范伟祥1, 曹艳丽1, 崔璐璐1, 逯晓寒1, 林海1,2

 , 孙淑红1,2,3
, 孙淑红1,2,3

1. 山东农业大学动物科技学院, 山东 泰安 271000;
2. 山东省动物生物工程与疾病防治重点实验室, 山东 泰安 271000;
3. 山东省畜禽疫病防制工程技术研究中心, 山东 泰安 271000
收稿日期:2020-03-01;修回日期:2020-05-21;网络出版日期:2020-06-08
基金项目:国家十三五重点研发计划(2016YFD0500510);山东省农业重大应用技术创新项目(SD2019XM009);山东省“双一流”计划(SYL2017YSTD11)
*通信作者:林海, E-mail:hailin@sdau.edu.cn;
孙淑红, E-mail:sunshuhong@sdau.edu.cn.
摘要:[目的] 旨在对鸡源丁酸梭菌进行分离鉴定与安全性评估。[方法] 利用厌氧培养方法对源自汶上芦花鸡与SPF鸡粪便样品进行丁酸梭菌的分离与纯化,挑选可疑菌落进行微生物质谱鉴定,进一步通过16S rRNA基因测序进行鉴定,16S rRNA测序结果与NCBI核苷酸数据库中丁酸梭菌的16S rRNA序列进行同源性分析;同时,进行所有分离株对氧氟沙星、头孢吡肟等9种药物的药敏试验,利用PCR方法进行mefA等23种耐药基因扩增,基于益生菌安全要求对样品进行alpha等4种梭菌毒素基因以及typeA等4种肉毒毒素基因的测定。[结果] 共分离鉴定了24株丁酸梭菌。24株均对氧氟沙星等7种抗生素表现为敏感,L-1、L-6、L-12仅对新霉素表现为中介,L-19仅对头孢吡肟表现为中介。全部菌株的mefA等16种耐药基因结果全部为阴性,sul2、flor、blaTEM 3种耐药基因全部呈阳性,tetC携带率为79.2%,cmlA携带率为45.8%,blaOXA携带率为37.5%,aadB携带率为12.5%,qnrA携带率为4.2%。PCR结果显示所有分离菌株的alpha、beta、epsilon、iota等4种梭菌毒素基因携带率0%。全部分离菌株均未携带typeA、typeB、typeE、typeF 4种肉毒毒素基因。[结论] 结果表明,从未饲喂抗生素和丁酸梭菌的汶上芦花鸡与SPF鸡群中获得的24株丁酸梭菌分离株达到预期的安全性要求,可作为益生添加菌的筛选参考株。
关键词:丁酸梭菌药敏试验耐药基因梭菌毒素基因肉毒毒素基因
Detection of resistance and virulence genes from 24 Clostridium butyricum strains isolated from chickens
Weixiang Fan1, Yanli Cao1, Lulu Cui1, Xiaohan Lu1, Hai Lin1,2

 , Shuhong Sun1,2,3
, Shuhong Sun1,2,3

1. College of Animal Science and Technology, Shandong Agricultural University, Tai'an 271000, Shandong Province, China;
2. Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Tai'an 271000, Shandong Province, China;
3. Shandong Provincial Engineering Technology Research Center of Animal Disease Control and Prevention, Tai'an 271000, Shandong Province, China
Received: 1 March 2020; Revised: 21 May 2020; Published online: 8 June 2020
*Corresponding author: Hai Lin, E-mail:hailin@sdau.edu.cn;
Shuhong Sun, E-mail:sunshuhong@sdau.edu.cn.
Foundation item: Supported by the Key National Research Developed Projects During the 13th Five Year Program (2016YFD0500510), by the Major Agricultural Application Technology Innovation Projects in Shandong Province (SD2019XM009) and by the "Double first class" Plan of Shandong Province (SYL2017YSTD11)
Abstract: [Objective] To isolate, identify and evaluate the safety of Clostridium butyricum from two breeding hen farms in Shandong province. [Methods] Anaerobic culture was used to isolate anaerobic bacterial strains from both Luhua chicken and SPF bird droppings originated in Shandong Province. Suspicious colonies were selected for mass spectrometry and then identified by 16S rRNA gene sequencing. The 16S rRNA sequencing results were analyzed for homology with 16S rRNA sequence of Clostridium butyricum in NCBI nucleotide data. Meanwhile, all isolates were tested for susceptibility to 9 drugs, such as ofloxacin and cefepime. PCR was used for the determination of 23 antimicrobial resistance genes such as mefA. Four clostridium toxin genes including alpha and four botulinum toxin genes including typeA were determined based on probiotic safety requirements. [Results] A total of 24 strains of Clostridium butyricum were identified, and they were sensitive to 7 antibiotics such as ofloxacin. L-1, L-6 and L-12 were moderated only for neomycin and L-19 was moderated only for cefepime. All strains of 16 drug-resistant genes were negative, and 3 drug-resistant genes of sul2, flor and blaTEM were positive, tetC carrying rate is 79.2%, cmlA carrying rate is 45.8%, blaOXA carrying rate is 37.5%, aadB carrying rate is 12.5%, qnrA carrying rate is 4.2%. PCR results showed that the alpha, beta, epsilon, iota clostridium toxin gene carrying rates of all isolated strains were 0%. The carrying rate of botulinum toxin genes typeA, typeB, typeE and typeF of all isolates was 0%. [Conclusion] The 24 isolates of Clostridium butyricum from tested chickens never fed with antibiotics and Clostridium butyricum met the expected safety requirements and could be used as a screening reference strain for probiotic added bacteria.
Keywords: Clostridium butyricumdrug sensitivity testresistance geneclostridium toxin genebotulinum toxin gene
丁酸梭菌归属于梭菌属,是一种产芽孢、周生鞭毛的革兰阳性菌,细胞直或稍弯,内生孢子卵圆,偏心或次端生,培养过程中发酵产酸产气[1]。丁酸梭菌对各种外界条件的抵抗能力强,具有耐酸、耐胆盐和耐高温等特性[2-3]。作为一种常见的人和动物肠道共生细菌,也经常在环境中发现。有文献指出部分丁酸梭菌会产梭菌毒素或肉毒毒素[4-5],且报道多与E型肉毒毒素有关,如携带typeE丁酸梭菌被认为是婴儿肉毒中毒或早产儿坏死性小肠结肠炎的原因之一[6]。丁酸梭菌是严格厌氧菌,已有研究表明丁酸梭菌出现耐药性并可能与E型肉毒毒素基因有关[7],非产毒菌株目前在亚洲被用作益生菌[8],丁酸可增强断奶仔猪肠屏障功能[9],在国内2020年起饲料中禁止添加抗生素[10]的大环境下,丁酸梭菌在畜禽生产中作为一种益生菌制剂具有良好的应用前景[11]。依据Isa等[12]对丁酸梭菌CBM588进行了7毒素基因的安全性评估,以及FAO/WHO2006年颁布的益生菌评价指南[13]中提出益生菌耐药谱和对人体安全的要求,我们对样品进行了更全面的8种毒力基因的PCR检测,CBM588在多次安全评估后于2014年获得欧盟批准作为食品原料。目前国内并未对丁酸梭菌进行毒素基因方面的安全性评估,所以仅以产量与抗逆性作为筛选标准可能会将携带毒素基因的菌株误作为益生添加,造成潜在风险。本研究对山东省汶上芦花鸡场与SPF鸡场分得的丁酸梭菌进行了耐药、梭菌毒素和肉毒毒素基因方面的安全性评价,分析两家鸡场内丁酸梭菌野株的耐药与毒素基因携带情况,为后续优良菌株的筛选打下基础,旨在保护动物健康。
1 材料和方法 1.1 试验材料
1.1.1 样品来源: 汶上芦花鸡的新鲜粪样24份,来自9月龄公母混养鸡群,采用随机采样;SPF鸡的新鲜粪样24份,来自231日龄公母混养鸡群,采用随机分样。两家种鸡饲料中均未添加丁酸梭菌及抗生素。丁酸梭菌A1株为从市场上某饲用菌粉中分离得到的菌株。
1.1.2 主要试剂和仪器: 强化梭菌培养基(RCM);DNA提取试剂盒(TIANGEN生化科技有限公司);抗生素药敏片(杭州微生物试剂有限公司);微生物质谱仪(德国BRUKER)。
1.2 菌株分离 称取每份采集的粪便样品5 g与生理盐水1:9比例加入到50 mL离心管中配成稀释液,振荡混匀,85 ℃水浴10 min后再次振荡,取稀释液1 mL与RCM培养液以1:4比例加入至5 mL离心管中,37 ℃厌氧增菌48 h,再次85 ℃水浴10 min,重复1次RCM增菌,以三线法接种于RCM固体培养基,厌氧培养24 h。
1.3 菌株鉴定
1.3.1 微生物质谱鉴定: 挑选菌落边缘不规则的疑似菌落进行微生物质谱鉴定,对经微生物质谱仪鉴定结果为丁酸梭菌的菌落进行纯化增菌。
1.3.2 16S rRNA鉴定: 提取细菌基因组DNA,16S通用引物(27F:5′-AGAGTTTGATCCTGGCT CAG-3′;1492R:5′-GGCTACCTTGTTACGACTT-3′)委托生工生物工程(上海)股份有限公司合成,对DNA进行16S rRNA的PCR扩增,PCR反应体系25 μL:MIX 12.5 μL,ddH2O 9.5 μL,上下游引物各1 μL,DNA模板1 μL。PCR反应条件为:95 ℃ 5 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 100 s,34个循环;72 ℃ 5 min。对PCR产物进行测序。16S rRNA测序结果在NCBI数据库中进行BLAST比对,记录与分离株比对结果最高的16S序列及其频次。
1.4 16S rRNA基因同源性分析 采用Megalign软件的CLUSTALW功能将16S rRNA测序结果与NCBI核苷酸数据中比对频次高的丁酸梭菌16S序列:KP944151.1 (脓,中国北京,2015)、KC195777.1 (沼气污泥,中国,2013)、MK156151.1 (海洋沉积,中国,2018)、MK764959.1 (中国,2019) HQ830243.1 (稻田,新加坡,2011)进行同源性分析。
1.5 药敏试验 选用9种对革兰阳性菌有效的抗生素药敏片:氧氟沙星、头孢吡肟、青霉素G、新霉素、呋喃妥因、氟苯尼考、红霉素、万古霉素、四环素,以无菌棉签将增菌24 h的菌液均匀涂布于RCM固体培养基上,每个20 mL培养基上等距离贴三种药敏纸片,培养基37 ℃厌氧培养24 h后量取抑菌圈直径,判定参考说明。
1.6 耐药基因测定 选用不同种类抗生素耐药基因引物(大环内酯类[14]:ermB、mefA、mrsD,多肽类[15]:vanA、vanB、vanC1,四环素类:tetC、tetD、tetE、tetG,磺胺类:sul1、sul2、sul3,酰胺醇类:cmlA、flor,β-内酰胺类:blaPSE、blaTEM、blaSHV、blaOXA,喹诺酮类:qnrS、qnrA、oqxA,氨基糖苷类:aac(3)-Ia、aadB,共24种耐药基因),进行PCR检测,以ddH2O为阴性对照。sul1、blaSHV、blaOXA参考文献[16],引物委托生工生物工程(上海)股份有限公司合成,引物序列及退火温度见表 1。
表 1. 耐药基因引物及退火温度 Table 1. Primer and annealing temperature of resistance gene
| Resistance | Gene | Sequence (5?→3?) | Fragment/bp | Annealing temp./℃ |
| Macrolides | mefA | F: AGTATCATTAATCACTAGTGC | 348 | 53 |
| R: TTCTTCTGGTACTAAAAGT | ||||
| ermB | F: GAAAAGGATCTCAACCAAATA | 639 | 53 | |
| R: AGTAACGGTACTTAAATTGTTT | ||||
| mrsD | F: GCCTTCCGGAGCTCCTACTT | 500 | 53 | |
| R: GCGTCCAATGTATCTCTAT | ||||
| Tetracyclines | tetC | F: GCTGTAGGCATAGGCTTGGTTA | 515 | 60 |
| R: CGCTCTCCCTTATGCGACTC | ||||
| tetD | F: GTTGCGGCTTCGGTAGTGG | 256 | 60 | |
| R: CTGCGCTTTCTTGTTTCTCGT | ||||
| tetE | F: CGGCGTTATTACGGGAGTTT | 821 | 60 | |
| R: CCAGCTTTGTGTAATGACCGC | ||||
| tetG | F: CTGCTGATCGTGGGTCTTGA | 761 | 60 | |
| R: GCTTGGAAGATCGCATGTGTT | ||||
| Peptides | vanA | F: GTAGGCTGCGATATTCAAAGC | 231 | 56 |
| R: CGATTCAATTGCGTAGTCCAA | ||||
| vanB | F: GTAGGCTGCGATATTCAAAGC | 330 | 56 | |
| R: GCCGACAATCAAATCATCCTC | ||||
| vanC1 | F: TGGTATTGGTATCAAGGAAACC | 447 | 56 | |
| R: AGATTGGAGCGCTGTTTTGTC | ||||
| Sulfa | sul1 | F: CTTCGATGAGAGCCGGCGG C | 238 | 58 |
| R: GCAAGGCGGAAACCCGCGCC | ||||
| sul2 | F: CGGCATCGTCAACATAAC C | 722 | 60 | |
| R: GTGTGCGGATGAAGTCAG | ||||
| sul3 | F: CATTCTAGAAAACAGTCGTAGTTCG | 990 | 50 | |
| R: CATCTGCAGCTAACCTAGGGCTTTGGA | ||||
| Amide alcohols | cmlA | F: GCTGCTACTCCCCGTTAAGTG | 351 | 60 |
| R: GCGACACCAATACCCACTAGC | ||||
| flor | F: GGTGATTTTTGGTCCGCTCTC | 779 | 60 | |
| R: CGGACACCGTGAAGACAATACC | ||||
| β-lactams | blaTEM | F: CAACATTTTCGTGTCGCCC T | 629 | 60 |
| R: TTATCCGCCTCCATCCAGTCT | ||||
| blaPSE | F: GCGTGCTTCGCAACTATGACT | 449 | 60 | |
| R: GCCATTGAAGCCTGTGTTTGA | ||||
| blaSHV | F: TTATCTCCCTGTTAGCCACC | 150 | 57 | |
| R: GATTTGCTGATTTCGCTCGG | ||||
| blaOXA | F: TCAACTTTCAAGATCGCA | 591 | 50 | |
| R: GTGTGTTTAGAATGGTGA | ||||
| Quinolones | qnrA | F: ATTTCTCACGCCAGGATTTG | 516 | 53 |
| R: GATCGGCAAAGGTTAGGTCA | ||||
| qnrS | F: ACGACATTCGTCAACTGCAA | 469 | 53 | |
| R: TAAATTGGCACCCTGTAGGC | ||||
| oqxA | F: GATCAGTCAGTGGGA TAGTTT | 670 | 55 | |
| R: TACTCGGCGTTAACTGATTA | ||||
| Aminoglycosides | Aac(3)-Ia | F: AAGTATGGGCATCATTCGCAC | 897 | 60 |
| R: CAATGACGCTTAGCACCTCTGA | ||||
| aadB | F: GGACACAACGCAGGTCACATT | 502 | 60 | |
| R: ACGCAAGACCTCAACCTTTTC |
表选项
1.7 梭菌毒素基因的测定 依据CBM588安全评估以及FAO/WHO2006年颁布的益生菌评价指南,选用4种梭菌毒素引物对分离菌株,进行PCR检测以进行安全性评估,4种梭菌毒素基因分别为:alpha毒素蛋白基因(CPA)、beta毒素蛋白基因(CPB)、epsilon毒素蛋白基因(CPE)、iota毒素蛋白基因(CPI),A1为参考,以ddH2O为阴性对照。引物委托生工生物工程(上海)股份有限公司合成,引物序列及退火温度[17]见表 2。阳性结果的PCR扩增产物委托上海生工进行测序,进一步鉴定。
表 2. 梭菌毒素引物序列及退火温度 Table 2. Primer sequence and annealing temperature of clostridium toxin
| Clostridium toxin gene | Sequence | Fragment/bp | Annealing temp./℃ |
| CPA | F: GTTGATAGCGCAGGACATGTTAAG | 402 | 55 |
| R: CATGTAGTCATCTGTTCCAGCATC | |||
| CPB | F: ACTATACAGACAGATCATTCAACC | 236 | 55 |
| R: TTAGGAGCAGTTAGAACTACAGAC | |||
| CPE | F: ACTGCAACTACTACTCATACTGTG | 541 | 55 |
| R: CTGGTGCCTTAATAGAAAGACTCC | |||
| CPI | F: GCGATGAAAAGCCTACACCACTAC | 317 | 55 |
| R: GGTATATCCTCCACGCATATAGTC |
表选项
1.8 肉毒毒素基因的测定 依据CBM588安全评估,选用4种肉毒毒素基因:A型肉毒毒素基因(typeA)、B型肉毒毒素基因(typeB)、E型肉毒毒素基因(typeE)、F型肉毒毒素基因(typeF),以PCR方法对分离株与A1进行两类毒力基因的测定,以ddH2O阴性对照。引物委托生工生物工程(上海)股份有限公司合成,引物序列及退火温度[12, 18]见表 3。
表 3. 肉毒毒素基因引物及退火温度 Table 3. Botox gene primers and annealing temperature
| Botulinum neurotoxin gene | Sequence | Fragment/bp | Annealing temp./℃ |
| typeA | F: AGCTACGGAGGCAGCTATGTT | 782 | 60 |
| R: CGTATTTGGAAAGCTGAAAAGG | |||
| typeB | F: CAGGAGAAGTGGAGCGAAAA | 205 | 60 |
| R: CTTGCGCCTTTGTTTTCTTG | |||
| typeE | F: CCAAGATTTTCATCCGCCTA | 389 | 60 |
| R: GCTATTGATCCAAAACGGTGA | |||
| typeF | F: CGGCTTCATTAGAGAACGGA | 543 | 60 |
| R: TAACTCCCCTAGCCCCGTAT |
表选项
2 结果和分析 2.1 菌株的鉴定
2.1.1 微生物质谱仪鉴定: 经微生物质谱鉴定结果显示分离到24株丁酸梭菌(Clostridiumbutyricum),部分菌株鉴定结果如表 4示。
表 4. 部分分离菌株质谱鉴定结果 Table 4. Identification results of partially isolated strains by mass spectrometry
| Analyte name | Organism (best match) | Score value | Organism(second best match) | Score value |
| L-1(++) | Clostridium butyricum | 2.171 | Clostridium butyricum | 2.150 |
| L-13(++) | Clostridium butyricum | 2.227 | Clostridium butyricum | 2.219 |
| L-14(++) | Clostridium butyricum | 2.145 | Clostridium butyricum | 2.113 |
| –: score value 0.000–1.699; +: 1.700–1.999 is; ++: 2.000–2.299; +++: 2.300–3.000. | ||||
表选项
2.1.2 16S rRNA鉴定结果: BLAST结果显示24株分离株均为丁酸梭菌,其中汶上芦花鸡场分离到21株,SPF鸡场分离到3株,统计比对结果最高的NCBIACCESSION VERSION与频次如表 5所示。
表 5. 部分丁酸梭菌菌株16S rRNA序列BLAST结果 Table 5. Results of BLAST of the 16S rRNA sequence of some strains of Clostridium butyricum
| NCBI version | Source | Country | Wen shang reed chicken farm | SPF |
| KP944151.1 | Pus | China | 7 | 1 |
| KC195777.1 | Biogas sludge | China | 6 | – |
| MK156151.1 | Marine deposit | China | 5 | 1 |
| MK764959.1 | – | China | 1 | 1 |
| HQ830243.1 | Rice field | Singapore | 2 | – |
| –: not detected. | ||||
表选项
2.2 菌株的培养特征 对分离到的24株丁酸梭菌,汶上芦花鸡源分离株分别标记为L-1–L-21,SPF鸡源分离株分别标记为S-22–S-24。菌株培养产物有臭味,菌落形态呈不规则,菌落颜色在RCM培养基呈白色到黄白色,表面光滑,部分分离株如图 1所示。
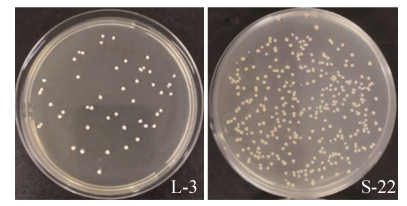 |
| 图 1 丁酸梭菌分离株菌落形态 Figure 1 Characteristics of Clostridium butyricum strain. Colony forms are irregular, colony color in RCM medium white to yellowish white, the surface is smooth. |
| 图选项 |
2.3 同源性分析 部分丁酸梭菌菌株与KP944151.1、KC195777.1、HQ30243.1、MK156151.1、MK764959.1同源性分析如图 2所示,结果表明丁酸梭菌在同源性上与地理源没有明显相关性。
 |
| 图 2 部分菌株同源性分析 Figure 2 Homology analysis of some strains. |
| 图选项 |
2.4 药敏试验结果 17株汶上芦花鸡场分离得到的丁酸梭菌与3株SPF鸡场分得的丁酸梭菌的药敏试验中对氧氟沙星、头孢吡肟、青霉素G、新霉素、呋喃妥因、氟苯尼考、红霉素、万古霉素、四环素全部表现为敏感。L-1、L-6、L-12仅对新霉素表现为中介,抑菌圈直径分别为16、15、16 mm,对其他药物均表现为敏感;L-19仅对头孢吡肟表现为中介,抑菌圈直径为16 mm,对其他药物表现为敏感。部分菌株药敏试验结果如图 3所示,统计情况如图 4所示。
 |
| 图 3 部分丁酸梭菌分离株的药敏试验图片 Figure 3 Photo of Clostridium butyricum antibiotic sensitivity test: L-4 (A) and L-6 (B). |
| 图选项 |
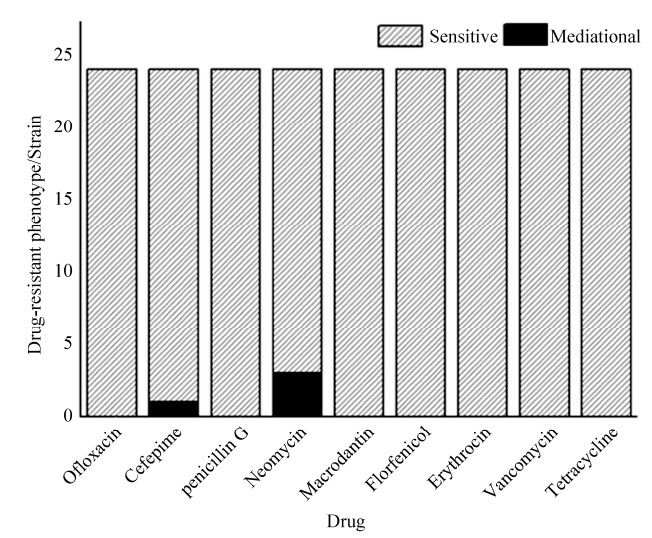 |
| 图 4 24株丁酸梭菌药敏试验结果统计 Figure 4 Statistical results of antibiotic sensitivity test for 24 strains of Clostridium butyricum. One strain of cefepime was intermediate, three strains of neomycin were intermediates, the rest are intermediary. |
| 图选项 |
2.5 耐药基因结果 PCR结果显示24株丁酸梭菌均不携带ermB、mefA、mrsD、vanA、vanB、vanC1、tetD、tetE、tetG、sul1、sul3、blaPSE、blaSHV、qnrS、oqxA、aac(3)-Ia 16种耐药基因。24株丁酸梭菌全部携带有磺胺类的sul2、酰胺醇类的flor以及β-内酰胺类的blaTEM 3种耐药基因;5种耐药基因结果显示为不同程度的携带,其中tetC有19株呈阳性,携带率为79.2%,cmlA有11株呈阳性,携带率为45.8%,blaOXA有9株携带,携带率为37.5%,aadB有3株呈阳性,携带率为12.5%,qnrA有1株呈阳性,携带率为4.2%,具体阳性株见表 6。
表 6. 不同程度携带耐药基因的菌株 Table 6. Strainscarrying resistance genes to varying degrees
| Gene | L-1 | L-2 | L-3 | L-4 | L-5 | L-6 | L-7 | L-8 | L-9 | L-10 | L-11 | L-12 | L-13 | L-14 | L-15 | L-16 | L-17 | L-18 | L-19 | L-20 | L-21 | S-22 | S-23 | S-24 | Negative |
| tetC | – | + | + | + | + | – | + | – | + | – | + | + | + | + | + | + | + | + | – | + | + | + | + | + | – |
| cmlA | – | – | + | + | + | + | + | + | – | + | + | – | – | – | – | – | + | – | – | + | – | – | + | – | – |
| blaOXA | – | – | + | – | + | + | – | – | + | – | + | + | – | – | + | + | + | – | – | – | – | – | – | – | – |
| aadB | – | – | – | + | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | + | – | – | – | – | – |
| qnrA | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – |
| +: carrying resistance genes; –: not carrying resistance genes. | |||||||||||||||||||||||||
表选项
2.6 梭菌毒素基因结果 PCR结果显示,汶上芦花鸡源与SPF鸡源的所有24株丁酸梭菌均不携带alpha、beta、epsilon、iota4种梭菌毒素基因中的任何一种,而从市场上某饲用菌粉中分离到的丁酸梭菌A1的alpha(α)毒素蛋白基因的PCR结果显示为阳性,且片段长度符合目的条带,如图 5所示。A1PCR扩增产物测序后在GenBank中进行BLAST,与α毒素蛋白基因同源性为99.43%(图略)。
 |
| 图 5 CPA基因PCR检测结果 Figure 5 PCR test results of CPA gene. The reference strain of Clostridium butyricum were positive, Clostridium butyricum isolates were negative. A1 is a strain isolated from a feeding powder on the market. |
| 图选项 |
2.7 肉毒毒素基因结果 PCR结果表明,24株分离得的丁酸梭菌不携带typeA、typeB、typeE、typeF4种肉毒毒素基因中的任何一种,typeEPCR结果见图 6。
 |
| 图 6 typeE肉毒毒素基因PCR检测结果 Figure 6 Results of PCR detection of typeE botulinum toxin gene. PCR showed that both samples and A1 were negative. |
| 图选项 |
3 讨论 本研究从汶上芦花鸡场与SPF鸡场分得的丁酸梭菌在RCM固体培养基上呈现为大小不等的白色到黄白色、边缘不规则菌落,这与目前研究[19]中丁酸梭菌的菌落特征相符。16SrRNA鉴定结果与质谱鉴定结果一致,表明质谱与16SrRNA均可以快速准确地完成丁酸梭菌鉴定。
16S rRNA测序结果表明,SPF鸡场的3株丁酸梭菌之间差异较显著,汶上芦花鸡场的21株丁酸梭菌之间的差异参差不齐,表明即使在同一鸡场中,丁酸梭菌也会表现出差异。易至等[20] 2020年发表的论文中采用比较基因组学的方法,发现宿主源和地理来源与丁酸梭菌的系统进化没有明显的相关性;本研究基于16S rRNA序列对丁酸梭菌分离株的同源性分析结果显示,宿主源和地理来源与丁酸梭菌的系统进化无明显相关性,与前者结果相符。为进行优良菌株筛选提供更多具有个性的选择。其中,L-17与KP944151.1相似性最高,为96.64%,樊晓璐等[21]研究中丁酸梭菌与CP016332.1的梭菌属菌株的相似性为100%,廖秀冬分离的丁酸梭菌相似性最高为99%[22]。
本次试验中丁酸梭菌分离株在耐药方面差异不大。3株SPF源丁酸梭菌对所有药物均表现敏感;汶上芦花鸡场中仅4株菌株存在对2种抗生素的中介。易至等[20]研究中受试菌株对万古霉素表现同样为敏感,但对四环素表现出了抗性。高文文等[19]研究中丁酸梭菌对青霉素有抗性,对红霉素中介,而此次分离得到的丁酸梭菌对青霉素G和红霉素均表现为敏感;在四环素、万古霉素结果相同为敏感。王腾浩等[23]研究中,丁酸梭菌对新霉素有抗性,对青霉素、红霉素、氧氟沙星表现为中介。本研究24株丁酸梭菌中,3株汶上芦花鸡源分离株对新霉素表现中介;对青霉素、红霉素、氧氟沙星表现敏感;在万古霉素、四环素表现敏感。Kaneko等研究结果表明分得的丁酸梭菌对选用的20种抗生素中的17种不具有耐药性[24]。而Ferraris等从早产儿粪便分得的丁酸梭菌对万古霉素与此次试验结果都表现为敏感,但其表现出青霉素G(15%)、四环素(7.5%)的耐药[25]。从药敏试验结果来看,证实汶上芦花鸡不饲用抗生素,与本研究前期调查结果一致,SPF鸡场也一定不使用抗生素。sul2、flor、blaTEM三种耐药基因在菌株中携带率为100%。董睿从鸡蛋源分离的沙门菌flor携带率为100%[26],试验结果与flor耐药基因目前只发现存在于革兰阴性菌[27]不符;近些年多种细菌的sul2耐药基因有较高携带率,李晴分离的大肠杆菌耐药基因中blaTEM携带率为100%[28]。5种不同程度携带的耐药基因tetC、cmlA、blaOXA、aadB、qnrA的来源以及是否会转移有待研究,易至等[20]就其研究中的四环素抗性结果提出丁酸梭菌可能携带有tet耐药基因,而本研究耐药基因检测结果显示tetC携带率为79.2%与其推论基本相符,但本研究中受试菌株对四环素未表现出耐药。sul2、flor、blaTEM等3种耐药基因,尤其flor是否能表达并发挥作用还需进一步验证。目前由于丁酸梭菌作为益生菌的广泛应用,本研究揭示,未来丁酸梭菌的筛选与使用上应避免作为其成为耐药基因的储存库,以减少有害菌获得新耐药基因的几率。
本研究分离鉴定的24株丁酸梭菌均未发现携带alpha、beta、epsilon、iota等4种梭菌毒素基因,但发现市场上某饲用菌粉中分离得到的菌株A1中携带alpha毒素蛋白基因。Sulthana等[29]在健康人粪便中未分离到携带梭菌毒素的丁酸梭菌,Isa等[17]在对丁酸梭菌标准株CMB588的梭菌内也未发现梭菌毒素基因。梭菌毒素基因的检测结果初步表明本研究24株丁酸梭菌比市场上的部分产品更具安全性。
已有研究表明肉毒毒素基因可通过质粒转移到梭菌中[30],目前针对丁酸梭菌携带毒素基因的研究也以肉毒毒素基因为主。肉毒毒素基因鉴定结果显示,本研究24株丁酸梭菌均未携带肉毒毒素基因,这与Ghoddusi等[31]从土壤等多处分离得的93株丁酸梭菌中筛选携带E型肉毒毒素基因的研究结果一致(在93个被检测的分离株中,均未发现typeE的存在);本研究的24株分离株的肉毒毒素基因携带率为0%,表明不会产生typeA、typeB、typeE或typeF肉毒毒素,也进一步验证了本研究24株丁酸梭菌的安全性。
4 结论 结果表明,从未饲喂抗生素和丁酸梭菌的汶上芦花鸡与SPF鸡获得的24株丁酸梭菌分离株达到预期的安全性要求,可作为益生添加菌的筛选参考株。
References
| [1] | 布坎南 RE, 吉本斯 NE. 伯杰细菌鉴定手册. 北京:科学出版社, 1984: 776-780. |
| [2] | Xie LJ, Wang L, Wang WH, Wang HK, Zhang RW. Antimicrobial activity and stress resistance of superior Clostridium butyricum LXYB-2. China Brewing, 2018, 37(6): 91-96. (in Chinese) 谢丽静, 王丽, 王伟华, 王海宽, 张仁文. 丁酸梭菌优良菌株LXYB-2抑菌活性及抗逆性研究. 中国酿造, 2018, 37(6): 91-96. |
| [3] | 贾丽楠.丁酸梭菌抗逆性能及其对肉仔鸡益生性能的体外研究.河北农业大学硕士学位论文, 2018. |
| [4] | Wang XM, Maegawa T, Karasawa T, Kozaki S, Tsukamoto K, Gyobu Y, Yamakawa K, Oguma K, Sakaguchi Y, Nakamura S. Genetic analysis of type E botulinum toxin-producing Clostridium butyricum strains. Applied and Environmental Microbiology, 2000, 66(11): 4992-4997. DOI:10.1128/AEM.66.11.4992-4997.2000 |
| [5] | Fenicia L, Franciosa G, Pourshaban M, Aureli P. Intestinal toxemia botulism in two young people, caused by Clostridium butyricum type E. Clinical Infectious Diseases, 1999, 29(6): 1381-1387. |
| [6] | Aureli P, Fenicia L, Pasolini B, Gianfranceschi M, McCroskey LM, Hatheway CL. Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. Journal of Infectious Diseases, 1986, 154(2): 207-211. |
| [7] | Camerini S, Marcocci L, Picarazzi L, Iorio E, Ruspantini I, Pietrangeli P, Crescenzi M, Franciosa G. Type E botulinum neurotoxin-producing Clostridium butyricum strains are aerotolerant during vegetative growth. mSystems, 2019, 4(2): e00299-18. DOI:10.1128/mSystems.00299-18 |
| [8] | Cassir N, Benamar S, La Scola B. Clostridium butyricum:from beneficial to a new emerging pathogen. Clinical Microbiology and Infection, 2016, 22(1): 37-45. DOI:10.1016/j.cmi.2015.10.014 |
| [9] | Li HH, Li YP, Zhu Q, Qiao JY, Wang WJ. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Journal of Applied Microbiology, 2018, 125(4): 964-975. |
| [10] | In 2020, the feed end of the total ban, feed enterprises and breeding enterprises how to deal with? Jiangxi Feed, 2018, (3): 50-51. (in Chinese) 2020 年饲料端全面禁抗, 饲料企业和养殖企业该如何应对?江西饲料, 2018, (3): 50-51. |
| [11] | He JJ, Yang XJ, Meng QX, Liu BX, Xie XX. Research progress and prospect of Clostridium butyrate in livestock production. Feed and Husbandry, 2019(11): 52-57. (in Chinese) 何家俊, 杨昕涧, 孟庆翔, 刘宝祥, 解祥学. 丁酸梭菌在畜牧生产上的研究进展及展望. 饲料与畜牧, 2019(11): 52-57. |
| [12] | Isa K, Oka K, Beauchamp N, Sato M, Wada K, Ohtani K, Nakanishi S, McCartney E, Tanaka M, Shimizu T, Kamiya S, Kruger C, Takahashi M. Safety assessment of the Clostridium butyricum MIYAIRI 588? probiotic strain including evaluation of antimicrobial sensitivity and presence of Clostridium toxin genes in vitro and teratogenicity in vivo. Human & Experimental Toxicology, 2016, 35(8): 818-832. |
| [13] | FAO/WHO. Probiotics in food: health and nutritional properties and guidelines for evaluation. FAO Food and Nutritional paper No.85, 2006. |
| [14] | Guan L, Wang DD, Zhu HD, Zhou JM, Sun K, Lv LX, Yu ZY, He KW, Li B, Ni YX. Analysis of drug resistance and resistance gene of Streptococcus suis serotype 9 isolates. Chinese Journal of Zoonoses, 2019, 35(11): 1015-1020. (in Chinese) 关琳, 王丹丹, 祝昊丹, 周俊明, 孙珂, 吕立新, 俞正玉, 何孔旺, 李彬, 倪艳秀. 猪链球菌9型分离株的耐药性及耐药基因分析. 中国人兽共患病学报, 2019, 35(11): 1015-1020. |
| [15] | Cheng C, Tan ZW, Liu YM, Zong S, Jiang F, Xie SS, Gu B. Genotype and analysis of drug resistance for vancomycin resistant enterococci. Chinese Journal of Clinical Laboratory Science, 2019, 37(7): 504-507. (in Chinese) 程晨, 谭枝微, 刘颖梅, 纵帅, 姜飞, 谢硕硕, 顾兵. 耐万古霉素肠球菌基因分型及耐药性分析. 临床检验杂志, 2019, 37(7): 504-507. |
| [16] | 杨杰.鸭源沙门菌流行特点及耐药特点分析.山东农业大学硕士学位论文, 2019. |
| [17] | Yoo HS, Lee SU, Park KY, Park YH. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. Journal of Clinical Microbiology, 1997, 35(1): 228-232. |
| [18] | Lindstr?m M, Keto R, Markkula A, Nevas M, Hielm S, Korkeala H. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Applied and Environmental Microbiology, 2001, 67(12): 5694-5699. |
| [19] | Gao WW, Shang JC, Zhou X, Fan XP, Li XR, Zhao A, Zhao PH, Zhao L, Meng XC. Isolation and identification of a strain of Clostridium butyricum with high yield of butyric acid and its biological characteristics. Science and Technology of Food Industry, 2020, 41(7): 82-88, 101. (in Chinese) 高文文, 尚佳萃, 周雪, 范小飘, 李欣芮, 赵桉, 赵鹏昊, 赵乐, 孟祥晨. 一株高产丁酸的丁酸梭菌分离鉴定及其生物学性质研究. 食品工业科技, 2020, 41(7): 82-88, 101. |
| [20] | Yi Z, Ding JQ, Wang HC, Lu WW, Zhao JX, Chen W, Zhang H. Genetic diversity and biological characteristics of Clostridium butyricum based on comparative genomics. Food and Fermentation Industries, 2020, 46(10): 1-7. (in Chinese) 易至, 丁洁琼, 王鸿超, 陆文伟, 赵建新, 陈卫, 张灏. 基于比较基因组学的丁酸梭菌遗传多样性及生物学特性. 食品与发酵工业, 2020, 46(10): 1-7. |
| [21] | Fan XL, Zhang WL, Wu YR, Zou Y, Li N. Isolation and identification of Clostridium butyricum and study of its basic characteristics. Liquor-Making Science & Technology, 2017(11): 31-37. (in Chinese) 樊晓璐, 张苇莉, 吴幼茹, 邹毅, 李楠. 丁酸梭菌的分离、鉴定与基本特性研究. 酿酒科技, 2017(11): 31-37. |
| [22] | 廖秀冬.丁酸梭菌的筛选及其对动物抗氧化能力和肉鸡肉品质影响的研究.中国农业大学博士学位论文, 2015. |
| [23] | Wang TH, Zong X, Song DG, Wang YZ. Screening, identification and in vitro functional study of Clostridium butyricum which produce antimicrobial protein. Chinese Journal of Animal Science, 2015, 51(13): 75-81. (in Chinese) 王腾浩, 宗鑫, 宋德广, 汪以真. 产抑菌蛋白的丁酸梭菌的筛选和鉴定及体外益生功能研究. 中国畜牧杂志, 2015, 51(13): 75-81. |
| [24] | Kaneko N, Nakayama T, Ichikawa N. Susceptibility of spore-forming butyric acid bacteria to antimicrobial agents. Yakugaku Zasshi, 2012, 132(7): 849-853. |
| [25] | Ferraris L, Butel MJ, Aires J. Antimicrobial susceptibility and resistance determinants of Clostridium butyricum isolates from preterm infants. International Journal of Antimicrobial Agents, 2010, 36(5): 420-423. |
| [26] | 董睿.鸡蛋源沙门菌的分离鉴定及其耐药基因和毒力基因的检测.西北农林科技大学硕士学位论文, 2012. |
| [27] | Wu FD. Florfenicol resistance and sequence analysis of floR gene in different bacteria. Chinese Agricultural Science Bulletin, 2019, 35(2): 122-126. (in Chinese) 吴方达. 不同菌属的氟苯尼考耐药特点及floR基因的序列分析. 中国农学通报, 2019, 35(2): 122-126. |
| [28] | 李晴.污水产ESBL大肠杆菌水平传递耐药性的研究.山东农业大学硕士学位论文, 2018. |
| [29] | Sulthana A, Thorramamidi A, Lakshmi SG, Madempudi RS. Whole-genome shotgun sequencing and characterization of probiotic strain Clostridium butyricum UBCB 70 to assess its safety. Microbiology Resource Announcements, 2019, 8(5): e01732-18. |
| [30] | Nawrocki EM, Bradshaw M, Johnson EA. Botulinum neurotoxin-encoding plasmids can be conjugatively transferred to diverse clostridial strains. Scientific Reports, 2018, 8(1): 3100. |
| [31] | Ghoddusi HB, Sherburn R. Preliminary study on the isolation of Clostridium butyricum strains from natural sources in the UK and screening the isolates for presence of the type E botulinal toxin gene. International Journal of Food Microbiology, 2010, 142(1/2): 202-206. |
