孙伟明1, 时晨4, 张梦涛1, 赵丹2, 陈璇1, 冯丽娜1, 罗永松4, 郭巍2,3

 , 邢单润1
, 邢单润1 1.河北科技师范学院农学与生物科技学院, 河北 秦皇岛 066000;
2.河北农业大学植物保护学院, 河北 保定 071001;
3.北京农学院植物科学技术学院, 北京 102206;
4.秦皇岛市疾病预防控制中心, 河北 秦皇岛 066000
收稿日期:2017-10-30;修回日期:2018-01-21;网络出版日期:2018-02-07
基金项目:河北省高等学校科学技术研究项目(QN2016069);河北省自然科学基金(C2017407006);河北省科技支撑计划(14226502D)
*通信作者:郭巍, Tel/Fax:+86-312-7528178, E-mail:1787421502@qq.com
摘要:[目的]为了分离鉴定对花生侵脉新赤壳菌果腐病病原菌Neocosmospora vasinfecta具有抑制作用的根际芽孢杆菌。[方法]利用平板稀释法从花生的根际土壤分离芽孢杆菌,再采用平板对峙法筛选出对N.vasinfecta具有抑制作用的根际芽孢杆菌,通过形态观察、生理生化特性和分子生物学相结合的多相分类方法对生防根际芽孢杆菌进行分类鉴定,检测脂肽类抗生素合成基因类型,并进行花生侵脉新赤壳菌果腐病的田间防治试验。[结果]从花生根际土壤中分离到28株芽孢杆菌,其中对花生果腐病病原菌具有明显抑制作用有8株。多相分类法结果显示2株为枯草芽孢杆菌(Bacillus subtilis),6株为解淀粉芽孢杆菌(B.amyloliquefaciens)。脂肽类抗生素合成基因检测显示,8株生防芽孢杆菌含有至少1种脂肽类抗生素,其中所有生防菌均含有丰原素B合成基因,推测这些芽孢杆菌对N.vasinfecta的抑制机制可能与脂肽类抗生素的合成相关。田间防病实验结果显示,B.amyloliquefaciens GF-3和GF-22制备的生物有机肥均能有效降低NPRP的发病指数,其防治效率分别为32.35%和79.41%,增产率分别为19.12%和25.85%。[结论]分离鉴定了2株对花生侵脉新赤壳菌果腐病具有明显防治效果的根际芽孢杆菌,这不仅为花生侵脉新赤壳菌果腐病的生防制剂研制提供了菌株,还为研究防治机理奠定了基础。
关键词: 花生侵脉新赤壳菌果腐病 根际细菌 芽孢杆菌
Isolation and identification of Bacillus spp. controlling Neocosmospora pod rot of peanut
Weiming Sun1, Chen Shi4, Mengtao Zhang1, Dan Zhao2, Xuan Chen1, Lina Feng1, Yongsong Luo4, Wei Guo2,3

 , Danrun Xing1
, Danrun Xing1 1.College of Agronomy and Biotechnology, Hebei Normal University of Science and Technology, Qinhuangdao 066000, Hebei Province, China;
2.College of Plant Protection, Hebei Agricultural University, Baoding 071001, Hebei Province, China;
3.College of Plant Science and Technology, Beijing University of Agriculture, Beijing 102206, China;
4.Qinhuangdao Center for Disease Control and Prevention, Qinhuangdao 066000, Hebei Province, China
Received 30 October 2017; Revised 21 January 2018; Published online 7 February 2018
*Corresponding author: Wei Guo, Tel/Fax:+86-312-7528178, E-mail:1787421502@qq.com
Supported by the Science and Technology Research Project of Hebei Universities (QN2016069), by the Natural Science Foundation of Hebei Province (C2017407006) and by the Science and Technology Support Plan Project of Hebei Province (14226502D)
Abstract: [Objective]To isolate and identify Bacillus spp. to control Neocosmospora pod rot of peanut (NPRP).[Methods]Bacillus spp. of peanut rhizosphere soil were isolated to inhibit Neocosmospora vasinfecta by plate confrontation method, and further classified by multiphase classification method. The lipopeptide synthesis gene type of biocontrol bacteria was detected by PCR. The control effect of Bacillus spp. on NPRP was tested in field trial.[Results]Twenty-eight Bacillus spp. strains were isolated and eight obviously inhibited N. vasinfecta. Two were identified as B. subtilis and the others were B. amyloliquefaciens. The biocontrol bacteria harbored one type of lipopeptide synthesis genes at least and all harbored FenB gene. We speculated that the inhibitory mechanism of the Bacillus spp. may be related to the synthesis of lipopeptide antibiotics. B. amyloliquefaciens GF-3 and GF-22 both could effectively reduce the disease index of NPRP and increase the yield of peanut in field trial.[Conclusion]Two strains B. amyloliquefaciens were isolated and identified to control NPRP. This study could provide strains to make biocontrol agents for prevention and control NPRP.
Key words: Neocosmospora pod rot of peanut rhizosphere bacteria Bacillus sp.
花生侵脉新赤壳菌果腐病(Neocosmospora pod rot of peanut,NPRP)是一种由侵脉新赤壳菌(Neocosmospora sp.)侵染引起的严重病害,其主要症状为花生荚果腐烂[1-5],可导致30%–100%的花生荚果腐烂,给种植户带来了巨大的经济损失,目前还没有有效的防治措施。
NPRP是近几年大面积突发于我国花生主产区的一种新型土传病害,只有少数科研人员对该病的防治进行了化学药剂田间防治实验,但由于化学药剂对土传病害的防治存在一些缺点,实际防治效果并不理想,而生防制剂在土传病害防治方面具有显著优势[6]。利用生防微生物不仅可以调节根部微生态环境,还可以参与生态位的竞争并限制土传病原真菌的繁殖和抑制土传病害的发生发展,因此,生防微生物在土传病害防治方面显示出巨大的应用潜力。芽孢杆菌由于具有内生芽孢、抗逆性强、繁殖速度快、营养要求简单和易于在植物根际定殖的特点,在病虫害的生物防治中被广泛地研究和应用。目前用于生防的芽孢杆菌种类有枯草芽孢杆菌、解淀粉芽孢杆菌、短小芽孢杆菌、蜡状芽孢杆菌和巨大芽孢杆菌等。生防芽孢杆菌的抑菌谱很广,能抑制大多数植物病原真菌,这是因为它们不仅能通过核糖体途径合成细菌素、细胞壁降解酶类和一些未鉴定的抗菌蛋白,还能利用非核糖体途径合成脂肽类抗生素[7-8]。本项目拟利用对峙实验和脂肽类抗生素合成基因检测获得对N. vasinfecta具有抑制作用的芽孢杆菌,并通过田间防病试验获得对NPRP具有良好防治效果的菌株,这不仅为NPRP的生物防治制剂的研发提供微生物资源,还为农用抗生素的开发奠定基础。
1 材料和方法 1.1 材料 本实验分离的73份花生根际土壤样品(沙壤土质)采自河北省邯郸市大名县的6个自然村、石家庄新乐市2个自然村和秦皇岛市昌黎县3个自然村的健康花生植株,对峙实验所用指示菌为Neocosmospora vasinfecta。
1.2 花生芽孢杆菌的分离、纯化 采用平板稀释涂布法[9-10]分离和纯化花生根际细菌,置于PDA平板28℃培养3 d,对菌体进行孔雀绿染色后镜检,纯化产芽孢的菌株作为初筛菌株。
1.3 抑菌活性测定 采用对峙法测定初筛菌株对NPRP病原菌的抑菌活性,每种菌株3个重复[11]。
1.4 生理生化特性、菌落特征和细胞形态特征的测定 生理生化特性利用梅里埃VITEK 2 Compact全自动细菌鉴定及药敏分析系统进行测试;菌落特征、革兰氏染色、芽孢染色和菌体大小按照常规方法进行测定[12]。
1.5 生防菌16S rDNA的扩增、克隆、序列测定分析 采用煮沸法提取细菌基因组DNA,PCR扩增16S rDNA。所用引物、扩增体系、反应条件见参考文献[11],PCR扩增目的片段经电泳检测后,回收、定量与T-easy载体(Promega公司)连接,转化感受态E. coli JM109。通过蓝白斑筛选和PCR检测获得阳性克隆。所筛选阳性克隆菌液送生工生物工程(上海)股份有限公司测序。根据测序结果,将扩增得到的序列在GenBank中进行BLAST分析。
1.6 特异基因鉴定 利用特异基因yyaR和tetB-tetL在B. subtilis和B. amyloliquefaciens的位置差异来进行分类鉴定[13-14]。
1.7 脂肽类抗生素合成基因检测 利用脂肽类抗生素合成相关基因特异引物进行PCR,琼脂糖凝胶检测扩增片段的大小,统计各菌株的脂肽类抗生素合成相关基因情况[8]。
1.8 田间防病试验 利用马铃薯葡萄糖液体培养基进行摇瓶液态发酵,获得生防芽孢杆菌的发酵液,计数后均匀喷洒于豆粕有机肥表面,制备成芽孢含量为0.5亿/g的豆粕生物有机肥。然后按照50 kg/亩的量穴施于冀花5号花生,每个处理3次重复,以未喷菌的豆粕有机肥作为空白对照。于收获前10 d进行五点取样病害分级调查,并测定产量。试验数据采用DPS7.05软件Duncan新复极差法进行差异性检验。
荚果分级标准:0级:荚果完好,无腐烂症状;1级:果皮有侵染斑点,果实完好;2级:荚果1/2腐烂;3级:荚果1/2以上腐烂,统计花生果各级腐烂数。病情指数、防治效率、干果亩产量和增产率分别用公式(1)、(2)、(3)和(4)表示。
 |
 |
 |
 |
 |
| 图 1 对峙实验 Figure 1 Confrontation experiment on PDA medium. |
| 图选项 |
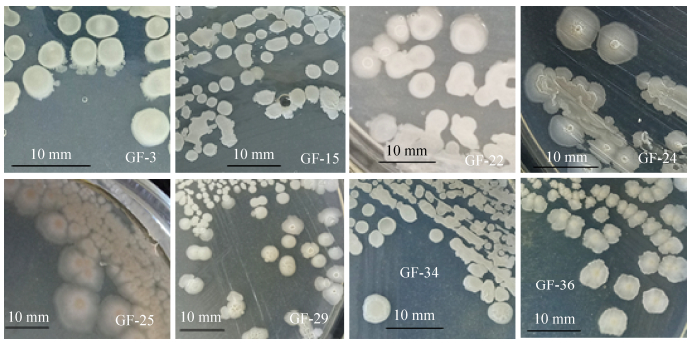 |
| 图 2 PDA培养基上培养48 h的菌落形态 Figure 2 Colony morphology for 48 h on PDA medium. |
| 图选项 |
表 1. 根际微生物的形态特征 Table 1. The morphological characteristics of rhizosphere
| Strains No. | Width of inhibition zone/cm | Microscopic observation | Locations |
| GF-22 | 0.46±0.09 | G+, bacilliform, (0.65–0.75) μm×(2.4–3.7)μm; Spore born in the middle, (0.60–0.70) μm×(1.4–1.6) μm | Daming County, Handan, Hebei (河北省邯郸市大名县) |
| GF-24 | 0.21±0.06 | G+, bacilliform, (0.65–0.80) μm×(2.2–3.2) μm; Spore born in the middle, (0.60–0.75) μm×(1.2–1.6) μm | |
| GF-25 | 0.46±0.07 | G+, bacilliform, (0.70–0.80) μm×(2.0–3.4) μm; Spore born in the middle, (0.55–0.70) μm×(1.0–1.2) μm | |
| GF-29 | 0.49±0.16 | G+, bacilliform, (0.70–0.80) μm×(2.2–3.1) μm; Spore born in the middle, (0.60–0.70) μm×(1.0–1.4) μm | Xinle County, Shijiazhuang, Hebei (河北石家庄新乐市) |
| GF-34 | 0.42±0.06 | G+, bacilliform, (0.60–0.75) μm×(2.1–3.1) μm; Spore born in the middle, (0.55–0.70) μm×(1.3–1.6) μm | |
| GF-36 | 0.50±0.12* | G+, bacilliform, (0.65–0.75) μm×(2.0–2.8) μm; Spore born in the middle, (0.60–0.70) μm×(1.1–1.4) μm | |
| GF-3 | 0.58±0.14 | G+, bacilliform, (0.65–0.80) μm×(2.2–3.3) μm; Spore born in the middle, (0.60–0.70) μm×(1.3–1.8) μm | Changli County, Qinhuangdao, Hebei (河北省秦皇岛市昌黎县) |
| GF-15 | 0.53±0.11 | G+, bacilliform, (0.65–0.75) μm×(2.2–3.3) μm; Spore born in the middle, (0.60–0.70) μm×(1.2–1.7) μm | |
| *: Represent a gradual decline of the width of inhibition zone. | |||
表选项
2.2 生理生化特性测定 利用BCL生化鉴定卡通过对46项生理生化特性进行了测定,结果显示GF-3、GF-22、GF-24、GF-25、GF-29和GF-36均为Bacillus subtilis/ amyloliquefaciens/atrophaeus,而GF-15和GF-34需进一步鉴定(表 2)。
表 2. 菌株生理生化实验结果 Table 2. The results of the physiological and biochemical test
| Items | Strains No. | ||||||||
| GF-3 | GF-15 | GF-22 | GF-24 | GF-25 | GF-29 | GF-34 | GF-36 | ||
| BXYL | + | + | + | + | + | + | – | + | |
| LysA | – | – | – | – | – | – | – | – | |
| AspA | – | – | – | – | (–) | – | – | – | |
| LeuA | – | – | + | – | + | – | – | – | |
| PheA | – | – | + | + | + | + | – | + | |
| ProA | – | – | – | – | – | – | – | – | |
| BGAL | – | – | – | – | + | – | – | – | |
| PyrA | + | (+) | + | + | + | + | – | + | |
| AGAL | + | + | + | + | + | + | – | + | |
| ALaA | – | – | – | – | – | – | – | – | |
| TyrA | (–) | – | + | – | + | (+) | – | – | |
| BNAG | – | – | – | – | – | – | – | – | |
| APPA | + | – | + | – | – | (+) | – | + | |
| CDEX | (+) | – | + | – | – | + | – | – | |
| dGAL | – | – | (+) | – | – | – | – | – | |
| GLYG | – | – | + | + | + | + | – | – | |
| INO | + | + | + | + | + | + | + | + | |
| MdG | + | + | + | + | + | + | – | + | |
| ELLM | – | – | – | – | – | – | – | – | |
| MdX | – | – | – | – | – | – | – | – | |
| AMAN | – | – | – | – | – | – | – | – | |
| MTE | + | + | + | + | + | + | + | + | |
| GlyA | – | – | – | – | + | – | – | – | |
| dMAN | + | + | + | + | + | + | + | + | |
| dMNE | + | + | + | + | + | + | + | + | |
| dMLZ | (+) | – | + | – | – | + | – | – | |
| NAG | – | – | (+) | – | – | – | – | – | |
| PLE | + | + | + | + | + | + | + | + | |
| IRHA | (–) | – | + | – | – | (–) | – | – | |
| BGLU | + | + | + | + | + | + | + | + | |
| BMAN | – | – | – | – | – | – | – | – | |
| PHC | + | + | + | + | + | – | + | – | |
| PVATE | + | + | + | + | + | + | (+) | + | |
| AGLU | + | (+) | + | (+) | + | + | – | (+) | |
| dTAG | – | – | – | – | – | – | – | – | |
| dTRE | – | + | + | + | + | + | + | + | |
| INU | – | – | + | – | + | (+) | – | – | |
| dGLU | + | + | + | + | + | + | + | + | |
| dRIB | + | + | + | + | + | + | + | + | |
| PSCNa | – | – | – | – | – | – | – | – | |
| 6.5% NaCl | + | + | + | + | + | + | + | + | |
| KAN | – | – | – | – | – | – | – | – | |
| OLD | – | – | – | – | – | – | – | – | |
| ESC | + | + | + | + | + | + | + | + | |
| TTZ | – | – | + | – | – | – | – | – | |
| POLYB_R | – | – | – | – | – | – | – | – | |
| (+) & (–) represent weakly positive result and weakly negative result, respectively. The abbreviations of the physiological and biochemical test refer to the BCL card instructions. | |||||||||
表选项
2.3 生防菌16S rDNA的生物信息学分析 测序结果经DNAMAN 6.0拼接、去除载体分析后,8株生防芽孢杆菌所克隆的16S rDNA序列大小均为1514 bp,提交GenBank,所得基因登录号分别为:GF-3(GenBank ID:MG264882)、GF-15(GenBank ID:MG264883)、GF-22(GenBank ID:MG264884)、GF-24(GenBank ID:MG264885)、GF-25(GenBank ID:MG264886)、GF-29(GenBank ID:MG264887)、GF-34(GenBank ID:MG264888)和GF-36(GenBank ID:MG264889)。利用NCBI网站BLASTn显示8株生防芽孢杆菌所克隆的16S rDNA序列与枯草芽孢杆菌(Bacillus subtilis,GenBank ID: NR102783 & NR112629 & NR113265 & NR027552)和解淀粉芽孢杆菌(Bacillus amyloliquefaciens,GenBank ID: NR075005)一致性均为99%,无法将8株生防芽孢杆菌进行分类,尚需进一步鉴定。
2.4 特异基因鉴定 B. subtilis和B. amyloliquefaciens的亲缘关系很紧密,利用形态特征、生理生化特性和16S rDNA序列并不能将其分开,而部分特异基因的差异可以用于两者的分类鉴定。以YyaO_F/TetB_R作为引物时,菌株GF-3、GF-15、GF-22、GF-24、GF-29和GF-34未能扩增出条带,而以YyaR_F/TetB_R作为引物时,菌株GF-3、GF-15、GF-22、GF-24、GF-29和GF-34能扩增出条带。以YyaO_F/TetB_R作为引物时,菌株GF-25和GF-36未能扩增出条带,而以YyaR_F/TetB_R作为引物时,菌株GF-25和GF-36能扩增出条带。结合16S rDNA的鉴定结果及部分生物学特性可以确定GF-25和GF-36菌为B. subtilis,GF-3、GF-15、GF-22、GF-24、GF-29和GF-34菌为B. amyloliquefaciens。
2.5 脂肽类抗生素合成基因分析 利用4对引物,分别对芽孢杆菌组DNA进行PCR扩增,结果显示:GF-25仅含有丰原素B一种合成基因;GF-3和GF-22均含有丰原素B和伊枯草菌素A两种合成基因;GF-15、GF-24、GF-29和GF-34均含有表面活性素、丰原素B和伊枯草菌素A三种合成基因;GF-36含有抗霉枯草菌素B、表面活性素和丰原素B三种合成基因(表 3)。B. amyloliquefaciens GF-3、GF-15、GF-22、GF-24、GF-29和GF-34均含有丰原素B和伊枯草菌素A合成基因。8株生防菌均含有丰原素B合成基因,推测这些芽孢杆菌对N. vasinfecta的抑制机制可能与丰原素的合成相关。
表 3. 脂肽类抗生素合成基因 Table 3. Lipopeptide antibiotic synthesis genes
| Gene | Target fragment size/bp | Strains No. | |||||||
| GF-3 | GF-15 | GF-22 | GF-24 | GF-25 | GF-29 | GF-34 | GF-36 | ||
| mycB | 2024 | – | – | – | – | – | – | – | + |
| fenB | 1400 | + | + | + | + | + | + | + | + |
| sfp | 675 | – | + | – | + | – | + | + | + |
| ituA | 1150 | + | + | + | + | – | + | + | – |
表选项
2.6 田间防病试验 利用生防芽孢杆菌制备生物有机肥进行田间试验,结果显示(表 4),在室内抑菌实验中对NPRP病原菌具有明显抑菌活性的8株芽孢杆菌,只有B. amyloliquefaciens GF-3和GF-22制备的生物有机肥均能有效降低NPRP的发病指数,其防治效率分别为32.35%和79.41%。B. amyloliquefaciens GF-22还能显著增加亩产量,其增产率高达25.85%。这表明,具有良好抑菌效果的芽孢杆菌要在田间实验中发挥显著的防病作用,除了与其脂肽类抗生素的合成有关,还受到其他条件制约。
表 4. 田间防病试验 Table 4. Field trial
| Strain | Disease index | Prevention rate/% | Yield/kg | Yield-increase rate/% |
| CK | 29.43 a | – | 328.46 b | – |
| GF-3 | 19.91 b | 32.35 | 391.25 ab | 19.12 |
| GF-22 | 6.06 c | 79.41 | 413.36 a | 25.85 |
| Different letters (a–c) represent significant differences between groups (P < 0.05). | ||||
表选项
3 讨论 花生侵脉新赤壳菌果腐病(NPRP)是近几年大面积突发于我国花生主产区的一种新型土传病害,目前多采用轮作等农业措施防治,还没有防治效果良好的药剂。芽孢杆菌属菌株是最普遍的植物生长促生根际细菌(PGPR),其中某些种能够产生次生代谢产物(如脂肽类抗生素),对植物病原菌具有较强的抑菌活性,常用于生物防治。本研究中从不同地区花生根部分离出8株对N. vasinfecta具有较强抑制活性的根际芽孢杆菌。其中,2株为B. subtilis,6株为B. amyloliquefaciens。所有生防菌含有至少1种脂肽类抗生素,其中均含有丰原素B合成基因,推测这些芽孢杆菌对N. vasinfecta的抑制机制可能与丰原素的合成相关。这不仅为花生侵脉新赤壳菌果腐病的防治机理奠定了理论基础,还为生防制剂的研制提供了菌株。
国内外研究均表明,芽孢杆菌制剂要发挥植物土传病害生物防治作用,首先芽孢杆菌要定殖于植物根际或体内,再通过竞争空间位点及营养、产生抗菌物质、诱导植物产生系统抗性和促进植物生长等作用机制达到防治植物病害的效果[15]。B. amyloliquefaciens可产生多种脂肽类抗生素,不仅能抑制植物病原菌,还可促进植物生长[16-17]。本研究从河北省花生主产区采集了73份健康花生的根际土壤,获得了6株对N. vasinfecta具有较强抑制活性的B. amyloliquefaciens GF-3、GF-15、GF-22、GF-24、GF-29和GF-34。尽管6株生防B. amyloliquefaciens均含有丰原素B和伊枯草菌素A合成基因,但田间防治效果显著的菌株只有B. amyloliquefaciens GF-3和GF-22。生防菌B. amyloliquefaciens GF-3和GF-22所制备的生物有机肥防治效率分别为32.35%和79.41%,B. amyloliquefaciens GF-22还能显著增加亩产量,其增产率高达25.85%。这说明不同B. amyloliquefaciens菌株之间田间防治效果和促生能力有显著差距,因此B. amyloliquefaciens的田间防治花生侵脉新赤壳菌果腐病的机制还需进一步研究。
References
| [1] | Pan R, Deng Q, Deng M, Guan M, Xu D, Gai Y, Chen W, Yang Y. First report of peanut foot rot caused by Neocosmospora vasinfecta in mainland China. Plant Pathology, 2010, 59(6): 1172. DOI:10.1111/j.1365-3059.2010.02360.x |
| [2] | Sun WM, Feng LN, Guo W, Liu DQ, Yang ZH, Liu LF, Ran LX, Meng QF. First report of Neocosmospora striata causing peanut pod rot in China. Plant Disease, 2012, 96(1): 146. |
| [3] | Sun WM, Feng LN, Guo W, Liu DQ, Li YN, Ran LX. First report of peanut pod rot caused by Neocosmospora vasinfecta in Northern China. Plant Disease, 2012, 96(3): 455. |
| [4] | Pan RQ, Gai YP, Deng MG, Chen XL, Xu DG, Ji CY. A notable new disease: Neocosmospora foot rot of peanut. Plant Protection, 2012, 38(3): 180-183. (in Chinese) 潘汝谦, 盖云鹏, 邓铭光, 陈兴龙, 徐大高, 纪春艳. 一种值得关注的花生新病害:花生新赤壳菌基腐病. 植物保护, 2012, 38(3): 180-183. DOI:10.3969/j.issn.0529-1542.2012.03.042 |
| [5] | Gai Y, Pan R, Ji C, Deng M, Chen W. First report of peanut foot rot caused by Neocosmospora vasinfecta var. africana in Jiangxi Province, China. Plant Disease, 2011, 95(11): 1480. |
| [6] | Yang FJ, Wang SL, Zhang L, Li CJ, Zhu TG, Liu HL, Zhou YP, Sun XP, Gao HY. Control effects of agent against peanut pod rot at high latitudes. Journal of Anhui Agricultural Sciences, 2015, 43(5): 140-141. (in Chinese) 杨富军, 王绍伦, 张丽, 李春洁, 朱统国, 刘海龙, 周玉萍, 孙晓苹, 高华援. 吉林省高纬度地区花生果腐病药剂防治效果. 安徽农业科学, 2015, 43(5): 140-141. DOI:10.3969/j.issn.0517-6611.2015.05.053 |
| [7] | Yang LR, Wang ZJ, Xue BG, Liu HY, Ma JG, Zheng YP. Clonging of antagonistic protein TasA gene in Bacillus amyloliquefaciens YN-1 and its prokaryotic expression. Genomics and Applied Biology, 2010, 29(5): 823-828. (in Chinese) 杨丽荣, 王正军, 薛保国, 刘红彦, 马建岗, 郑亚平. 解淀粉芽孢杆菌YN-1抑菌蛋白TasA基因的克隆及原核表达. 基因组学与应用生物学, 2010, 29(5): 823-828. |
| [8] | Deng JL, Liu HY, Liu YX, Liu XT, Ni YX, Gao SX, Li GQ. Identification of the antifungal substances from Bacillus amyloliquefaciens strain YN-1. Acta Phytopathologica Sinica, 2010, 40(2): 202-209. (in Chinese) 邓建良, 刘红彦, 刘玉霞, 刘新涛, 倪云霞, 高素霞, 李国庆. 解淀粉芽孢杆菌YN-1抑制植物病原真菌活性物质鉴定. 植物病理学报, 2010, 40(2): 202-209. |
| [9] | Ma Y, Rajkumar M, Luo YM, Freitas H. Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. Journal of Hazardous Materials, 2011, 195: 230-237. DOI:10.1016/j.jhazmat.2011.08.034 |
| [10] | Miller KI, Chen Q, Man-Yuen Sze D, Roufogalis BD, Neilan BA. Culturable endophytes of medicinal plants and the genetic basis for their bioactivity. Microbial Ecology, 2012, 64(2): 431-449. DOI:10.1007/s00248-012-0044-8 |
| [11] | Zhao LF, Xu YJ, Sun R, Deng ZS, Yang WQ, Wei GH. Identification and characterization of the endophytic plant growth prompter Bacillus cereus strain MQ23 isolated from Sophora alopecuroides root nodules. Brazilian Journal of Microbiology, 2011, 42(2): 567-575. DOI:10.1590/S1517-83822011000200022 |
| [12] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册. 北京: 科学出版社, 2001. |
| [13] | Reva ON, Dixelius C, Meijer J, Priest FG. Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiology Ecology, 2004, 48(2): 249-259. DOI:10.1016/j.femsec.2004.02.003 |
| [14] | Quan CS, Wang JH, Xu HT, Fan SD. Identification and characterization of a Bacillus amyloliquefaciens with high antifungal activity. Acta Microbiologica Sinica, 2006, 46(1): 7-12. (in Chinese) 权春善, 王军华, 徐洪涛, 范圣第. 一株抗真菌解淀粉芽孢杆菌的分离鉴定及其发酵条件的初步研究. 微生物学报, 2006, 46(1): 7-12. DOI:10.3321/j.issn:0001-6209.2006.01.003 |
| [15] | 戴秀华. 解淀粉芽胞杆菌Lx-11防治水稻细菌性条斑病的促生、控病机理研究. 南京农业大学硕士学位论文, 2015. |
| [16] | Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Süssmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nature Biotechnology, 2007, 25(9): 1007-1014. DOI:10.1038/nbt1325 |
| [17] | Wang YG, Wang JH, Quan CS, Fan SD. Purification and antifungal characterization of an antifungal substance from Bacillus amyloliquifaciens. China Biotechnology, 2007, 27(12): 41-45. (in Chinese) 王英国, 王军华, 权春善, 范圣第. 解淀粉芽孢杆菌抗菌活性物质的分离纯化及抑菌活性研究. 中国生物工程杂志, 2007, 27(12): 41-45. |
