snow over Tibetan Plateau Glaciers. Extremophiles, 2009, 13(3): 411-423. DOI:10.1007/s00792-009-0227-5 |
| [61] | Liu YQ, Yao TD, Jiao NZ, Kang SC, Zeng YH, Liu XJ. Snow bacteria in two glaciers at the Tibetan Plateau and their relationship with climate and environment. Frontiers of Earth Science in China, 2009, 3: 80-90. DOI:10.1007/s11707-009-0016-6 |
| [62] | Cameron KA, Hodson AJ, Osborn AM. Carbon and nitrogen biogeochemical cycling potentials of supraglacial cryoconite communities. Polar Biology, 2012, 35(9): 1375-1393. DOI:10.1007/s00300-012-1178-3 |
| [63] | Varin T, Lovejoy C, Jungblut AD, Vincent WF, Corbeil J. Metagenomic analysis of stress genes in microbial mat communities from Antarctica and the high Arctic. Applied and Environmental Microbiology, 2012, 78(2): 549-559. |
| [64] | del Giorgio PA, Cole JJ, Cimbleris A. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature, 1997, 385(6612): 148-151. DOI:10.1038/385148a0 |
| [65] | Rutigliano FA, D'Ascoli R, Virzo De Santo A. Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biology and Biochemistry, 2004, 36(11): 1719-1729. DOI:10.1016/j.soilbio.2004.04.029 |
| [66] | Fierer N, Colman BP, Schimel JP, Jackson RB. Predicting the temperature dependence of microbial respiration in soil:a continental-scale analysis. Global Biogeochemical Cycles, 2006, 20(3): GB3026. |
| [67] | Stibal M, Tranter M, Telling J, Benning LG. Speciation, phase association and potential bioavailability of phosphorus on a Svalbard glacier. Biogeochemistry, 2008, 90(1): 1-13. DOI:10.1007/s10533-008-9226-3 |
| [68] | Telling J, Anesio AM, Tranter M, Irvine-Fynn T, Hodson A, Butler C, Wadham J. Nitrogen fixation on Arctic glaciers, Svalbard. Journal of Geophysical Research-Biogeosciences, 2011, 116(G3): G03039. |
| [69] | Tranter M, Fountain AG, Fritsen CH, Lyons WB, Priscu JC, Statham PJ, Welch KA. Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrological Processes, 2004, 18(2): 379-387. DOI:10.1002/hyp.5217 |
| [70] | Anesio AM, Sattler B, Foreman C, Telling J, Hodson A, Tranter M, Psenner R. Carbon fluxes through bacterial communities on glacier surfaces. Annals of Glaciology, 2010, 51(56): 32-40. DOI:10.3189/172756411795932092 |
| [71] | Telling J, Anesio AM, Tranter M, Stibal M, Hawkings J, Irvine-Fynn T, Hodson A, Butler C, Yallop M, Wadham J. Controls on the autochthonous production and respiration of organic matter in cryoconite holes on high Arctic glaciers. Journal of Geophysical Research:Biogeosciences, 2012, 117(G1): G01017. |
| [72] | Hodson A, Anesio AM, Ng F, Watson R, Quirk J, Irvine-Fynn T, Dye A, Clark C, McCloy P, Kohler J, Sattler B. A glacier respires:quantifying the distribution and respiration CO2 flux of cryoconite across an entire Arctic supraglacial ecosystem. Journal of Geophysical Research:Biogeosciences, 2007, 112(G4): G04S36. |
| [73] | Hodson A, B? ggild C, Hanna E, Huybrechts P, Langford H, Cameron K, Houldsworth A. The cryoconite ecosystem on the Greenland ice sheet. Annals of Glaciology, 2010, 51(56): 123-129. DOI:10.3189/172756411795931985 |
| [74] | Telling J, Anesio AM, Hawkings J, Tranter M, Wadham JL, Hodson AJ, Irvine-Fynn T, Yallop ML. Measuring rates of gross photosynthesis and net community production in cryoconite holes:a comparison of field methods. Annals of Glaciology, 2010, 51(56): 153-162. DOI:10.3189/172756411795932056 |
| [75] | Chandler DM, Alcock JD, Wadham JL, Mackie SL, Telling J. Seasonal changes of ice surface characteristics and productivity in the ablation zone of the Greenland Ice Sheet. Cryosphere, 2015, 9(2): 487-504. DOI:10.5194/tc-9-487-2015 |
| [76] | Stibal M, Telling J, Cook J, Mak KM, Hodson A, Anesio AM. Environmental controls on microbial abundance and activity on the Greenland ice sheet:a multivariate analysis approach. Microbial Ecology, 2012, 63(1): 74-84. DOI:10.1007/s00248-011-9935-3 |
| [77] | Bagshaw EA, Tranter M, Wadham JL, Fountain AG, Mowlem M. High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrological Processes, 2011, 25(18): 2868-2877. DOI:10.1002/hyp.8049 |
| [78] | Hodson A, Cameron K, B? ggild C, Irvine-Fynn T, Langford H, Pearce D, Banwart S. The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier:Longyearbreen, Svalbard. Journal of Glaciology, 2010, 56(196): 349-362. DOI:10.3189/002214310791968403 |
| [79] | Stibal M, Tranter M. Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. Journal of Geophysical Research:Biogeosciences, 2007, 112(G4): G04S33. |
| [80] | Hodson A, Paterson H, Westwood K, Cameron K, Laybourn-parry J. A blue-ice ecosystem on the margins of the East Antarctic ice sheet. Journal of Glaciology, 2013, 59(214): 255-268. DOI:10.3189/2013JoG12J052 |
| [81] | Telling J, Anesio AM, Tranter M, Fountain AG, Nylen T, Hawkings J, Singh VB, Kaur P, Musilova M, Wadham JL. Spring thaw ionic pulses boost nutrient availability and microbial growth in entombed Antarctic Dry Valley cryoconite holes. Frontiers in Microbiology, 2014, 5: 694. |
| [82] | Immerzeel WW, van Beek LPH, Bierkens MFP. Climate change will affect the Asian water towers. Science, 2010, 328(5984): 1382-1385. DOI:10.1126/science.1183188 |
