胡智慧, 谌柄旭, 于爱群, 肖冬光


天津科技大学生物工程学院, 教育部工业发酵微生物重点实验室, 天津 300457
收稿日期:2017-11-02;修回日期:2018-01-24;网络出版日期:2018-03-13
基金项目:天津市教委科研计划(2017ZD03);天津市自然科学基金(17JCYBJC40800);天津科技大学"海河****"培育计划引进人才基金;南开大学分子微生物学与技术教育部重点实验室开放课题
*通信作者:肖冬光, Tel:+86-22-60600019, E-mail:xiao99@tust.edu.cn
摘要:植物萜类化合物是以异戊二烯为结构单位的一大类植物天然的次生代谢产物。D-柠檬烯属于单萜类化合物,由于它具有抑菌、增香、抗癌、止咳、平喘等多种功能,已被广泛应用于食品、香料、医疗等行业。目前D-柠檬烯的工业生产主要是从植物的果皮或者果肉中提取的,但提取方法存在着分离纯化复杂、产率低、能耗大等缺点。而本世纪初合成生物学技术的兴起,为微生物异源合成天然活性化合物带来了全新的理念与工具,打破了物种间的界限,使微生物异源合成D-柠檬烯成为现实。构建定向、高效的异源合成D-柠檬烯的微生物细胞工厂,实现微生物发酵法替换传统的植物提取法,具有重要的经济与社会效益。本文主要回顾了近几年利用代谢工程改造酿酒酵母异源合成萜类化合物取得的成就,阐述了以酿酒酵母作为底盘微生物,利用代谢工程和合成生物学的手段构建高产D-柠檬烯的合成策略。
关键词: 代谢工程 酿酒酵母 D-柠檬烯 合成策略
Strategies of metabolic engineering Saccharomyces cerevisiae to produce plant-derived D-limonene
Zhihui Hu, Bingxu Chen, Aiqun Yu, Dongguang Xiao


Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science & Technology, Tianjin 300457, China
Received 2 November 2017; Revised 24 January 2018; Published online 13 March 2018
*Corresponding author: Dongguang Xiao, Tel:+86-22-60600019, E-mail:xiao99@tust.edu.cn
Supported by the Research Foundation of Tianjin Education Committee (2017ZD03), by the Natural Science Foundation of Tianjin (17JCYBJC40800), by the Startup Fund for "Haihe Young Scholars" of Tianjin University of Science and Technology and by the Open Fund of Ministry of Education Key Laboratory of Molecular Microbiology and Technology, Nankai University
Abstract: Plant terpenoids are the natural secondary metabolites derived from units of isoprene with a molecular formula of C5H8 from different types of plants. D-Limonene is recognized as monoterpene and widely used in food and medical industry because of many functions, such as anti-bacteriostasis, aroma enhancement, anti-cancer and anti-cough. At present, the industrial production of D-limonene is generally obtained by extraction from the peel or pulp of plants. However, the extraction of D-limonene from plants suffers from complex separation and purification, low efficiency and high energy consumption. At the beginning of this century, the rise of synthetic biology technology has brought new ideas and tools for the synthesis of natural active compounds, which has broken the boundary between species and has become a reality of synthesis of D-limonene from microbes. It is of great economic and social benefit to construct a targeted and efficient microbial cell factory for the synthesis of D-limonene, and to replace the traditional method of plant extraction with microbial fermentation. We reviewed recent achievements of metabolic engineering of Saccharomyces cerevisiae to synthesize terpenoids and elaborated Saccharomyces cerevisiae as microbial chassis, using the method of metabolic engineering and synthetic biology to build high heterologous production of D-limonene synthetic strategies.
Key words: metabolic engineering Saccharomyces cerevisiae D-limonene synthesis strategies
萜类化合物,又称类异戊二烯,由异戊二烯单元组成的化合物及其衍生物构成,是植物的次生代谢产物。根据所含异戊二烯数目的不同可以分为单萜(C10)、倍半萜(C15)、二萜(C20)、三萜(C30)、四萜(C40)和多萜等[1]。
柠檬烯又称苧烯、苎烯,属于单环单萜类化合物,化学式为C10H16,是一种来源于植物的天然活性化合物。它主要存在于柑橘类(如柠檬、橙子、柑橘等)的果皮和果肉中,并且已被美国食用香料与提取物制造商协会(FEMA)认定其毒性属GRAS级(一般公认安全),并已被FDA批准食用[2]。
柠檬烯因结构内只含有一个手性碳原子,所以有两种光学异构体:右旋柠檬烯(D-柠檬烯,如图 1-A所示)和左旋柠檬烯(L-柠檬烯,如图 1-B所示),此外还存在一种外消旋体(D/L-柠檬烯,如图 1-C所示)[3]。
 |
| 图 1 D-柠檬烯[4](A)、L-柠檬烯[5](B)、D/L-柠檬烯[6](C)的化学结构式 Figure 1 The chemical structures of D-limonene[4](A), L-limonene[5](B) and D/L-limonene[6](C). |
| 图选项 |
D-柠檬烯具有类似柠檬或甜橙的香味,已被应用于香精领域,如作为添加剂添加到冰激凌、巧克力等来调整口感;亦可在橙香、果香、柠檬等香型的配方中作为修饰剂[7]。D-柠檬烯同时还具有防腐保鲜、抑菌、抗氧化、抗炎症、抗肿瘤、祛痰平喘、利胆溶石等多种生理活性,因此已被应用于食品保鲜、医疗领域[7]。此外,D-柠檬烯的衍生物如紫苏醇、松油醇、香芹酮、香芹醇等也具有重要的应用价值[8]。
D-柠檬烯具有重要的生理功能和很高的应用价值,但由于大量提取D-柠檬烯受来源与工艺的限制,合成生物学为更高效地获取D-柠檬烯提供了新思路。酿酒酵母作为底盘微生物,具有遗传背景清晰、遗传改造技术成熟并存在合成萜类化合物的甲羟戊酸(MEV)途径等优势,已经被广泛应用到构建合成萜类化合物的底盘微生物中。将D-柠檬烯合成途径的关键酶——D-柠檬烯合成酶,利用合成生物学的手段将其在酿酒酵母中异源表达,并对重构后的酵母细胞代谢流进行相应调控,最后完成整个合成途径的优化。本文阐述了异源构建高产D-柠檬烯的合成策略并着重介绍了D-柠檬烯合成酶的定向进化及代谢途径的改造思路,为其他植物萜类化合物异源合成提供借鉴。
1 D-柠檬烯合成酶基因的克隆 近些年研究者已经从多种不同的植物中,通过提取植物的总RNA后,反转录形成cDNA文库,通过文库筛选或者使用特异性引物克隆得到了多个D-柠檬烯合成酶的基因(表 1)。
表 1. 多种植物中克隆出的D-柠檬烯合成酶的基因 Table 1. The cloning of D-limonene synthase genes from a variety of plants
| Plants | Gene cloning methods | GenBank Code | PDB number | References |
| Schizonepeta tenuifolia | RNA—cDNA—PCR amplification —selection—target gene | AF282875.2 | NO | [9] |
| Citrus limon | RNA— cDNA library construction— library screening—target gene | AF514287.1 | NO | [10] |
| Citrus unshiu | RNA—cDNA library construction— library screening—target gene | AB110636.1 AB110637.1 | NO | [11-12] |
| Citrus sinensis | RNA—cDNA—PCR amplification— selection—target gene | KU746814 | 5UV0 | [13] |
表选项
2 代谢工程改造酿酒酵母异源合成萜类化合物的实例 目前,已有很多科研工作者把植物里特有的萜类化合物的合成途径,利用合成生物学的手段导入到酿酒酵母中异源合成植物特有的萜类化合物。以下简单地介绍了近几年利用酿酒酵母异源合成植物萜类化合物的实例(表 2),主要采用代谢工程的手段,过表达萜类合成途径中的关键基因、全局转录因子,强化代谢通路的流量;采用氨基酸抑制型启动子、铜离子抑制型启动子、弱启动子等替换原有基因的启动子,弱化前体物的竞争路径,减少前体物的流失;从其他植物中挖掘代谢途径的关键酶,采用合成生物学的手段,合成密码子优化后的外源基因并异源过量表达,提高目的产物的产量;采用酶的定向进化策略,对萜类合成酶进行定向进化,从中筛选出高催化活性的萜类合成酶。
表 2. 近年来通过改造酿酒酵母生产植物萜类物质的研究进展 Table 2. Recent researches about producing terpenoids in plants by engineering Saccharomyces cerevisiae
| Terpenes | Metabolic engineering strategies | Titer/ (mg/L) | References |
| Artemisinic acid | Overexpression upc2-1, a global transcription factor regulating the biosynthesis of sterols (Figure 2-18) Co-overexpression native tHMGR, ERG20 gene and heterologous codon-optimized ADS, CYP71AV1, CPR gene from Artemisia annua Downregulation ERG9 gene expression using a methionine-repressible promoter (PMET3) replace native promoter of ERG9 (Figure 2-16) | 100.00 | [14] |
| Ginsenoside | Co-overexpression heterologous PgDDS, PgPPDS, AtCPR1 gene through codon optimization from Panax ginseng and endogenous tHMGR, ERG2, ERG9, ERG1 gene | 1548.00 | [15] |
| D-limonene | Co-overexpression heterologous codon-optimized D-limonene synthase gene from Citrus limon and endogenous mutation ERG20K197G gene | 0.12 | [16] |
| Lycopene | To mining solely phytoene synthase function, the lycopene cyclase function of the bifunctional enzyme CrtYB from Xanthophyllomyces dendrorhous was inactivated by deletion of functional domain and directed evolution to obtain target mutants To further increase the FPP competitiveness of the lycopene synthesis pathway, enhancing the catalytic performance of CrtE by directed evolution and creating a series of pathway variants by varying the copy number of Crt genes Co-overexpression of the resulting CrtYB11M mutant along with the heterologous mutation CrtE03M and CrtI genes from X. dendrorhous, and the native tHMG1 gene | 1610.00 | [17] |
表选项
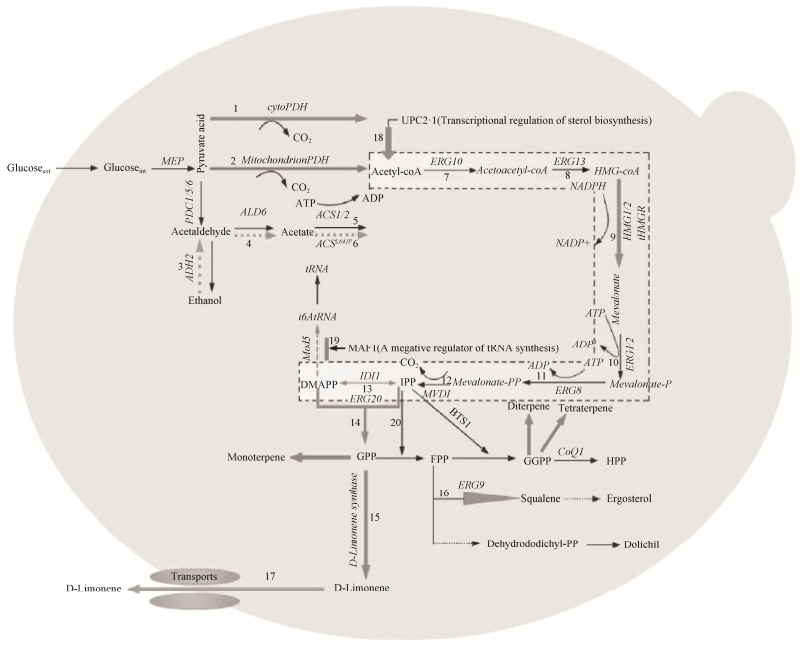 |
| 图 2 代谢工程改造酿酒酵母异源合成D-柠檬烯的策略图 Figure 2 The strategy diagram of metabolic pathways that lead to the production of D-limonene in Saccharomyces cerevisiae. CytoPHD: a pyruvate dehydrogenase in the cytosol of Saccharomyces cerevisiae; mitochondrionPHD: a pyruvate dehydrogenase in the mitochondrion of Saccharomyces cerevisiae; PDC1/5/6: pyruvate decarboxylase; UPC2-1: a transcriptional regulation of sterol biosynthesis; MAF1: a negative regulator of tRNA synthesis; HMG-CoA: 3-hydroxy-3-methylglutaryl-CoA; Mev-P: (R)-5-phosphomevalonate; Mev-PP: (R)-5-diphosphomevalonate; IPP: isopentenyl diphosphate; DMAPP: dimethylallyl diphosphate; GPP: geranyl diphosphate; FPP: farnesyl pyrophosphate; GGPP: geranylgeranyl diphosphate; HPP: hexaprenyl diphosphate; ERG10: acetoacetyl-CoA thiolase; ERG13: HMG-CoA synthase; HMG1/2: HMG-CoA reductase; ERG12: mevalonate kinase; ERG8: phosphomevalonate kinase; MVD1: mevalonate pyrophosphate decarboxylase; IDI1: isopentenyl diphosphate isomerase 1; ERG20: FPP synthase; ERG9: squalene synthase; COQ1: hexaprenyl-diphosphate synthase; ADH2: alcohol dehydrogenase; ALD6: acetaldehyde dehydrogenase; ACS1/2: acetyl-CoA synthase; The dotted line represents the MEV pathway (Figure 2-7–13). |
| 图选项 |
3 酿酒酵母中异源合成D-柠檬烯的策略 酿酒酵母中异源合成D-柠檬烯的策略见图 2,主要是从提高乙酰-CoA的供应量、调节MEV途径的代谢通量、D-柠檬烯合成酶的定向进化、酶的融合表达和定位等几个方面进行介绍的,为植物萜类化合物的异源合成提供参考。
3.1 增加乙酰-CoA的供应量 D-柠檬烯的合成需要大量的乙酰-CoA,酿酒酵母中乙酰-CoA主要来源于线粒体的PDH途径,其次来源于细胞质基质内的PDH旁路,由于酿酒酵母内缺少将线粒体内的乙酰-CoA转运到基质内的转运蛋白,因此细胞质基质内的PDH旁路对D-柠檬烯的合成至关重要。目前,主要通过调控和改造酿酒酵母内源代谢途径或者引入外源酶来提高乙酰-CoA的供应量(表 3)。
表 3. 酿酒酵母中增加乙酰-CoA供应量的措施 Table 3. The measures to increase the supply of acetyl-coA in Saccharomyces cerevisiae
| Position in the Figure 2 | Metabolic engineering strategies | Results | References |
| Figure 2-5 | Overexpression acetyl-CoA synthase gene (ACS1, ACS2) | The content of Acetyl-CoA and ATP increased The eight key gene expression levels of MEV pathway were also significantly increased | [18] |
| Figure 2-3, 4, 6 | Co-overexpression native alcohol dehydrogenase gene (ADH2), aldehyde dehydrogenase gene (ALD6) and heterologous codon-optimized acetyl-CoA synthase mutation gene (ACSL641P) from Salmonella enterica To decrease consumption of acetyl-CoA, knockouting peroxisomal citrate synthase gene (CIT2) and cytosolic malate synthase gene (MLS1) | Reducing the consumption of acetyl-CoA and greatly increasing the content of acetyl-CoA in cytosol, and then pulling acetyl-CoA towards the products of interest | [19] |
| Figure 2-1 | To reconstitution the high energy input requirement and feedback inhibition of PDH metabolic bypass, reconstruction four PDH pathways in the cytosol of yeast | The efficiency of acetyl-CoA utilization was greatly improved | [20] |
表选项
3.2 调节MEV途径的代谢通量 酿酒酵母中MEV途径涉及多步反应、多个限速酶(图 2虚线框内所示),单一调控手段很难实现MEV途径的代谢平衡,只有协调好关键基因的过量表达与竞争途径的弱化表达二者之间的关系,才能实现MEV途径的代谢平衡。可采取对外源基因进行密码子优化、梯度调控启动子强度[21-22]、调节RBS结合强度[23]等方法过量表达MEV途径的关键基因tHMGR、IDI1、ERG20,强化MEV途径的通量,积累足够的GPP[24](图 2-9、13、14);过量表达甾醇合成的全局转录因子upc2-1,进一步强化MEV途径的通量[14](图 2-18);过量表达tRNA合成的负调控因子MAF1,减少DMAPP被分流,强化DMAPP流向GPP[25-26] (图 2-19)。
FPP对形成细胞膜至关重要,因此不能敲除ERG20基因,只能采取弱化GPP竞争途径的策略,香叶基焦磷酸合成酶(ERG20F96W-N127W)的突变体对GPP的亲和力高于FPP,更利于合成GPP,进而弱化了GPP分流到FPP[24](图 2-20)。
3.3 D-柠檬烯合成酶的定向进化 在植物萜类合成途径中,单萜合成酶是决定单萜类次生代谢产物的关键酶,也是单萜代谢工程中最常用的靶酶,单萜合成酶的催化效率往往决定单萜的产量[27]。鉴于此,如何提高D-柠檬烯合成酶的催化效率,解除限速步骤?限速步骤的解除可采用两种方法:一种是在基因水平上构建强启动子的表达盒或者选择游离型高拷贝质粒来提高D-柠檬烯合成酶的表达量,该法操作相对简单、方便,也是目前基因过量表达主要采用的方法,但是该法主要是通过增加D-柠檬烯合成酶的浓度来强化酶的催化效率;另一种是提高D-柠檬烯合成酶的酶活,相比较而言,提高关键酶的酶活能够更有效地解除限速步骤。酶的定向进化可以有效地提高酶的活性和稳定性,已有的文献报道表明酶的活性和底物选择性与酶活性中心区域相关,酶的稳定性与酶活性中心附近或酶表面的氨基酸相关[28]。D-柠檬烯合成酶的定向进化可采用半理性的进化策略,将来源于不同植物的D-柠檬烯合成酶与已知晶体结构的D-柠檬烯合成酶(PDB: 5UV0)进行同源建模,找到酶与底物(GPP)结合口袋作用比较大的位点,将底物结合口袋附近位阻大的氨基酸突变为位阻小的氨基酸(G、A),减少底物进入酶活性中心区域的阻力,扩大结合口袋,进而提高酶的催化效率,但筛选是制约D-柠檬烯合成酶定向进化改造的瓶颈,孙周通等开发了系列快速半理性设计方法,如SCSM和TCSM,通过建立“小而精”的高质量突变体文库来解决筛选问题,为酶的改造和应用提供了新策略[29]。
3.4 酶的融合表达与定位 通过对D-柠檬烯合成酶基因的密码子优化、启动子和终止子的选取,构建了强表达盒。为了提高D-柠檬烯的产量,可采取将D-柠檬烯合成途径的香叶基焦磷酸合成酶与D-柠檬烯合成酶融合表达;也可将D-柠檬烯合成途径的多个关键酶利用蛋白支架串联融合表达,以上方法均可在空间上缩短酶与酶、酶与底物之间的距离,增加酶与底物的局部浓度,减少其他代谢物的干扰,进一步提高酶的催化效率,达到提高D-柠檬烯产量的目的。线粒体是独立的亚细胞结构,具有前体物乙酰-CoA供应充足、ATP含量丰富等优势,可作为萜类合成调控的第二场所。将D-柠檬烯合成途径的酶整体定位于线粒体中并融合表达,再结合细胞质基质内的D-柠檬烯合成途径,同时调控基质和线粒体的D-柠檬烯合成途径,进而提高D-柠檬烯的产量。近年来采用酶的融合表达与定位的策略,改造酿酒酵母异源合成萜类化合物的实例(表 4)。
表 4. 酶的融合表达与定位 Table 4. The fusion expression and localization of enzymes
| Methods | The fusion strategies of enzymes | Results | References |
| The fusion of two enzymes | Co-fusion expression endogenous farnesyl diphosphate synthase mutation (ERG20F96W-N127W) and heterologous codon-optimized geraniol synthase (CrGES truncated at S43, t3CrGES) from Catharanthus roseus | Enhancing the combination efficiency of GPP and geraniol synthase, and then the yield of geraniol was greatly improved | [24] |
| Co-fusion expression endogenous farnesyl diphosphate synthase mutation (ERG20K197E) and heterologous codon-optimized (S)-linalool synthase (AaLS1) from Actinidia arguta | The yield of (S)-linalool increased by 69.7% and the final yield was 0.24 mg/L | [30] | |
| The fusion of multi-enzymes | To further closer the distance of enzymes in space, the protein GBD, SH3 and PDZ were used as proetin scaffold, and then three enzymes of the MEV pathway (AtoB, HGMS and HMGR) were connected on the scaffold | By changing the number of scaffolds to optimize enzyme expression quantity, when AtoB, HGMS and HMGR by 1:2:2 combined, the content of mevalonic acid increased 77 times to 5 mmol/L | [31] |
| To further closer the distance of enzymes in space, nonimmunoglobulin affinity proteins were used as protein scaffolds, which were based on the recognition of affibodies to their anti-idiotypic partners in vivo, such as ZTaq:anti-ZTaq; ZIgA:anti-ZIgA. They were employed for co-localization of farnesyl diphosphate synthase (FPPSyn) and farnesene synthase (FarnSyn) in S. cerevisiae | Optimizing the ratio of enzymes and scaffolds, the yield of farnesene increased by 135% | [32] | |
| The relocalization of enzymes | In order to improve the efficiency of acetyl-CoA utilization, mitochondrial localization peptide (MLS) was used to relocate and fuse expression the enzymes of MEV pathway (ERG10, HMGS, tHMG1, ERG12, PMK, MVD1, IDI1) in mitochondria | Proposing dual metabolic engineering of cytoplasmic and mitochondrial regulation acetyl-CoA utilization to boost isoprene synthesis in S. cerevisiae and the final yield of isoprene reached 2.527 g/L | [33] |
表选项
4 其他调控策略 D-柠檬烯等萜类物质在酿酒酵母中过量的积累对其自身具有毒害作用。为了减弱D-柠檬烯对细胞持续的毒害作用,可采用半乳糖诱导型启动子(GAL1)来驱动D-柠檬烯合成酶基因的表达,在酿酒酵母处于对数后期时加入诱导剂,启动诱导系统并开始合成D-柠檬烯。
D-柠檬烯是如何从细胞内快速地转运到细胞外?这也是亟待解决的问题。在酿酒酵母中引入外源ABC转运蛋白(ATP-binding cassette transporters)显著地提高了对D-柠檬烯的耐性[34]。目前已在酿酒酵母中发现与毒性物质转运有关的一些蛋白,如Pdr5p、Pdr10p、Pdr15p、Tpo1p、SNQ2p、FLR1p (图 2-17)等,过表达上述蛋白有助于保护微生物细胞膜和细胞壁,并提高了对有毒物质的耐性[35]。
D-柠檬烯发酵过程中,在培养基中加入有机溶剂(十二烷、十四酸异丙酯、邻苯二甲酸丁二酯等)作为萃取剂,将发酵液中的D-柠檬烯萃取出来,双相发酵可以有效地减弱D-柠檬烯对酵母的毒害作用[24, 36]。
D-柠檬烯发酵培养基的组成和发酵条件的优化可以使酿酒酵母更好地生长与代谢,更利于D-柠檬烯的合成与积累。
5 讨论与展望 随着合成生物学和代谢工程的发展,将不同来源的生物元件导入到酿酒酵母中,重构了D-柠檬烯的合成途径。要想实现D-柠檬烯的异源高产,满足工业化的需求,仍然存在诸多问题:(1)如何筛选到高活性的D-柠檬烯合成酶;(2)异源途径的进入打破了酿酒酵母原有的代谢网络,如何保持酵母内的代谢平衡,如何调控新的代谢网络以达到最佳状态;(3) D-柠檬烯的转运机制知之甚少,这些是目前限制D-柠檬烯产量的重要原因。
随着诸如基因组学、转录组学、蛋白质组学以及代谢组学等现代“组学”技术的建立与发展,越来越多具有天然活性成分(植物萜类、生物碱类、黄酮类等)的化合物的合成途径与关键酶被解析,将会极大地促进合成生物学的发展与应用。利用合成生物学和代谢工程等多种手段,将会解决萜类合成途径的瓶颈,有望实现更多的萜类化合物及其衍生物在微生物中定向、高效的异源合成。
References
| [1] | Baunach M, Franke J, Hertweck C. Terpenoid biosynthesis off the beaten track: Unconventional cyclases and their impact on biomimetic synthesis. Angewandte Chemie International Edition, 2015, 54(9): 2604-2626. DOI:10.1002/anie.201407883 |
| [2] | 刘树文. 合成香料技术手册. 第2版. 北京: 中国轻工业出版社, 2009. |
| [3] | 杜府亮. 大肠杆菌MEP途径的改造及用于(-)-柠檬烯生物合成的研究. 华东理工大学硕士学位论文, 2014. |
| [4] | National Center for Biotechnology Information. PubChem substance database[2017-06-05]. https://pubchem.ncbi.nlm.nih.gov/substance/319222027. |
| [5] | National Center for Biotechnology Information. PubChem substance database[2017-06-05]. https://pubchem.ncbi.nlm.nih.gov/substance/249928896. |
| [6] | National Center for Biotechnology Information. PubChem substance database[2017-06-05]. https://pubchem.ncbi.nlm.nih.gov/substance/319078. |
| [7] | Cao T, Liu XY, Ding X, Bai WD, Zeng XF, Deng QH, Ren JP, Gu ZD. Advances in research and application of limonene. Farm Products Processing, 2017(8): 51-54. (in Chinese) 曹甜, 刘晓艳, 丁心, 白卫东, 曾晓房, 邓其海, 任嘉平, 顾振东. 柠檬烯的研究与应用进展. 农产品加工, 2017(8): 51-54. |
| [8] | Duetz WA, Bouwmeester H, van Beilen JB, Witholt B. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Applied Microbiology and Biotechnology, 2003, 61(4): 269-277. DOI:10.1007/s00253-003-1221-y |
| [9] | Maruyama T, Ito M, Kiuchi F, Honda G. Molecular cloning, functional expression and characterization of D-limonene synthase from Schizonepeta tenuifolia. Biological and Pharmaceutical Bulletin, 2001, 24(4): 373-377. DOI:10.1248/bpb.24.373 |
| [10] | Lücker J, El Tamer MK, Schwab W, Verstappen FWA, van der Plas LHW, Bouwmeester HJ, Verhoeven HA. Monoterpene biosynthesis in lemon (Citrus limon). The FEBS Journal, 2002, 269(13): 3160-3171. |
| [11] | Shimada T, Endo T, Fujii H, Hara M, Ueda T, Kita M, Omura M. Molecular cloning and functional characterization of four monoterpene synthase genes from Citrus unshiu Marc. Plant Science, 2004, 166(1): 49-58. DOI:10.1016/j.plantsci.2003.07.006 |
| [12] | Shimada T, Endo T, Fujii H, Omura M. Isolation and characterization of a new D-limonene synthase gene with a different expression pattern in Citrus unshiu Marc. Scientia Horticulturae, 2005, 105(4): 507-512. DOI:10.1016/j.scienta.2005.02.009 |
| [13] | Morehouse BR, Kumar RP, Matos JO, Olsen SN, Entova S, Oprian DD. Functional and structural characterization of a (+)-limonene synthase from Citrus sinensis. Biochemistry, 2017, 56(12): 1706-1715. DOI:10.1021/acs.biochem.7b00143 |
| [14] | Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho K, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature, 2006, 440(7086): 940-943. DOI:10.1038/nature04640 |
| [15] | Dai ZB, Liu Y, Zhang XA, Shi MY, Wang BB, Wang D, Huang LQ, Zhang XL. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metabolic Engineering, 2013, 20: 146-156. DOI:10.1016/j.ymben.2013.10.004 |
| [16] | Jongedijk E, Cankar K, Ranzijn J, van der Krol S, Bouwmeester H, Beekwilder J. Capturing of the monoterpene olefin limonene produced in Saccharomyces cerevisiae. Yeast, 2015, 32(1): 159-171. |
| [17] | Xie WP, Lv XM, Ye LD, Zhou PP, Yu HW. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metabolic Engineering, 2015, 30: 69-78. DOI:10.1016/j.ymben.2015.04.009 |
| [18] | Chen FJ, Zhou JW, Shi ZP, Liu LM, Du GC, Chen J. Effect of acetyl-CoA synthase gene overexpression on physiological function of Saccharomyces cerevisiae. Acta Microbiologica Sinica, 2010, 50(9): 1172-1179. (in Chinese) 陈孚江, 周景文, 史仲平, 刘立明, 堵国成, 陈坚. 乙酰辅酶A合成代谢对酿酒酵母生理功能的影响. 微生物学报, 2010, 50(9): 1172-1179. |
| [19] | Chen Y, Daviet L, Schalk M, Siewers V, Nielsen J. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metabolic Engineering, 2013, 15: 48-54. DOI:10.1016/j.ymben.2012.11.002 |
| [20] | Lian JZ, Zhao HM. Functional reconstitution of a pyruvate dehydrogenase in the cytosol of Saccharomyces cerevisiae through lipoylation machinery engineering. ACS Synthetic Biology, 2016, 5(7): 689-697. DOI:10.1021/acssynbio.6b00019 |
| [21] | Hammer K, Mijakovic I, Jensen PR. Synthetic promoter libraries-tuning of gene expression. Trends in Biotechnology, 2006, 24(2): 53-55. DOI:10.1016/j.tibtech.2005.12.003 |
| [22] | Blazeck J, Liu LQ, Redden H, Alper H. Tuning gene expression in Yarrowia lipolytica by a hybrid promoter approach. Applied and Environmental Microbiology, 2011, 77(22): 7905-7914. DOI:10.1128/AEM.05763-11 |
| [23] | Smolke CD, Martin VJJ, Keasling JD. Controlling the metabolic flux through the carotenoid pathway using directed mRNA processing and stabilization. Metabolic Engineering, 2001, 3(4): 313-321. DOI:10.1006/mben.2001.0194 |
| [24] | Jiang GZ, Yao MD, Wang Y, Zhou L, Song TQ, Liu H, Xiao WH, Yuan YJ. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae. Metabolic Engineering, 2017, 41: 57-66. DOI:10.1016/j.ymben.2017.03.005 |
| [25] | Kamińska J, Grabińska K, Kwapisz M, Sikora J, Smagowicz WJ, Palamarczyk G, ?o? dek T, Boguta M. The isoprenoid biosynthetic pathway in Saccharomyces cerevisiae is affected in a maf1-1 mutant with altered tRNA synthesis. FEMS Yeast Research, 2002, 2(1): 31-37. |
| [26] | Brown S, Clastre M, Courdavault V, O'Connor SE. De novo production of the plant-derived alkaloid strictosidine in yeast. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(11): 3205-3210. DOI:10.1073/pnas.1423555112 |
| [27] | Han JL, Li ZQ, Liu BY, Wang H, Li GF, Ye HC. Metabolic engineering of terpenoids in plants. Chinese Journal of Biotechnology, 2007, 23(4): 561-569. (in Chinese) 韩军丽, 李振秋, 刘本叶, 王红, 李国凤, 叶和春. 植物萜类代谢工程. 生物工程学报, 2007, 23(4): 561-569. DOI:10.3321/j.issn:1000-3061.2007.04.001 |
| [28] | 谢渊. 脂肪酶活性中心区域进化提高酶动力学稳定性和催化活性. 吉林大学博士学位论文, 2014. |
| [29] | Qu G, Zhao J, Zheng P, Sun JB, Sun ZT. Recent advances in directed evolution. Chinese Journal of Biotechnology, 2018, 34(1): 1-11. (in Chinese) 曲戈, 赵晶, 郑平, 孙际宾, 孙周通. 定向进化技术的最新进展. 生物工程学报, 2018, 34(1): 1-11. DOI:10.13345/j.cjb.170273 |
| [30] | Deng Y, Sun MX, Xu S, Zhou JW. Enhanced (S)-linalool production by fusion expression of farnesyl diphosphate synthase and linalool synthase in Saccharomyces cerevisiae. Journal of Applied Microbiology, 2016, 121(1): 187-195. DOI:10.1111/jam.2016.121.issue-1 |
| [31] | Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, Prather KLJ, Keasling JD. Synthetic protein scaffolds provide modular control over metabolic flux. Nature Biotechnology, 2009, 27(8): 753-759. DOI:10.1038/nbt.1557 |
| [32] | Tippmann S, Anfelt J, David F, Rand JM, Siewers V, Uhlén M, Nielsen J, Hudson EP. Affibody scaffolds improve sesquiterpene production in Saccharomyces cerevisiae. ACS Synthetic Biology, 2017, 6(1): 19-28. DOI:10.1021/acssynbio.6b00109 |
| [33] | Lv XM, Wang F, Zhou PP, Ye LD, Xie WP, Xu HM, Yu HW. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nature Communications, 2016, 7: 12851. DOI:10.1038/ncomms12851 |
| [34] | Li YF, Prinz WA. ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. Journal of Biological Chemistry, 2004, 279(43): 45226-45234. DOI:10.1074/jbc.M407600200 |
| [35] | Verwaal R, Jiang Y, Wang J, Daran JM, Sandmann G, van den Berg JA, van Ooyen AJJ. Heterologous carotenoid production in Saccharomyces cerevisiae induces the pleiotropic drug resistance stress response. Yeast, 2010, 27(12): 983-998. DOI:10.1002/yea.v27.12 |
| [36] | Zhao JZ, Bao XM, Li C, Shen Y, Hou J. Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 2016, 100(10): 4561-4571. DOI:10.1007/s00253-016-7375-1 |
