王明元1

 , 侯式贞1, 董涛2, 张敏瑜1, 刘建福1, 徐志周1
, 侯式贞1, 董涛2, 张敏瑜1, 刘建福1, 徐志周1 1. 华侨大学园艺系, 福建 厦门 361021;
2. 广东省农业科学院果树研究所, 广东 广州 510640
收稿日期:2018-09-29;修回日期:2018-12-21;网络出版日期:2019-03-12
基金项目:农业部“948”重点项目子课题(2011-G16);华侨大学中青年教师科研提升资助计划(ZQN-YX507)
*通信作者:王明元, Tel/Fax:+86-592-6162300, E-mail:w_mingyuan@163.com.
摘要:[目的] 将香蕉假茎生物炭施加到土壤,探讨香蕉假茎生物炭对香蕉根际土壤微生物的影响。[方法] 以香蕉假茎生物炭(BPB)0、1%、2%、3%的质量比与土壤均匀混合。盆栽培养3个月后分离香蕉根际土壤。采用16S rRNA高通量测序技术对根际土壤细菌群落结构和丰度进行表征。[结果] 提高BPB施用量可增加土壤有机质、有效钾、有效磷含量,提高土壤pH值,但降低有效氮浓度。在1% BPB样品中获得2278个OTUs,其显示细菌群落中的最大多样性。施加3%的BPB处理土壤,拟杆菌门、疣微菌门和厚壁菌门的相对丰度显著增加;放线菌门、酸杆菌门、芽单胞菌门明显减少。主成分分析发现,1% BPB和2% BPB处理的样本之间土壤细菌群落相似。[结论] 施加不同比例BPB改变了根际土壤中细菌丰度和群落结构,且高比例添加改变更加明显。
关键词:细菌群落香蕉假茎生物炭不同比例高通量测序
Effects of banana pseudostem biochar on bacterial abundance and community structure in rhizosphere soil
Mingyuan Wang1

 , Shizhen Hou1, Tao Dong2, Minyu Zhang1, Jianfu Liu1, Zhizhou Xu1
, Shizhen Hou1, Tao Dong2, Minyu Zhang1, Jianfu Liu1, Zhizhou Xu1 1. Horticultural Department, Huaqiao University, Xiamen 361021, Fujian Province, China;
2. Institute of Fruit Tree Research, Guangdong Academy of Agricultural Sciences, Guangzhou 510640, Guangdong Province, China
*Corresponding author: Mingyuan Wang, Tel/Fax:+86-592-6162300, E-mail:w_mingyuan@163.com.
Foundation item: Supported by the Recommend International Advanced Agricultural Science and Technology Plan of the "948" Project of Ministry of Agriculture of China (2011-G16) and by the Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (ZQN-YX507)
Abstract: [Objective] To study the effect of banana pseudostem biochar on soil microorganisms in banana rhizosphere, we added the biochar into soil. [Methods] We mixed banana pseudostem biochar (BPB) with soil uniformly at the mass ratio of 0, 1%, 2% and 3%. Banana rhizosphere soil was separated after 3 months of pot culture. The bacterial community structure and abundance in rhizosphere soil were characterized by 16S rRNA high throughput sequencing. [Results] Increasing BPB application could increase soil organic matter, available potassium, available phosphorus contents and increase soil pH value, but decrease available nitrogen concentration. In total 2278 OTUs were obtained from 1% BPB samples, showing the greatest diversity of bacterial communities. When 3% BPB was applied to the soil, the relative abundance of Bacteroidates, Verrucomicrobia and Firmicutes increased significantly, whereas that of Actinobacteria, Acidobacteria and Gemmatimonade decreased significantly. Principal component analysis showed that soil bacterial communities were similar between 1% BPB and 2% BPB treated samples. [Conclusion] We changed the bacterial abundance and community structure in rhizosphere soil by applying different proportion of BPB, and the change in high proportion of BPB was more obvious.
Keywords: bacterial communitybanana pseudostem biochardifferent proportionshigh throughput sequencing
生物炭是在无氧条件下,生物质(如作物秸秆,有机废物,生物能源作物)通过缓慢高温(350–500 ℃)热解的一类难溶的、稳定的、高度芳香化的、富含碳的固体物质[1]。生物炭具有高度多孔结构,较大的比表面积和离子交换能力,可以直接提高土壤养分含量,提升土壤养分的生物利用率。生物炭已被用于改良不同非生物胁迫下的土壤质量[2]。
生物炭的添加对土壤微生物的生长、繁殖起到促进作用。生物炭为微生物提供水分、生存空间和养分、促进微生物的生长。其中生物炭的热解衍生芳香化大分子结构可以促进土壤微生物的生长、生物量的增加和活性增强[3]。研究表明,生物炭的应用增加细菌多样性,降低真菌数量,显著改变细菌和真菌群落结构[4]。Kelly等报道添10%的柳枝稷生物炭,细菌为主要的优势群落[5]。Ippolito等也发现在2%和10%水平的硬木生物炭中细菌为主要群落构成[6]。Steinbeiss等[7]实验表明源于葡萄型的生物炭施加会促进革兰氏阴性菌生长,酵母型的生物炭能促进土壤中真菌的生长。吕伟波[8]测定分析土壤中微生物量,发现生物炭能够提高微生物量,且两者成正比关系。Muhammad等[9]实验得到不同的生物炭及施加量均能明显提升细菌、放线菌、革兰氏阴性菌、硫酸还原菌的磷酸脂肪酸含量。添加生物炭对土壤养分和微生物群落的影响存在差异。Anderson等表明,在田间试验中添加生物炭的土壤和未添加生物炭的土壤,细菌群落结构没有明显的差异[10]。Imparato等报道,添加小麦秸秆气化生物炭对丹麦温带农业生态系统土壤微生物群落的功能和结构多样性影响较小[11]。在3 kg/m2水平上没有发现松树生物炭对细菌群落结构的影响[10]。原因可能是由于土壤性质的变化,真菌和细菌之间的营养竞争,或者是来自生物炭的潜在有毒化合物影响等。因此,不同比例生物炭的添加对于细菌群落的生物量、多样性和分布需要更深入的研究。Kolton等[12]通过研究细菌群落的群落变化,得到施加生物炭后,不同的优势属对于生物炭的施加反应不同,但这些变化都有助于植物生长和病害防治。
为进一步探讨生物炭对土壤微生物群落的影响,本试验盆栽香蕉小苗,同时添加不同比例香蕉假茎生物炭(banana pesudostem biochar,BPB),监测土壤养分和群落结构的变化,并通过Illumina MiSeq/HiSeq测序平台分析微生物群落的丰度,为香蕉假茎生物炭的应用提供理论依据。
1 材料和方法 1.1 试验材料 从香蕉园的耕作层收集土壤。土壤的基本性质如下:有机质5.25 g/kg,pH 6.70,阳离子交换容量(CEC) 8.13 cmol/kg,有效氮153.76 mg/kg,速效钾105.28 mg/kg,有效磷3.67 mg/kg。
香蕉假茎生物炭(BPB)是香蕉假茎在无氧高温400 ℃下热解而制成的。生物炭和试验用香蕉苗均由广东省农业科学院果树研究所提供。
土壤与不同比例的BPB (0、1%、2%、3%,W/W)均匀混合,上盆,每盆总重5 kg。每盆定殖一株香蕉幼苗,每处理重复5次,共20盆。置于华侨大学园艺系温室,幼苗在18–20 ℃/26–28 ℃夜间/白天培养。3个月后,收集根际土壤,首先用剪刀切断地上植物,然后通过手摇从土壤中去除根系表面土壤,将紧密附着在根上的土壤作为根际土壤样品收集,每组6次重复。将相同处理中的土壤样品保存在灭菌的密封袋中,然后在–80 ℃下储存,用于细菌群落检测和土壤DNA提取测定。
测定土壤有机质、有效氮、速效钾和有效磷[13]。用BaCl2-H2SO4强制交换方法后测量阳离子交换容量[14]。采用HI8424NEW pH仪测量土壤pH。
1.2 DNA提取、扩增和测序 使用土壤基因组DNA试剂盒(天根生化科技(北京)有限公司),通过CTAB/SDS方法提取每个样品的总基因组DNA。用土壤微生物DNA纯化试剂盒纯化提取基因组DNA。提取的DNA用1%琼脂糖凝胶电泳进行检测。通过16S rRNA基因的实时定量荧光PCR进行比较,检测所有样品的细菌总量。
用引物515F (5′-GTGCCAGCMGCCGCGG-3′)和806R (5′-GGACTACHVGGGTWTCTA AT-3′)扩增16S rRNA基因。每个样品3个重复,在50 μL体积中,包含每种引物0.4 μL (10 μmol/L),5倍稀释的模板DNA 4 μL,20 μL PremixEx Taq (TaKaRa Biotechnology)和25.2 μL无菌水。扩增条件:94 ℃ 3 min,6个循环;94 ℃ 45 s,65 ℃至58 ℃ 60 s,72 ℃ 70 s;94 ℃ 45 s,58 ℃ 60 s,72 ℃ 60 s,22个循环;72 ℃延伸10 min。使用Wizard SV凝胶和PCR纯化系统(Promega,San Luis Obispo,CA,USA)纯化PCR产物。然后使用QIAquick PCR纯化试剂盒(QIAGEN,Hilden,Germany)纯化PCR产物,并使用Qubit?2.0荧光计(Invitrogen,USA)定量DNA。然后使用TruSeq DNA无DNA样品制备试剂盒(Illumina,USA)构建文库。通过Agilent 2100 Bioanalyzer Instruments (Agilent Technologies Co. Ltd,USA)测定文库的最终质量和浓度,并使用KAPA Library Quantification Kits (Kapa Biosystems,USA)测定。用于测序的文库的所有制备均在中国北京诺禾致源生物信息科技有限公司的Illumina MiSeq平台上进行。
1.3 序列分析 序列分析采用Caporaso等方法[15]。除去低质量序列和错配引物序列并留下清洁标签[16]。对修剪的序列进行聚类,使用EzTaxon-e数据库将每个聚类中的代表性序列鉴定为分类位置[17]。通过随机采样对土壤样品中的所有读数进行归一化,并使用MOTHUR软件计算多样性指数[18]。根据先前的描述[19]去除嵌合序列。操作分类单位(operational taxonomic units,OTU)是基于软件Uparse的97%相似性确定的[15]。不同的16S rRNA序列的相似性高于97%就可以把它定义为1个OTU。OTU使用RDA分类器(V2.2)[20]和GreenGene数据[21]进行注释。将OTU均质化并使用Mothurm (V1.32)进行进一步的α和β分析[18]。
用Chao 1指数分析群落丰富度,并计算社区多样性的Shannon-Weiner指数。Good的覆盖率用于估计克隆文库是否反映了样品中的细菌多样性。所有这些指数均由Mothur (V1.32)计算。聚类分析之前是主成分分析(PCA),应用于使用R软件(版本2.15.3)中的FactoMineR包和ggplot2包来减少原始变量的维数。
2 结果和分析 2.1 添加不同比例BPB对土壤理化性质的影响 添加生物炭,对香蕉苗株高、茎围、青叶数及最大叶长、叶宽方面均有促进作用,显著促进香蕉的生长。添加2% BPB的土壤植物生长状况更加明显(表 1)。
表 1. 香蕉假茎生物炭对香蕉苗生长的影响 Table 1. Effects of adding banana pesudostem biochar on banana seedlings growth
| Plant height/cm | Stem circumference (top)/cm | Stem circumference (middle)/cm | Stem circumference (below)/cm | Green level number/one | Maximum leaf length/cm | Maximum leaf width/cm | |
| CK | 38.84±5.34b | 3.24±0.14b | 4.16±0.31b | 5.26±0.42b | 8.20±0.45a | 27.84±1.78c | 10.17±0.64b |
| 1% | 47.58±3.33a | 3.43±0.58ab | 4.67±0.55a | 6.41±0.97a | 8.20±1.30a | 30.80±1.14ab | 11.48±0.91a |
| 2% | 52.08±1.40a | 4.07±0.66a | 5.15±0.34a | 6.88±1.03a | 8.60±0.55a | 31.96±0.60a | 12.19±0.78a |
| 3% | 47.22±3.10a | 3.74±0.68ab | 4.91±0.23a | 6.45±0.31a | 9.40±0.89a | 29.76±0.86b | 11.93±0.69a |
| All values are means±SD (n=5). Different letters in the same column represent significant differences (P < 0.05). | |||||||
表选项
添加不同比例BPB对土壤理化性质的影响见表 2。BPB的质量比越大,根际土壤有机质、速效钾、有效磷和pH指数均越高。土壤有效氮含量随着BPB添加量的增加而降低。土壤中阳离子交换量在2% BPB处理土样中最高(表 2)。
表 2. 不同比例BPB处理土壤的理化性质 Table 2. Soil physical-chemical parameters in substrate under different proportion of banana pesudostem biochar to soil
| Biochar addition | 0 | 1% | 2% | 3% |
| Organic matter/(g/kg) | 4.69±0.37d | 8.17±0.09c | 10.01±0.52b | 12.74±0.59a |
| pH | 6.68 | 7.13 | 7.70 | 7.90 |
| CEC/(cmol/kg) | 7.57±0.94d | 25.46±1.66b | 31.99±1.99a | 20.87±1.55b |
| Available nitrogen/(mg/kg) | 127.32±9.20a | 70.88±1.67b | 54.58±4.88c | 31.73±2.14d |
| Available potassium/(mg/kg) | 93.45±0.96d | 117.27±1.75c | 212.87±0.18b | 239.45±0.63a |
| Available phosphorus/(mg/kg) | 3.25±0.12d | 3.89±0.12c | 6.92±0.33b | 12.15±0.13a |
| All values are means±SD (n=5). Different letters in the same column represent significant differences (P < 0.05). | ||||
表选项
2.2 添加不同比例BPB对根际土壤细菌群落组成的影响 在细菌门水平,BPB处理土壤样本和非BPB处理土壤样本之间的相对丰度没有显著差异(图 1)。变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、拟杆菌门(Bacteroidates)、酸杆菌门(Acidobacteria)在大多数细菌群落中存在,在所有处理中约占80%。变形菌门(Proteobacteria)是在所有处理中在细菌群落中所占比例最多。其次是放线菌门、拟杆菌门和酸杆菌门。随着BPB比例的增加,变形菌、放线菌和酸杆菌的数量逐渐减少。拟杆菌随着BPB的增加而增加。不同比例的BPB处理土样之间各个细菌的丰度存在显著差异。1% BPB处理土样相较于0% BPB土样,变形菌门、绿弯菌门(Chloroflexi)和芽单胞菌门(Gemmatiomonadetes)相对丰度增加。与低水平BPB加入相比,在加入高浓度3% BPB土壤样品中,拟杆菌门、疣微菌门(Verrucomicrobia)和厚壁菌门(Firmicutes)的相对丰度显著增加,而放线菌门,绿弯菌门、酸杆菌门、芽单胞菌门和浮霉菌门(Planctomycetes)明显减少。3% BPB处理土样相较于2% BPB处理土样,厚壁菌门、疣微菌门和放线菌门显著增加。
 |
| 图 1 不同BPB比例样品中的细菌群落(门水平) Figure 1 Comparison of bacterial community in different samples at phylum level. |
| 图选项 |
2.3 添加不同比例BPB对细菌菌群α多样性的影响 试验分析了2000个序列后的OTU数。从稀释曲线中得到,24236个标准化序列可用于检测细菌群落的完整信息。由图 2可得,1% BPB处理土样中,物种丰富度最高,3% BPB处理土样中物种丰富度最少。
 |
| 图 2 细菌群落DNA片段的稀释曲线 Figure 2 A set of bacterial communications' DNA rarefaction curves. |
| 图选项 |
维恩分析显示,1%和2% BPB处理土样本共享1802个OTUs。在0和1% BPB处理土样之间共享有1607个OTUs,其次是0和2% BPB处理土壤中共享有1588个OTUs。在0和3% BPB处理的土样中最低共享有1266个OTUs。数据表明,任何2个生物群落之间共享的OTUs多于任何3个生物群落(图 3)。
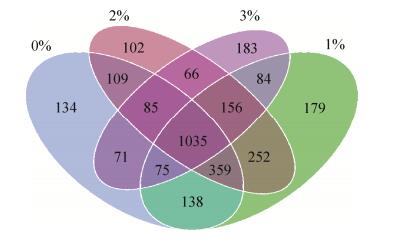 |
| 图 3 维恩图表显示不同处理的关系、土壤样本中细菌群落的共享和独特OTU数量 Figure 3 The relationship of different treatments by Venn diagrams showing numbers of shared andunique OTUs (97% similarity cut off) of bacterial communities in soil samples (three experimental, one control). |
| 图选项 |
4个处理的土壤样品分析总共217331个读数(生物炭样品的平均读数为52767,0% BPB土样为59031个读数)。在添加BPB和不添加BPB样品之间比较OTU的平均数量,生物炭比例(1%和2% BPB)的OTU丰度最高(表 3)。
表 3. 焦磷酸测序获得的细菌群落多样性指数 Table 3. Summary of diversity indices in bacterial communities obtained from pyrosequencing
| BPB | Analyzed read | Observed OTUs | Shannon | Estimated OTUs (Chao1) | Good’s coverage |
| 0 | 59031 | 2006 | 8.659 | 2483.955 | 0.977 |
| 1% | 65760 | 2278 | 9.397 | 2954.621 | 0.973 |
| 2% | 38384 | 2164 | 8.936 | 2550.104 | 0.975 |
| 3% | 54156 | 1755 | 8.277 | 1926.694 | 0.984 |
表选项
2.4 添加不同比例BPB对根际土壤细菌群落的影响 1% BPB处理土样和2% BPB处理土样分别解释了40.32%和33.59%的数据方差。细菌群落在BPB添加量较低时最相似(1%和2%)(图 4)。
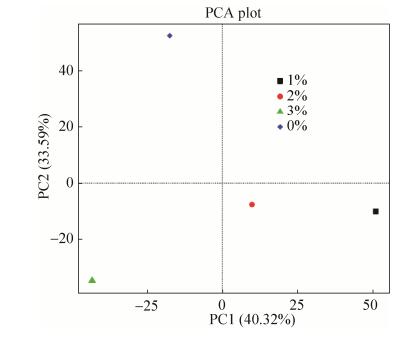 |
| 图 4 细菌群落的主成分分析 Figure 4 Principal component analysis of the bacteria community. |
| 图选项 |
香蕉根际土壤细菌群落在属水平上选取丰度高的前35个属,根据土壤样品的丰度信息,从物种和样品2个层面进行聚类后绘制成丰度聚类热图。土壤样品及不同细菌属间的系统进化树分别在聚类热图的上方和左方。由图 5可知,1% BPB和2% BPB处理土样组亲缘关系更近,0% BPB和3% BPB处理土样组的相关项相对较近。在0% BPB处理组,Burkholderia、Pseudomona、Promicromonospora和Rhodanobacter丰富度较高,随着BPB的量增加土壤中细菌丰度降低,两者呈负相关关系。增添1% BPB,Thermomonas、Sterioidabacter、Arthrobater和Nitrospira的丰度提高。随着BPB的添加量增大,Sediminibacterium、Sinomonas、Dyella、Bradyrhizobium和Candidatus_ Xiphinematobacter的丰度逐渐增高,与添加量成正比。添加3% BPB时,Planctomyces、Opitutuus、Rhodoplanes、Arenimonas、Candidatus_Solibacter、Anaeromyxobacter Luteolibacter、Sediminibacterium、Sinomonas、Dyella、Bradyrhizobium和Candidatus_ Xiphinematobacter丰度提高,其中Planctomyces Opitutuus、Bradyrhizobium、Candidatus_ Xiphinematobacterd的丰度在2% BPB处理土样中最高。因此,生物炭施加并非促进所有细菌属丰度。
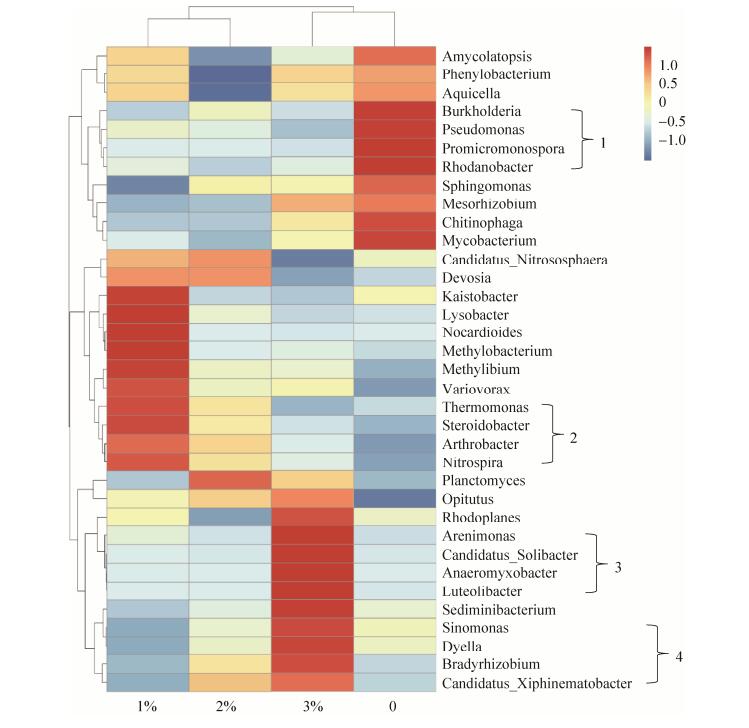 |
| 图 5 不同样品细菌的热力图分析 Figure 5 Heatmap analysis of frequently detected bacterial genera on different samples. |
| 图选项 |
3 讨论 土壤中变形菌门、放线菌门、拟杆菌门和酸杆菌门为土壤中的优势菌。本研究发现,生物炭对香蕉根际土壤的微生物群落具有促进作用,促进香蕉的生长。添加单比例生物炭,能够影响土壤中细菌群落结构的改变。然而,PLFA或变性梯度凝胶电泳(DGGE)的技术研究表明,不同比例的生物炭添加对微生物群落的精确变化依然不清楚。土壤pH是影响细菌群落的重要环境因素之一,在大多数研究[22]以及本研究中均观察到生物炭提高了根际土壤pH。研究表明,土壤pH与酸性细菌丰度之间存在负相关关系[23]。Sun等[24]研究显示添加生物炭酸杆菌门(Acidobacteria)丰富度提高,pH降低。本研究中,空白对照组土壤中的酸杆菌门丰度要明显高于生物炭处理的土壤,表现出酸杆菌门与pH变化的显著相关性。土壤的pH变化对细菌群落丰度产生影响,接种1% BPB和2% BPB的土壤接近中性环境,其细菌群落的丰度大于CK和3% BPB处理,可能是由于细菌群落中,较多的细菌种类适宜在中性pH环境中生长。本结果也证实BPB的添加对细菌群落有明显的影响。特别是,酸杆菌门和放线菌的丰度随着BPB的添加而下降,结果与前人一致。生物炭的施加会抑制放线菌的生长,这与Sun[24]的研究发现相一致,但却与Hu[25]的研究结论相反,这种差异可能与土壤类型、其固有细菌群落、土壤植物和土壤的理化性质等多种因素有关。Kishimoto[26]在研究生物炭对火山灰土中种植大豆和玉米的研究发现,以0.5 kg/m2和1.0 kg/m2的比例在田间施加生物炭后,大豆和玉米的产量降低,其主要的原因可能是生物炭的施加提高土壤pH,而高pH降低了某些微量元素的有效性。所以,对于不同的植物,生物炭的促进作用以及最佳的施用量需要进一步探讨。
在添加低至中等比例BPB的土壤中,细菌的丰度最高。显然,较低比例的BPB添加,增加了细菌的碳资源、多孔性和水的可利用性[27],然而,BPB比例越高,土壤pH值越高,细菌丰度逐渐降低。添加生物炭对细菌有不同的刺激,并优先刺激几种细菌的活性。Watzinger等[28]观察到在农业土壤中添加小麦壳生物炭后革兰氏阳性细菌几乎没有变化,但革兰氏阴性菌浓度很高。Gomez等[29]报道,添加橡木生物炭,土壤中细菌群落主要为革兰氏阴性菌。本研究同样证实生物炭的添加增加了革兰氏阴性菌伯克氏菌(Burkholderia),假单胞菌(Pseudomonas),原小单孢菌(Promicromonospora)和罗思河小杆菌(Rhodanobacter)的丰度。生物炭对土壤细菌群落的积极效应是因为生物炭施加后促进土壤中固有机质的释放。而细菌中的变形菌门和放线菌门[30]能够迅速利用这些释放出的营养,从而使细菌群落得到了优化。
生物炭添加对土壤氮循环的影响取决于生物炭原料、土壤性质和土壤环境。例如,添加生物炭可以增加土壤氮固定,加速有机氮转化为氨,促进氨的释放,以及刺激土壤硝化[31]。在本试验中,有效氮浓度降低,可能与氮循环活性的增加相关。添加生物炭后,土壤有机质、有效磷和有效钾含量增加,阳离子交换能力增加。可能是由于其较高的表面电荷密度和孔隙度,提高碳的储存能力,提高土壤肥力,降低土壤养流失[32],结果支持了生物炭添加可以补偿有机物质损失的论点,因此可能有助于改善土壤的物理、化学和生物特性[33]。生物炭施加对细菌如拟杆菌门和厚壁菌门均具有积极作用,拟杆菌门的大多数细菌都具有纤维素降解能力,可有效将土壤中的纤维素降解、循环,对于土壤中碳元素的循环具有积极意义;厚壁菌门中的细菌大多能产生孢子应对恶劣环境,同时还具有调控土壤微生物数量和病害生物防控功能[34]。
综上所述,添加生物炭对土壤养分、pH值和微生物群落有显著影响,但不同比例的添加影响结果差异明显。因此,生物炭作为一种土壤改良剂,在应用领域必须重视其添加比例,以便更好地解决土壤问题。
References
| [1] | Fang YY, Singh B, Singh BP. Effect of temperature on biochar priming effects and its stability in soils. Soil Biology and Biochemistry, 2015, 80: 136-145. DOI:10.1016/j.soilbio.2014.10.006 |
| [2] | Lashari MS, Liu YM, Li LQ, Pan WN, Fu JY, Pan GX, Zheng JF, Zheng JW, Zhang XH, Yu XY. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crops Research, 2013, 144: 113-118. DOI:10.1016/j.fcr.2012.11.015 |
| [3] | Rondon MA, Lehmann J, Ramírez J, Hurtado M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biology and Fertility of Soils, 2007, 43(6): 699-708. DOI:10.1007/s00374-006-0152-z |
| [4] | Zheng JF, Chen JH, Pan GX, Liu XY, Zhang XH, Li LQ, Bian RJ, Cheng K, Zheng JW. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Science of the Total Environment, 2016, 571: 206-217. DOI:10.1016/j.scitotenv.2016.07.135 |
| [5] | Kelly CN, Calderón FC, Acosta-Martínez V, Mikha MM, Benjamin J, Rutherford DW, Rostad CE. Switchgrass biochar effects on plant biomass and microbial dynamics in two soils from different regions. Pedosphere, 2015, 25(3): 329-342. DOI:10.1016/S1002-0160(15)30001-1 |
| [6] | Ippolito JA, Stromberger ME, Lentz RD, Dungan RS. Hardwood biochar influences calcareous soil physicochemical and microbiological status. Journal of Environmental Quality, 2014, 43(2): 681-689. DOI:10.2134/jeq2013.08.0324 |
| [7] | Steinbeiss S, Gleixner G, Antonietti M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biology and Biochemistry, 2009, 41(6): 1301-1310. DOI:10.1016/j.soilbio.2009.03.016 |
| [8] | Lv WB. Effect of biochar on soil microbial biomass, respiration and community structure. Master Dissertation of Zhejiang University, 2012. (in Chinese) 吕伟波.生物炭对土壤微生物生态特征的影响.浙江大学硕士学位论文, 2012. |
| [9] | Muhammad N, Dai ZM, Xiao KC, Meng J, Brookes PC, Liu XM, Wang HZ, Wu JJ, Xu JM. Changes in microbial community structure due to biochars generated from different feedstocks and their relationships with soil chemical properties. Geoderma, 2014, 226-227: 270-278. DOI:10.1016/j.geoderma.2014.01.023 |
| [10] | Anderson CR, Hamonts K, Clough TJ, Condron LM. Biochar does not affect soil N-transformations or microbial community structure under ruminant urine patches but does alter relative proportions of nitrogen cycling bacteria. Agriculture, Ecosystems & Environment, 2014, 191: 63-72. |
| [11] | Imparato V, Hansen V, Santos SS, Nielsen TK, Giagnoni L, Hauggaard-Nielsen H, Johansen A, Renella G, Winding A. Gasification biochar has limited effects on functional and structural diversity of soil microbial communities in a temperate agroecosystem. Soil Biology and Biochemistry, 2016, 99: 128-136. DOI:10.1016/j.soilbio.2016.05.004 |
| [12] | Kolton M, Harel YM, Pasternak Z, Graber ER, Elad Y, Cytryn E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Applied and Environmental Microbiology, 2011, 77(14): 4924-4930. DOI:10.1128/AEM.00148-11 |
| [13] | 鲍士旦. 土壤农化分析. 第3版. 北京: 中国农业出版社, 2007. |
| [14] | Novak JM, Bauer PJ, Hunt PG. Carbon dynamics under long-term conservation and disk tillage management in a Norfolk loamy sand. Soil Science Society of America Journal, 2007, 71(2): 453-456. DOI:10.2136/sssaj2005.0284N |
| [15] | Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pe a AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QⅡME allows analysis of high-throughput community sequencing data. Nature Methods, 2012, 7(5): 335-336. |
| [16] | Avershina E, Frisli T, Rudi K. De novo semi-alignment of 16S rRNA gene sequences for deep phylogenetic characterization of next generation sequencing data. Microbes and Environments, 2013, 28(2): 211-216. DOI:10.1264/jsme2.ME12157 |
| [17] | Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi HN, Won S, Chun J. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. International Journal of Systematic and Evolutionary Microbiology, 2012, 62: 716-721. DOI:10.1099/ijs.0.038075-0 |
| [18] | Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, van Horn DJ, Weber CF. Introducing mothur: open-source, platform- independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 2009, 75(23): 7537-7541. DOI:10.1128/AEM.01541-09 |
| [19] | Mago? T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics, 2011, 27(21): 2957-2963. DOI:10.1093/bioinformatics/btr507 |
| [20] | Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 2013, 10(10): 996-998. DOI:10.1038/nmeth.2604 |
| [21] | Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 2011, 27(16): 2194-2200. DOI:10.1093/bioinformatics/btr381 |
| [22] | Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR. Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia, 2011, 54(5-6): 309-320. DOI:10.1016/j.pedobi.2011.07.005 |
| [23] | Dimitriu PA, Grayston SJ. Relationship between soil properties and patterns of bacterial β-diversity across reclaimed and natural boreal forest soils. Microbial Ecology, 2010, 59(3): 563-573. |
| [24] | Sun DQ, Meng J, Xu EG, Chen WF. Microbial community structure and predicted bacterial metabolic functions in biochar pellets aged in soil after 34 months. Applied Soil Ecology, 2016, 100: 135-143. DOI:10.1016/j.apsoil.2015.12.012 |
| [25] | Hu L, Cao LX, Zhang RD. Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World Journal of Microbiology and Biotechnology, 2014, 30(3): 1085-1092. DOI:10.1007/s11274-013-1528-5 |
| [26] | Kishimoto S, Sugiura G. Charcoal as a soil conditioner. International Achievements for the Future, 1985, 5: 12-23. |
| [27] | Brewer CE, Unger R, Schmidt-Rohr K, Brown RC. Criteria to select biochars for ?eld studies based on biochar chemical properties. BioEnergy Research, 2011, 4(4): 312-323. DOI:10.1007/s12155-011-9133-7 |
| [28] | Watzinger A, Feichtmair S, Kitzler B, Zehetner F, Kloss S, Wimmer B, Zechmeister-Boltenstern S, Soja G. Soil microbial communities responded to biochar application in temperate soils and slowly metabolized 13C-labelled biochar as revealed by 13C PLFA analyses: results from a short-term incubation and pot experiment. European Journal of Soil Science, 2014, 65(1): 40-51. |
| [29] | Gomez JD, Denef K, Stewart CE, Zheng J, Cotrufo MF. Biochar addition rate in?uences soil microbial abundance and activity in temperate soils. European Journal of Soil Science, 2014, 65(1): 28-39. |
| [30] | Fierer N, Bradford MA, Jackson RB. Toward an ecological classification of soil bacteria. Ecology, 2007, 88(6): 1354-1364. DOI:10.1890/05-1839 |
| [31] | Prommer J, Wanek W, Hofhansl F, Trojan D, Offre P, Urich T, Schleper C, Sassmann S, Kitzler B, Soja G, Hood-Nowotny RC. Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS One, 2014, 9(1): e86388. DOI:10.1371/journal.pone.0086388 |
| [32] | Liu XY, Zhang AF, Ji CY, Joseph S, Bian RJ, Li LQ, Pan GX, Paz-Ferreiro J. Biochar's effect on crop productivity and the dependence on experimental conditions-a meta-analysis of literature data. Plant and Soil, 2013, 373(1-2): 583-594. DOI:10.1007/s11104-013-1806-x |
| [33] | Arriagada C, Almonacid L, Cornejo P, Garcia-Romera I, Ocampo J. In?uence of an organic amendment comprising saprophytic and mycorrhizal fungi on soil quality and growth of Eucalyptus globulus in the presence of sewage sludge contaminated with aluminium. Archives of Agronomy and Soil Science, 2014, 60(9): 1229-1248. DOI:10.1080/03650340.2013.878455 |
| [34] | Ni T. Effects of different fertilizing treatments on the microbial flora in rice-wheat rotation field. Master Dissertation of Nanjing Agricultural University, 2013. (in Chinese) 倪添.稻麦轮作体系下不同施肥措施对土壤微生物区系的影响.南京农业大学硕士学位论文, 2013. |
