胡云霏, 陈华海, 尹业师


湘南优势植物资源综合利用湖南省重点实验室, 湖南科技学院化学与生物工程学院, 湖南 永州 425199
收稿日期:2018-10-09;修回日期:2018-12-18;网络出版日期:2019-03-14
基金项目:国家自然科学基金(31100097,31800119);湖南省自然科学基金(2018JJ3200)
*通信作者:尹业师, Tel:+86-746-6381164, Fax:+86-746-2382989, E-mail:yinyeshi@126.com.
摘要:大豆食品中通常富含染料木素和大豆苷元等异黄酮素,人和动物肠道中的某些细菌具有将异黄酮素代谢转化为S-雌马酚的能力。到目前为止,S-雌马酚被认为是一种具有潜在健康调节作用的化合物。啮齿类动物均具备产雌马酚的能力,但不同人群之间存在差异,产雌马酚细菌是否存在可能是造成这种差异的重要原因;不同产雌马酚细菌的代谢机制可能不同,并影响机体最终产雌马酚的能力。本文对已知的各种产雌马酚细菌及其细菌的雌马酚合成机制进行综述,以期为进一步了解雌马酚产生个体差异、雌马酚代谢转化效率、体外雌马酚的发酵生产,以及临床产雌马酚细菌的应用等提供理论参考。
关键词:雌马酚肠道细菌大豆苷元染料木素代谢机制
Research progress in equol-producing bacteria and their metabolism
Yunfei Hu, Huahai Chen, Yeshi Yin


Key Laboratory of Comprehensive Utilization of Advantage Plants Resources in Hunan South, College of Chemistry and Bioengineering, Hunan University of Science and Engineering, Yongzhou 425199, Hunan Province, China
Received: 9 October 2018; Revised: 18 December 2018; Published online: 14 March 2019
*Corresponding author: Yeshi Yin, Tel:+86-746-6381164, Fax:+86-746-2382989, E-mail:yinyeshi@126.com.
Foundation item: Supported by the National Natural Science Foundation of China (31100097, 31800119) and by the Hunan Natural Science Foundation (2018JJ3200)
Abstract: Soybean contains high content of isoflavones such as genistein and daidzein, both can be converted to equol by intestinal microflora in human and animals. Equol may play an important role in human and animal health. Rodent species consistently produce high levels of equol, however, not all humans can produce equol. Equol-producing bacteria may be the key factors for this difference. Equol-producing bacteria may also have different equol-producing mechanism and capacity. In this paper, all equol-producing bacteria and their equol synthesis mechanism are reviewed, for further understanding the factors of equol-producing difference, the transformation of equol-producing ability, equol fermentation in vitro and applying to human clinical studies.
Keywords: equolintestinal bacteriadaidzingenisteinmetabolism
雌马酚可由人和动物胃肠道中的产雌马酚细菌通过代谢大豆异黄酮类物质(主要是大豆苷元)转化而成[1-2],S-雌马酚(S-equol)和去氧甲基安哥拉紫檀素(ODMA)是大豆苷元的最终代谢产物。ODMA和R-雌马酚(雌马酚的另一种对映体形式[1])由于没有显著的生物学活性,对它们的关注较少。已有研究表明,S-雌马酚在激素相关疾病(如乳腺癌、前列腺癌、更年期综合症、衰老)、心脏病和神经系统疾病、抗氧化应激和炎症相关疾病,甚至在皮肤病、脱发、肥胖、热潮红和骨质疏松症等方面都具有潜在的调节作用[3-6],尽管它的安全性和副作用仍有待进一步评价,但S-雌马酚以及相关复合物已经被添加到少数日常饮食中,显示了较好的应用前景[7]。
啮齿类动物都具有产雌马酚的能力[8-9],但对人而言,不同个体产生雌马酚的能力具有很大的差异性。在西方人群中只有约30%的个体能够产生雌马酚[10],而对于亚洲地区或者素食主义群体,雌马酚产生率可达60%[11-12]。根据对不同产雌马酚细菌的分离及其异黄酮素代谢情况研究发现,个体能否转化生产雌马酚,以及其产雌马酚能力的强弱主要取决于肠道细菌中是否存在雌马酚代谢相关细菌,以及产雌马酚细菌的雌马酚代谢效率。本文针对近年来分离的各种雌马酚产生菌情况及其已知细菌的雌马酚合成机制进行综述,以期为进一步了解产雌马酚个体差异的原因、产雌马酚能力的强弱和加速体外雌马酚合成与应用等提供参考。
1 产雌马酚细菌发现及分类 1.1 产雌马酚细菌的发现 在对雌马酚的早期研究中发现,食用大豆类食品的无菌动物和新生儿体内无法检测到雌马酚的存在,而将大豆原料与部分人群的粪便进行共培养却可以检测到雌马酚的生成[13-14]。进一步的研究发现,对小鼠和部分人群的粪便进行厌氧培养时,这些粪便培养物能将异黄酮素或异黄酮衍生物代谢为雌马酚[15-16]。另外,抗生素干预可以影响雌马酚的生成,进一步说明雌马酚的生成可能与粪便中的细菌有关[17-18]。尽管食用大豆苷元的啮齿类动物粪便中都能检测到雌马酚的产生,但对人而言,只有部分群体的粪便样品中能检测到雌马酚[8-9, 11-12]。直到第一株能将二氢大豆苷元(DHD)代谢为S-雌马酚的革兰氏阴性杆菌(SNU Julong 732)从人的肠道粪便中被分离出来,才明确了雌马酚代谢与肠道细菌的直接关系[2]。随后,研究人员从大鼠的盲肠内容物中分离获得一株能将大豆苷元转化为雌马酚的革兰氏阳性杆菌[19]。随着研究的不断深入,越来越多的与雌马酚转化相关的细菌被分离和鉴定出来。通过对这些细菌的研究,现已基本明确了产雌马酚细菌的存在及其与雌马酚代谢的直接相关性。
1.2 非独立产雌马酚细菌 在早期产雌马酚细菌的研究中发现,一些肠道菌代谢异黄酮素的最终产物并不是雌马酚,而是雌马酚代谢通路中的一些中间产物;另一些细菌却只能利用雌马酚中间代谢产物合成雌马酚,仅参与雌马酚代谢通路的其中某些过程,这些细菌都不能独立将异黄酮素代谢为雌马酚。SNU Julong 732是最早被发现的非独立产雌马酚细菌,该菌只能利用DHD合成雌马酚,并且只能将包含有外消旋混合物的DHD代谢为S-雌马酚[2]。Maruo等[20]从人的粪便中也分离到两株只能利用DHD转化为雌马酚的革兰氏阳性球菌——FJC-A10和FJC-A161。MRG-1则是一株从人粪便中分离的只能将大豆苷元、染料木素等异黄酮素转化为R-二氢异黄酮素的革兰氏阴性杆菌[21]。此外,与MRG-1类似的还有TM-40[22]、HGH6[23]、INIA P333[24]、INIA P540[24]和Bifidobacterium MB[25]。除了人源性的细菌以外,还从牛瘤胃内容物中分离获得了Niu-O16[26],从卤臭豆腐中分离获得了SNR45DH-1和SNR48DH-1[27]。
为了进一步研究这些非独立产雌马酚细菌的功能,研究人员尝试通过混合不同菌株进行异黄酮素的发酵,测试这些细菌在雌马酚代谢过程中的作用和相关性。例如Wang等[28]将Niu-O16(只能利用大豆苷元合成DHD)与Julong 732 (只能利用DHD合成S-雌马酚)进行混合培养,在24 h内可将大豆苷元彻底转化为S-雌马酚。通过将不同的非独立产雌马酚细菌组合培养,成功实现了从大豆苷元转化为雌马酚的过程。Tamura等[29]把TM-40 (只能利用大豆苷元合成DHD)加入到含有产雌马酚细菌的粪便中,可提高粪便微生物群产雌马酚的效率。由此看出,虽然非独立产雌马酚细菌并不能独立将大豆苷元合成雌马酚,但它们作为雌马酚代谢生物群的一部分,或是促进其他产雌马酚菌合成雌马酚,或是通过协同作用参与雌马酚的代谢。另一方面,由于这些细菌只参与整个雌马酚代谢过程的某一部分,对他们各自代谢通路的研究,将有利于我们更好地了解雌马酚代谢的完整机制。
1.3 独立产雌马酚细菌 人和小鼠肠道产雌马酚细菌的研究是目前关注的重点。已知的鼠源产雌马酚菌主要有3株——do03、LH-52和Mt1B8。Minamida等[19]首次从大鼠的盲肠内容物中分离获得一株能将大豆苷元转化为雌马酚的革兰氏阳性杆菌do03。随后,Guo等从大鼠肠道中筛选分离到一株能产S-雌马酚的革兰氏阴性奇异变形杆菌LH-52,该菌具有兼性厌氧的特点,可在有氧条件下培养[30]。Mt1B8则是分离自小鼠的、能将大豆苷元转化为雌马酚和能将染料木素转化为5-羟基雌马酚的严格厌氧型革兰氏阳性菌[31]。相比于鼠源性产雌马酚菌,从人的粪便中分离的产雌马酚细菌数量相对较多。例如,YY7918是一株从健康人体粪便中分离的严格厌氧产雌马酚菌,它能将大豆苷元和DHD转化为S-雌马酚[32]。Adlercreutzia equolifaciens (FJC-B9)是Maruo等[20]从人的粪便中分离的能将异黄酮素转化为雌马酚的革兰氏阳性球菌。DZE也是从粪便中分离出的产雌马酚菌,在厌氧条件下,它既能将大豆苷元转化为S-雌马酚,也能将染料木素转化为5-羟基雌马酚。
这些能独立将相应的异黄酮素转化为雌马酚的细菌称为独立产雌马酚细菌,它们的发现确定了肠道细菌与雌马酚代谢的直接关系。此外,在DZE分离实验中,研究人员还发现一株命名为PUE的细菌,该菌能将葛根素转化为大豆苷元,并且PUE和DZE在体外共培养条件下,可将葛根素转化为S-雌马酚[33],说明多种产雌马酚相关细菌的协同作用可提高雌马酚的代谢效率,即个体产雌马酚能力大小还可能受到非独立产雌马酚细菌的影响;同时也提示个体之间的产雌马酚能力差异可能与多种细菌的参与和协同调节有关,这为进一步分析不同个体的产雌马酚差异提供了参考依据。
1.4 产雌马酚细菌的分布范围和基本特征 近年来,随着对肠道微生物资源关注度的增加,被发现的产雌马酚细菌数量也在逐渐增多,细菌的宿主来源范围从人和小鼠扩展到了猪、鸡和牛等物种,甚至从食物中也分离到了产雌马酚细菌[27],说明参与雌马酚代谢的细菌种类以及代谢途径多样。本文对各类产雌马酚细菌进行了汇总整理,从表 1可以看出雌马酚代谢相关细菌主要来源于肠道,它们中的多数都属于严格厌氧型细菌,但也有兼性厌氧产雌马酚细菌的存在,除了鼠源性LH-52外,人源性乳球菌20-92也是一株兼性厌氧菌,但是该菌在有氧条件下不能将大豆苷元转化为雌马酚[34]。由此推断,厌氧条件可能是影响雌马酚代谢转化的一个重要因素。进一步对雌马酚代谢转化条件进行优化摸索,将有利于健康膳食指导和体外雌马酚的发酵生产与应用。
表 1. 雌马酚代谢转化相关细菌 Table 1. Equol-producing bacterial strains
| Origin | Name | Classification | GenBank | Substrates | Unusable substrate | Final product | Conversion efficiency/time |
| Stinky tofu | SNR[27] | Coriobacteriaceae | AB752500 | Daidzein | Daidzin, glycitein | S-equol | 12%–90%/24 h |
| Genistein | DHG | NA | |||||
| Rat | LH-52[30] | Proteus mirabilis | JN861767 | Daidzein | NA | S-equol | NA |
| Rat | do03[19] | Eggerthella | AB266102 | Daidzein | NA | equol | 17%/96 h |
| Mouse | Mt1B8[31] | Coriobacteriaceae | AM747811 | Daidzein | NA | equol | 100%/18 h |
| Genistein | 5-OH equol | 100%/25 h | |||||
| Human | NATTS[35] | Coriobacteriaceae | AB505075 | Daidzein | NA | equol | NA |
| Human | TM-30[36] | Coriobacteriaceae | AB727353 | Daidzein | NA | S-equol | 52%/72 h |
| Human | SNU Julong 732[2] | Eggerthella | AY310748 | DHD | Daidzein | S-equol | > 80%/96 h |
| Human | Eggerthella sp. YY7918[32] | Eggerthella | AB379693 | Daidzein | Daidzin, glycitein, genistein, formononetin | S-equol | 100%/72 h |
| Human | Adlercreutzia equolifaciens[20] | Eubacterium | AB306661 | Daidzein | NA | equol | NA |
| Human | FJC-A10/FJC-A161[20] | Eubacterium | NA | DHD | NA | equol | NA |
| Human | DZE[33] | Eggerthella | EU377663 | Daidzein | NA | S-equol | 85.6%/120 h |
| Genistein | 5-OH equol | NA | |||||
| Human | PUE, DZE[33] | Eggerthella | EU377662 | Puerarin | NA | S-equol | 85%/120 h |
| Human | Slackia isoflavoniconvertens (HE8)[37] | Coriobacteriaceae | EU826403 | Daidzein | NA | equol | 61.9%/14 h |
| Genistein | 5-OH equol | ~34%/38 h | |||||
| Human | Lactococcus strain 20-92[34] | Lactococcus | AY699289 | Daidzein | NA | equol | 89.4%/1 h |
| Human | Bifidobacterium breve 15700[38] | Bifidobacterium | NA | Daidzein | NA | equol | 78.5%/96 h |
| Human | Bifidobacterium longum BB536[38] | NA | 77.2%/96 h | ||||
| Human | LJ-G1[25] | Enterobacter amnigenus | NA | Isoflavone | NA | equol | NA |
| Human | MRG-1[21] | Coprobacillus | HQ687764 | Isoflavonoids | Flavanoids | R-dihydro isoflavones | 100%/2 h |
| Human | TM-40[22] | Coprobacillus | AB249652 | Daidzin | NA | DHD | 61.1%/24 h |
| Human | HGH6[23] | NA | NA | Daidzein | Flavanoids | DHD | 9.3%/7 d |
| Genistein | DHG | 90.4%/7 d | |||||
| Human | INIA P333[24] | Enterococcus | NA | Soybean Extract | NA | DHD | NA |
| Human | INIA P540[24] | Lactobacillus | NA | Soybean Extract | Genistin, glycitin | DHD | NA |
| Human | Bifidobacterium MB[25] | Bifidobacterium | NA | Daidzin | NA | Daidzein | ~48%–65%/7 d |
| Bovine | Niu-O16[26] | NA | AY263505 | Daidzein | NA | R/S-DHD | 100%/40 h |
| Genistein | R/S-DHG | 100%/40 h | |||||
| Pig | D1/D2[39] | Eubacterium | DQ904563 DQ904564 | Daidzein | NA | Equol | 1.75%/48 h |
| Chicken | C1[40] | Clostridium | NA | Daidzein | NA | S-equol | NA |
| Chicken | AUH-JLC257[41] | Eggerthella | KM277366 | Genistein | NA | 5-OH equol | 83.1%/3 d |
| DHG: Dihydrogenistein; DHD: Dihydrodaidzein; NA: not available. | |||||||
表选项
2 产雌马酚细菌的16S rRNA基因进化树分析 很多产雌马酚细菌虽然分离自不同物种,但这些细菌的16S rRNA基因同源性很高,都属于红蝽菌科(Coriobacteriaceae)。例如鼠源性do03与人源性SNU Julong 732的同源性高达99%,均属于红蝽菌科Eggerthella细菌[2, 19];鼠源产雌马酚菌Mt1B8在基因进化树上与Eggerthella也很相近[31],这两个不同物种的肠道中存在相似的雌马酚代谢相关细菌。其他属于Eggerthella的人源性产雌马酚菌还包括YY7918和DZE,它们与Eggerthella相关细菌的16S rRNA基因同源性分别为93.3%和85%[20, 32-33](图 1)。此外,属于红蝽菌科的产雌马酚菌还有人源HE8、NATTS、TM-30、鸡源AUH-JLC257和分离自卤臭豆腐的SNR。
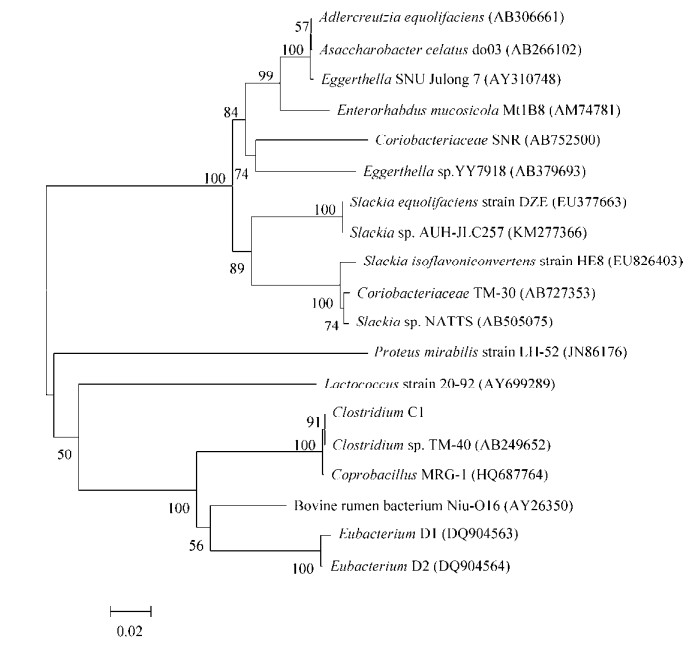 |
| 图 1 部分产雌马酚相关细菌16S rRNA基因进化树分析 Figure 1 Phylogenetic tree analysis of 16S rRNA gene of some equol-producing bacteria. The accession number is shown in parenthesis; Numbers at the branch points indicated the bootstrap values (> 50%); The scale bar corresponds to 0.02 substitutions per nucleotide position. |
| 图选项 |
其他种属的雌马酚代谢相关细菌包括乳球菌属(Lactococcus)、乳杆菌属(Lactobacillus)、优杆菌属(Eubacterium)、梭状芽孢杆菌属(Clostridium)、双歧杆菌属(Bifidobacterium)、奇异变形杆菌属(Proteusmirabilis)、棒状杆菌属(Coprobacillus)、肠球菌属(Enterococcus)和肠杆菌属(Enterobacter)等(表 1)。结合16S rRNA基因进化树和细菌种属分类可以看出,雌马酚代谢相关细菌可能包含一个庞大的生物群系,而红蝽菌科的肠道细菌可能是一类优势的产雌马酚细菌。
3 产雌马酚细菌的代谢机制 3.1 代谢过程 通过HPLC等检测方法对产雌马酚细菌代谢产物进行检测,发现不同的产雌马酚细菌合成雌马酚的过程具有相似性,即首先将大豆苷元或染料木素转化为雌马酚的中间产物——DHD或DHG,然后再进一步转化为雌马酚[42]。但不同产雌马酚细菌对异黄酮素及其衍生物代谢机制又不尽相同,各细菌可利用的底物会有差异,代谢最终产物也会存在差异。有些细菌只能利用某一种底物进行雌马酚代谢,如YY7918只能将大豆苷元和DHD转化为S-雌马酚[32]。而有些细菌则能利用多种异黄酮素,如Mt1B8、DZE和HE8可同时利用大豆苷元和染料木素,但最终代谢产物却分别为雌马酚和5-羟基雌马酚[31-33, 37]。同时,即使是对大豆苷元的代谢,不同细菌甚至同一细菌的代谢产物也存在R/S-对映异构体的差异,如MRG-1只能生成R-DHD[22]、Niu-O16却能合成S-DHD和R-DHD[26]。
对同一细菌而言,不同底物的代谢速率会存在差异。研究发现,大豆苷元随着细菌共培养的同时便开始被代谢,而染料木素的代谢则呈现明显延迟现象,甚至在培养20 h后才开始代谢。当细菌生长达到平台期后,加入对应的异黄酮素,只能检测到微量的雌马酚,而预先在细菌迟缓期就加入少量异黄酮素,待细菌进入平台期后再添加异黄酮素,能显著增加细菌产雌马酚的效率,说明细菌的异黄酮素代谢需要在异黄酮素的预先诱导下才能进行[31, 33, 37, 43]。异黄酮素的预加入一般不会影响细菌的生长,目前只发现Niu-O16的生长会受影响,但不影响该菌对异黄酮素的代谢效率[26]。
对不同细菌而言,雌马酚代谢效率也存在较大差异,并且这种差异与细菌种属、物种来源之间没有相关性。例如鼠源性Mt1B8产雌马酚效率可达100%/18 h,而另一株鼠源性do03的产雌马酚效率仅为17%/96 h;人源性YY7918的雌马酚合成效率为100%/72 h,但另一株人源性TM-30则只能达到52%/72 h。这些细菌的16S rRNA基因进化树分析也并不能解释代谢效率的差异性。非独立产雌马酚细菌的代谢效率也具有相似的特点(表 1、图 1)。在体内实验中,不同的物种间雌马酚代谢情况也存在较大差异,大豆苷、染料木苷在啮齿类动物中能高效的转化为雌马酚,而在人和猪体内的转化效率则很低[2, 31]。由此推测,体内外条件的差异,不同的肠道环境(同一物种或不同物种),或是细菌本身代谢机制、代谢条件的不同,都可能是导致个体产雌马酚差异的影响因素。目前对于这方面的信息还了解不多,进一步研究雌马酚的代谢机制,是解释这些差异的主要途径之一。
3.2 雌马酚代谢转化关键酶的作用机制 Shimada等[44]在产雌马酚乳球菌20-92的大豆苷元发酵液上清中发现了一个NADP(H)依赖性大豆苷元还原酶(L-DZNR),通过体外实验确定了L-DZNR可将大豆苷元转化为S-DHD。随后,通过克隆表达L-DZNR基因簇上下游的基因,成功筛选到能在体外实现R-DHD和S-DHD的转换并发挥外消旋酶作用的外消旋酶——L-DDRC,以及能分别将DHD转化为四氢大豆苷元(THD)、将THD转化为雌马酚的还原酶——DHDR和THDR,并发现DHDR发挥活性需要NADPH的参与,而THDR同时具备反向转化的活性,即可以将THD转化为DHD。
体外酶活性分析发现,4种重组蛋白混合酶(L-DDRC、L-DZNR、L-DHDR和L-THDR)能将大豆苷元转化为雌马酚,转化率可达89.4%,而缺少L-DDRC时,雌马酚转化率仅为15.3%。另一方面,即使没有L-DDRC的参与,也有少量的S-雌马酚生成,可能大豆苷元还能通过其他途径转化为S-雌马酚。通过此研究发现,体外大豆苷元合成S-雌马酚至少需要4种关键代谢酶,其基本代谢机制流程如图 2[34, 43]。
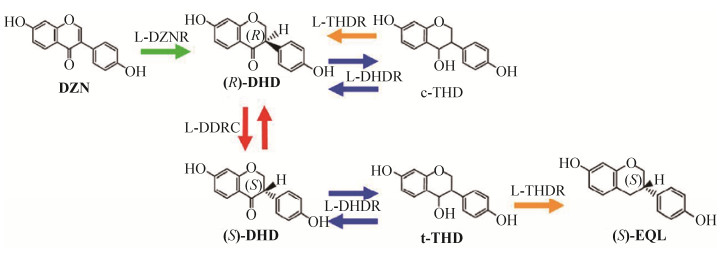 |
| 图 2 大豆苷元转化为S-雌马酚的基本代谢机制[34] Figure 2 The metabolism of daidzein to S-equol[34]. |
| 图选项 |
此后,Schroder等[45]在HE8菌中也发现了与Shimada研究相似的DZNR、DHDR和THDR雌马酚代谢酶,并发现DZNR也能将染料木素转化为DHG,且DZNR转化DHG的效率要高于转化DHD的效率,但最终未检测到5-羟基雌马酚的生成。虽然在体外酶活性实验中,只需DZNR、DHDR和THDR (同时添加NADPH)就能将大豆苷元转化为S-雌马酚,但是其代谢效率要明显低于该细菌的S-雌马酚转化效率;同时还发现大豆苷元和染料木素经DZNR转化后主要形成R-二氢异黄酮素,仅有少量的S-二氢异黄酮素生成,因此推测在HE8雌马酚代谢过程中也可能存在将R-DHD转化为S-DHD的消旋酶。Kawada等[46]在YY7918中找到DZNR、DHDR和THDR基因与已知20-92的相关基因相似性高达99%。将这三种酶与大豆苷元混合发酵后,可检测到THD的生成,但未检测到雌马酚,整个体外发酵过程效率很低,推测YY7918的雌马酚代谢过程中可能还有其他未知的关键酶参与。
HE8、NATTS、YY7918和20-92的DHDR和THDR氨基酸序列相似性很高,但DZNR氨基酸序列相似性较低(约40%)。DZNR的差异,可能是影响S-雌马酚代谢效率的一个重要因素。本实验室分离的产雌马酚C1菌[40],其DZNR基因与20-92的DZNR基因相似性仅为47%,具有DHD还原酶活性,但在C1菌中并没有找到与已报道的其他3个雌马酚代谢关键酶相似的基因,推测C1菌具有不同的雌马酚代谢机制。目前对雌马酚代谢机制的研究较少,虽然已经了解了部分肠道细菌产雌马酚的基本代谢途径,但不同细菌之间的雌马酚代谢机制仍可能存在差异,甚至可能有不同的代谢通路,仍有待进一步研究。
4 结论 多种动物体内,甚至豆腐中都存在着产雌马酚细菌,包括非独立产雌马酚菌和独立产雌马酚菌,它们的存在与个体是否能产雌马酚具有直接相关性。不同细菌的雌马酚代谢效率存在差异,代谢机制也可能不同,而且这种差异与细菌种属并没有直接相关性。此外,雌马酚的代谢可能还与多种细菌的协同作用有关。这些因素如何影响雌马酚转化生产效率仍有待进一步研究。目前对雌马酚的代谢机制研究有限,仅掌握了少数细菌的雌马酚代谢相关关键酶信息,进一步研究雌马酚的代谢机制,将有利于我们掌握肠道菌群在雌马酚代谢中所发挥的重要作用。另外,对饮食能否调节个体产雌马酚的能力、通过补充产雌马酚细菌能否实现非雌马酚产生者的转变、个体产雌马酚的能力是否会随着时间的推移发生变化等研究结论仍不清楚[47-52],这些都是值得关注和研究的方向。
References
| [1] | Setchell KDR, Clerici C. Equol: history, chemistry, and formation. The Journal of Nutrition, 2010, 140(7): 1355S-1362S. DOI:10.3945/jn.109.119776 |
| [2] | Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Applied and Environmental Microbiology, 2005, 71(1): 214-219. DOI:10.1128/AEM.71.1.214-219.2005 |
| [3] | Choi EJ. Evaluation of equol function on anti- or prooxidant status in vivo. Journal of Food Science, 2010, 74(2): H65-H71. |
| [4] | Xiao YQ, Zhang S, Tong HB, Shi SR. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytotherapy Research, 2018, 32(3): 384-394. DOI:10.1002/ptr.5966 |
| [5] | Yee S, Burdock GA, Kurata Y, Enomoto Y, Narumi K, Hamada S, Itoh T, Shimomura Y, Ueno T. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food and Chemical Toxicology, 2008, 46(8): 2713-2720. DOI:10.1016/j.fct.2008.04.026 |
| [6] | Setchell K. The history and basic science development of soy isoflavones. Menopause, 2017, 24(12): 1338-1350. DOI:10.1097/GME.0000000000001018 |
| [7] | Wang JY, Li L, Yin YS, Gu ZK, Chai RY, Wang YL, Sun GH. Equol, a clinically important metabolite, inhibits the development and pathogenicity of Magnaporthe oryzae, the causal agent of rice blast disease. Molecules, 2017, 22(10): 1799. DOI:10.3390/molecules22101799 |
| [8] | Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. The Journal of Nutrition, 2002, 132(12): 3577-3584. DOI:10.1093/jn/132.12.3577 |
| [9] | Gu LW, House SE, Prior RL, Fang NB, Ronis MJJ, Clarkson TB, Wilson ME, Badger TM. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. The Journal of Nutrition, 2006, 136(5): 1215-1221. DOI:10.1093/jn/136.5.1215 |
| [10] | Rowland IR, Wiseman H, Sanders TAB, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutrition and Cancer, 2000, 36(1): 27-32. |
| [11] | Setchell KDR, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. The Journal of Nutrition, 2006, 136(8): 2188-2193. DOI:10.1093/jn/136.8.2188 |
| [12] | Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, Fujioka T, Mori M, Kim WJ, Song JM, Pantuck AJ. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Japanese Journal of Clinical Oncology, 2004, 34(2): 86-89. DOI:10.1093/jjco/hyh015 |
| [13] | Brown NM, Galandi SL, Summer SS, Zhao XH, Heubi JE, King EC, Setchell KDR. S-(-)equol production is developmentally regulated and related to early diet composition. Nutrition Research, 2014, 34(5): 401-409. DOI:10.1016/j.nutres.2014.03.005 |
| [14] | Setchell KDR, Brown NM, Summer S, King EC, Heubi JE, Cole S, Guy T, Hokin B. Dietary factors influence production of the soy isoflavone metabolite S-(-)equol in healthy adults. The Journal of Nutrition, 2013, 143(12): 1950-1958. DOI:10.3945/jn.113.179564 |
| [15] | Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Archives of Microbiology, 2005, 183(1): 45-55. DOI:10.1007/s00203-004-0747-4 |
| [16] | Atkinson C, Berman S, Humbert O, Lampe JW. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. The Journal of Nutrition, 2004, 134(3): 596-599. DOI:10.1093/jn/134.3.596 |
| [17] | Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora-implications for health. Molecular Nutrition and Food Research, 2007, 51(7): 765-781. DOI:10.1002/mnfr.200600262 |
| [18] | Halm BM, Franke AA, Ashburn LA, Hebshi SM, Wilkens LR. Oral antibiotics decrease urinary isoflavonoid excretion in children after soy consumption. Nutrition and Cancer, 2008, 60(1): 14-22. |
| [19] | Minamida K, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. Journal of Bioscience and Bioengineering, 2006, 102(3): 247-250. DOI:10.1263/jbb.102.247 |
| [20] | Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. International Journal of Systematic and Evolutionary Microbiology, 2008, 58(5): 1221-1227. DOI:10.1099/ijs.0.65404-0 |
| [21] | Park HY, Kim M, Han J. Stereospecific microbial production of isoflavanones from isoflavones and isoflavone glucosides. Applied Microbiology and Biotechnology, 2011, 91(4): 1173-1181. DOI:10.1007/s00253-011-3310-7 |
| [22] | Tamura M, Tsushida T, Shinohara K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe, 2007, 13(1): 32-35. DOI:10.1016/j.anaerobe.2006.10.001 |
| [23] | Hur HG, Lay Jr JO, Beger RD, Freeman JP, Rafii F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Archives of Microbiology, 2000, 174(6): 422-428. DOI:10.1007/s002030000222 |
| [24] | Gaya P, Peirotén , Medina M, Landete JM. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. International Journal of Food Sciences and Nutrition, 2016, 67(2): 117-124. DOI:10.3109/09637486.2016.1144724 |
| [25] | Raimondi S, Roncaglia L, De Lucia M, Amaretti A, Leonardi A, Pagnoni UM, Rossi M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Applied Microbiology and Biotechnology, 2009, 81(5): 943-950. DOI:10.1007/s00253-008-1719-4 |
| [26] | Wang XL, Shin KH, Hur HG, Kim SI. Enhanced biosynthesis of dihydrodaidzein and dihydrogenistein by a newly isolated bovine rumen anaerobic bacterium. Journal of Biotechnology, 2005, 115(3): 261-269. DOI:10.1016/j.jbiotec.2004.08.014 |
| [27] | Abiru Y, Ueno T, Uchiyama S. Isolation and characterization of novel S-equol-producing bacteria from brines of stinky tofu, a traditional fermented soy food in Taiwan. International Journal of Food Sciences and Nutrition, 2013, 64(8): 936-943. DOI:10.3109/09637486.2013.816936 |
| [28] | Wang XL, Kim HJ, Kang SI, Kim SI, Hur HG. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Archives of Microbiology, 2007, 187(2): 155-160. DOI:10.1007/s00203-006-0183-8 |
| [29] | Tamura M, Hori S, Nakagawa H. Dihydrodaidzein-producing Clostridium-like intestinal bacterium, strain TM-40, affects in vitro metabolism of daidzein by fecal microbiota of human male equol producer and non-producers. Bioscience and Microflora, 2011, 30(3): 65-71. DOI:10.12938/bifidus.30.65 |
| [30] | Matthies A, Clavel T, Gutschow M, Engst W, Haller D, Blaut M, Braune A. Conversion of daidzein and genistein by an anaerobic bacterium newly isolated from the mouse intestine. Applied and Environmental Microbiology, 2008, 74(15): 4847-4852. DOI:10.1128/AEM.00555-08 |
| [31] | Guo YY, Huang YY, Ye J, Zhang XQ, Xiao MT. Screening and identification of a bacterium capable of converting daidzein to S-equol. Acta Microbiologica Sinica, 2012, 52(6): 696-702. (in Chinese) 郭远洋, 黄雅燕, 叶静, 张学勤, 肖美添. 一株转化大豆苷元为S-雌马酚菌株的筛选和鉴定. 微生物学报, 2012, 52(6): 696-702. |
| [32] | Yokoyama SI, Suzuki T. Isolation and characterization of a novel equol-producing bacterium from human feces. Bioscience, Biotechnology, and Biochemistry, 2008, 72(10): 2660-2666. DOI:10.1271/bbb.80329 |
| [33] | Jin JS, Nishihata T, Kakiuchi N, Hattori M. Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biological & Pharmaceutical Bulletin, 2008, 31(8): 1621-1625. |
| [34] | Shimada Y, Takahashi M, Miyazawa N, Abiru Y, Uchiyama S, Hishigaki H. Identification of a novel dihydrodaidzein racemase essential for biosynthesis of equol from daidzein in Lactococcus sp. strain 20-92. Applied and Environmental Microbiology, 2012, 78(14): 4902-4907. DOI:10.1128/AEM.00410-12 |
| [35] | Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Archives of Microbiology, 2010, 192(4): 279-287. DOI:10.1007/s00203-010-0546-z |
| [36] | Tamura M, Hori S, Nakagawa H. Intestinal bacterium TM-30: an S-equol-producing bacterium isolated from human feces is involved in estrogen metabolism in vitro. Food Science and Technology International, 2014, 20(2): 309-316. |
| [37] | Matthies A, Blaut M, Braune A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Applied and Environmental Microbiology, 2009, 75(6): 1740-1744. DOI:10.1128/AEM.01795-08 |
| [38] | Elghali S, Mustafa S, Amid M, Manap MYA, Ismail A, Abas F. Bioconversion of daidzein to equol by Bifidobacterium breve 15700 and Bifidobacterium longum BB536. Journal of Functional Foods, 2012, 4(4): 736-745. DOI:10.1016/j.jff.2012.04.013 |
| [39] | Yu ZT, Yao W, Zhu WY. Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiology Letters, 2008, 282(1): 73-80. DOI:10.1111/j.1574-6968.2008.01108.x |
| [40] | Yin YS, Fang D, Zhu LY, Liu W, Wu J, Wang X. Protective effect of an equol-producing Clostridium C1 against Salmonella infection in chicken. Acta Agriculturae Zhejiangensis, 2016, 28(2): 234-239. (in Chinese) 尹业师, 方丹, 朱立颖, 刘伟, 吴健, 王欣. 产雌马酚梭菌C1对小鸡沙门氏菌攻毒保护效果. 浙江农业学报, 2016, 28(2): 234-239. DOI:10.3969/j.issn.1004-1524.2016.02.09 |
| [41] | Xie YJ, Liu ZG, Gao YN, Wang XL, Hao QH, Yu XM. Bioconversion of genistein to (-)-5-hydroxy-equol by a newly isolated cock intestinal anaerobic bacterium. Journal of Chinese Pharmaceutical Sciences, 2015, 24(7): 442-448. |
| [42] | Murota K, Nakamura Y, Uehara M. Flavonoid metabolism: the interaction of metabolites and gut microbiota. Bioscience, Biotechnology, and Biochemistry, 2018, 82(4): 600-610. DOI:10.1080/09168451.2018.1444467 |
| [43] | Shimada Y, Takahashi M, Miyazawa N, Ohtani T, Abiru Y, Uchiyama S, Hishigaki H. Identification of two novel reductases involved in equol biosynthesis in Lactococcus strain 20-92. Journal of Molecular Microbiology and Biotechnology, 2011, 21(3/4): 160-172. |
| [44] | Shimada Y, Yasuda S, Takahashi M, Hayashi T, Miyazawa N, Sato I, Abiru Y, Uchiyama S, Hishigaki H. Cloning and expression of a novel NADP(H)-dependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus strain 20-92. Applied and Environmental Microbiology, 2010, 76(17): 5892-5901. DOI:10.1128/AEM.01101-10 |
| [45] | Schr?der C, Matthies A, Engst W, Blaut M, Braune A. Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Applied and Environmental Microbiology, 2013, 79(11): 3494-3502. DOI:10.1128/AEM.03693-12 |
| [46] | Kawada Y, Yokoyama S, Yanase E, Niwa T, Suzuki T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Bioscience of Microbiota, Food and Health, 2016, 35(3): 113-121. DOI:10.12938/bmfh.2015-023 |
| [47] | Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Experimental Biology and Medicine (Maywood), 2005, 230(3): 155-170. DOI:10.1177/153537020523000302 |
| [48] | Franke AA, Halm BM, Ashburn LA. Urinary isoflavones are increased in adults, but decreased in children, consuming soy when on oral antibiotic therapy. Nutrition and Cancer, 2008, 60(5): 627-635. DOI:10.1080/01635580802065310 |
| [49] | Lampe JW. Is equol the key to the efficacy of soy foods?. The American Journal of Clinical Nutrition, 2009, 89(5): 1664S-1667S. DOI:10.3945/ajcn.2009.26736T |
| [50] | Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Short-term soy and probiotic supplementation does not markedly affect concentrations of reproductive hormones in postmenopausal women with and without histories of breast cancer. The Journal of Alternative and Complementary Medicine, 2005, 11(6): 1067-1074. DOI:10.1089/acm.2005.11.1067 |
| [51] | Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Probiotic consumption does not enhance the cholesterol-lowering effect of soy in postmenopausal women. The Journal of Nutrition, 2004, 134(12): 3277-3283. DOI:10.1093/jn/134.12.3277 |
| [52] | Bonorden MJL, Greany KA, Wangen KE, Phipps WR, Feirtag J, Adlercreutz H, Kurzer MS. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. European Journal of Clinical Nutrition, 2004, 58(12): 1635-1642. DOI:10.1038/sj.ejcn.1602020 |
