姚志超, 白帅, 张宏宇


华中农业大学植物科学技术学院, 城市与园艺昆虫研究所, 湖北 武汉 430070
收稿日期:2017-07-27;修回日期:2017-11-02;网络出版日期:2017-11-28
基金项目:国家自然科学基金(31572008);国家现代农业(柑橘)产业技术体系(CARS-27);国际原子能署IAEA项目(D42016)
作者简介:张宏宇, 1965年4月出生, 博士, 教授, 博士生导师, 国家现代农业(柑橘)产业技术体系果实虫害防控岗位科学家。先后任International Foundation for Science科学顾问、中国粮油学会理事、中国昆虫学会昆虫微生物学组专业委员会副主任委员, 外来物种及检疫专业委员会、药剂与毒力专业委员会、城市昆虫专业委员会委员、中国植物保护学会园艺病虫害防治专业委员会委员、湖北植保学会副理事长等职务。1992年在华中农业大学留校任教, 主要研究方向为昆虫微生物学与分子生物学、昆虫免疫学、植物害虫检疫学。近年来一直研究实蝇、蛴螬肠道微生物分子多态性、群落组成及其对昆虫的生理功能, 以及肠道微生物群落稳态平衡机制。目前累计发表学术论文百余篇, 其中SCI收录70多篇, 授权专利8项
*通信作者:张宏宇, Tel:+86-27-87286962, E-mail:hongyu.zhang@mail.hzau.edu.cn
摘要:在长期的进化过程中,昆虫形成了独特的肠道防御系统,主要由物理屏障和免疫系统共同作用来抵御外来微生物的入侵。如大部分后生动物一样,昆虫肠道上皮细胞无时无刻不与微生物接触,其种类从有益的共生菌、随食物进入的微生物到影响宿主生命的病原菌。在这样一种复杂的环境中,为了实现防御肠道病原微生物的同时又能维持共生微生物稳定的目的,宿主肠道上皮细胞必须在免疫应激和免疫耐受之间保持一种稳态平衡。Duox-ROS免疫系统和免疫缺陷(immune deficiency,Imd)信号通路作为肠道免疫反应的基本途径,必然参与调节此过程。本文从昆虫肠道防御组成、肠道免疫信号通路作用分子机制以及肠道免疫系统在肠道微生物群落稳态维持中的作用的最新研究进展进行综述。
关键词: 肠道微生物 围食膜 肠道免疫 Duox-ROS免疫系统 Imd信号通路
Intestinal defense system and mechanism of maintenance of microbiota homeostasis in insects
Zhichao Yao, Shuai Bai, Hongyu Zhang


Institute of Urban and Horticultural Entomology, College of Plant Sciences and Technology, Huazhong Agricultural University, Wuhan 430070, Hubei Province, China
Received 27 July 2017; Revised 2 November 2017; Published online 28 November 2017
*Corresponding author: Hongyu Zhang, Tel:+86-27-87286962, E-mail:hongyu.zhang@mail.hzau.edu.cn
Supported by the National Natural Science Foundation of China (31572008), by the Earmarked Fund for the China Agricultural Research System (CARS-27) and by the International Atomic Energy Agency's Coordinated Research Project (D42016)
Abstract: In the long-term evolution process, insects have formed a unique intestinal defense system. The combination of physical barrier and the immune system resists invasive microbes. Alike most metazoans, the guts of insects are in permanent contact with the microbial realm that includes beneficial symbionts, food-borne microbes and life-threatening pathogens. Thus, gut epithelium can tolerate a certain amount of commensal microbes proliferation for the beneficial gut-microbe interactions, accompanied by the proficient elimination of detrimental microbes. Based on the function of Duox-ROS system and immune deficiency pathway in the intestinal immune response, these immune systems are involved in the regulation of gut microbiota homeostasis. In this article, we reviewed recent advances in insect intestinal defense mechanisms, combined with the intestinal immune signaling pathways and the regulation mechanism of intestinal immune system on the gut microbiota homeostasis.
Key words: intestinal microbiota peritrophic matrix intestinal immunity Duox-ROS immune system Imd signaling pathway
昆虫肠道是宿主与外部环境之间的重要交界面,栖息着大量的微生物。昆虫肠道持续不断地与微生物接触,而宿主与微生物之间的这种相互作用在其生命活动中具有重要作用:肠道有益微生物参与宿主代谢,提供营养物质[1],参与有害物质降解[2],保护宿主免受不利因子如天敌、寄生物和病原菌等的侵害[3-4],增强物种内和物种间的不断交流[5],影响疾病媒介昆虫传播疾病的效率[6-7],调控宿主交配与繁殖能力[8],促进宿主的生长发育[9]、免疫形成和成熟[10]等。然而,在自然开放性的环境中,昆虫取食同样会带来包括病原微生物在内的复杂多样的外来微生物。鉴于昆虫肠道微生物对于宿主昆虫的重要作用,肠道微生物结构组成、丰度以及功能的改变会引发宿主疾病的产生[11-12]。因此,维持肠道微生物群落稳态平衡、抵抗和消除外来病原菌对于昆虫生长发育与繁殖等的影响是至关重要的。很多因素参与肠道微生物结构的组成,如肠道pH、含氧量、氧化还原电位、养分有效性以及免疫系统等,但大量研究发现肠道免疫系统在宿主肠道微生物结构组成及区域限定中起重要作用[13]。本文结合作者相关研究工作,侧重于阐述昆虫肠道防御机制组成,以及肠道免疫信号通路Duox-ROS免疫体系和Imd信号通路作用的分子机制,并对两条免疫信号通路在肠道微生物群落稳态调控中的重要作用进行综合阐述。
1 昆虫肠道防御机制 昆虫在取食过程中,一部分微生物会定殖于肠道上皮组织成为宿主肠道共生微生物,而有些则会成为宿主潜在病原微生物。为了保障宿主的正常存活,昆虫肠道进化出了很多防御机制抵御外来微生物的入侵,主要包括:物理屏障、双氧化酶DUOX介导产生的活性氧(reactive oxygen species,ROS)和Imd信号通路产生的抗菌肽(antimicrobial peptides,AMPs)[14-15]。不同于系统免疫反应以保障宿主体腔和血淋巴的无菌环境,肠道免疫反应在有效清除病原菌的同时必须保障肠道共生微生物的存在。对于宿主而言,肠道免疫反应平衡的维持是一个很大的挑战,肠道免疫系统必须能够很好地区分病原微生物和肠道共生微生物,至少能够使肠道共生微生物适应其恰当的免疫反应。
1.1 物理屏障 昆虫围食膜是由几丁质聚合分子和蛋白质组成的半透性膜状结构,是昆虫肠道上皮细胞和食物之间的一个物理屏障,防御肠道病原微生物的第一道防线(图 1)[16]。围食膜不但能够阻挡细菌,而且可以阻止有害物质、细菌毒素、食物颗粒等和肠道上皮细胞的直接接触,如滴滴涕(DDT)、苏云金芽孢杆菌(Bacillus thuringiensis)毒素等[16-18],从而有效地保护肠道上皮细胞。最近研究发现果蝇围食膜的防御作用受某些基因调控,晶状体蛋白(Crystallin)基因的突变会导致肠道围食膜厚度下降一半,致使宿主对虫媒假单胞菌(Pseudomonas entomophila)和粘质沙雷氏菌(Serratia marcescens)的敏感性增加[19-20]。虫媒假单胞菌通过分泌一种成孔毒素(monalysin)最终导致宿主死亡[21-22]。
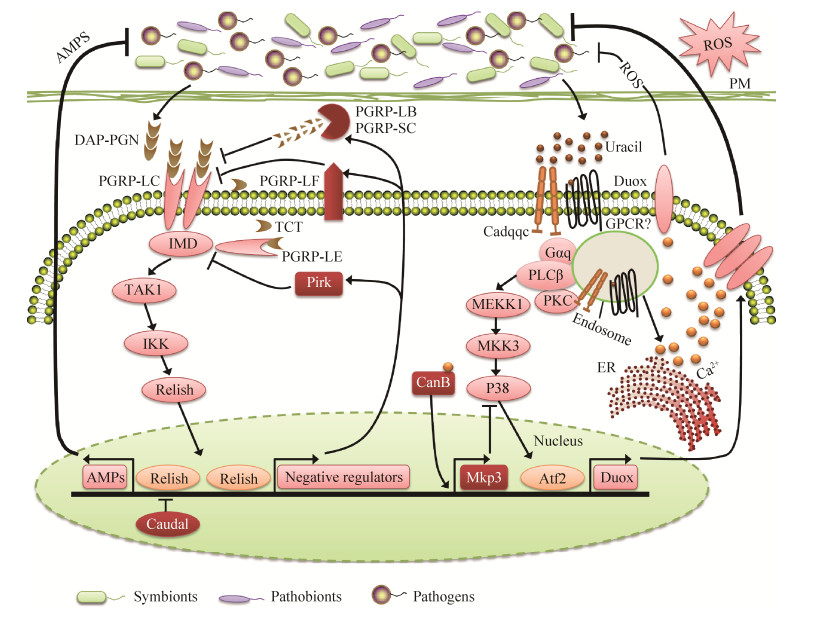 |
| 图 1 肠道Imd和Duox信号通路介导AMPs和ROS的产生 Figure 1 Production of AMPs and ROS by the Imd and Duox pathway in the gut. In the presence of high microbial load (infection or dysbiosis conditions), the Imd pathway is triggered when the membrane receptor PGRP-LC recognize diaminopimelic acid-type peptidoglycan (DAP-PGN) or by the intracellular receptor PGRP-LE which identify DAP-PGN monomers (tracheal cytotoxin, TCT). Vigorous initiation of the Imd pathway leads to the extreme nuclear translocation of Relish, which overcome caudal suppression and permit the occurrence of transcription of genes encoding AMPs. In addition, the large amount of uracil generated by pathogens/pathobionts strongly induces the Duox expression. Duox expression is mediated by the PLCβ, which activates the MEKK1/MEK3/p38 MAPK pathway and triggers Activating Transcription Factor 2 (ATF2), enormous quantities of DUOX results in the sufficient production of ROS required to fight against pathobionts and infectious microbes. During the routine situations, degradation of peptidoglycan and TCT is catalyzed by the negative regulators PGRP-LB and PGRP-SC; Pirk and PGRP-LF prevent the activity of recognition receptors PGRP-LE and PGRP-LC, transcriptional response of Relish is modulated by Caudal, suppress the Imd pathway activation. Under normal conditions the Duox system is also tightly regulated, Duox expression can be down-regulated by MAPK phosphatase 3 (Mkp3); PLCβ and Calcineurin B (CanB) are responsible to induce Mkp3 transcription under routine conditions. PM, peritrophic matrix; ER, endoplasmic reticulum; Tak1, transforming growth factor-β-activated kinase 1; IKK, IkB kinase; GPCR, G protein-coupled receptor; Cadqqc, Cadherin qqc; Mekk1, MAPK and ERK kinase kinase 1; MKK3, MAPK kinase 3. |
| 图选项 |
在脊椎动物里,由多糖和粘蛋白组成的粘液层将肠道上皮细胞与肠腔内含物隔开,从而限制微生物进入到肠道内腔。部分昆虫肠道的围食膜具有脊椎动物粘液层相似的功能,但果蝇中肠肠细胞上同样具有粘液层[19]。尽管果蝇中已注释有30多种基因编码粘蛋白类似物,然而这些基因的功能以及粘蛋白层在宿主昆虫防御中的作用还是未知的[23]。胡萝卜软腐欧文氏菌(Erwinia carotovora subsp. carotovora 15,Ecc 15)侵染的果蝇转录组分析发现,很多跟围食膜新陈代谢和粘液产生相关的基因被激活,说明两层栅栏组织在被侵染过程中进行了重建[24]。肠道上皮细胞结构的完整性在宿主肠道免疫防御中同样具有重要作用,big bang基因的突变会影响肠上皮细胞隔膜连接强度,Myo61F (编码肌球蛋白IB)基因的突变会改变肠道上皮细胞绒毛结构,肠道上皮细胞结构的改变最终引起宿主对细菌侵染的敏感性增强[25-26]。此外,在一些昆虫如索诺兰沙漠龟蚁(Cephalotes rohweri)中,其前胃(贲门瓣)可以有效地阻止细菌及大于0.2 μm的颗粒进入中肠及后肠,但可以允许营养物的进入,贲门瓣的这种结构和过滤作用为宿主防御病原微生物提供了一个新颖的物理防御机制[27]。
1.2 Imd信号通路 目前,通过Imd信号通路介导产生AMPs来清除病原菌是昆虫重要的免疫防御机制之一[28]。当微生物通过体表或肠道黏膜伤口进入宿主体腔(血腔)后便会迅速激活Imd信号通路。虽然昆虫缺乏适应性免疫系统,但却能够根据微生物种类的不同,启动相对应的免疫反应诱导表达不同种类抗菌肽进行防御,表明宿主自身存在着多样而特异性的微生物识别受体和免疫防御机制。宿主通过模式识别受体(PRRs)识别微生物相关的分子模式(MAMPs),从而激活信号转导通路,进而表达效应分子来裂解入侵微生物。Imd信号通路主要存在两种肽聚糖识别受体(recognition receptors of the peptidoglycan recognition protein,PGRP):一种是结合于细胞膜的细胞表面受体LC (PGRP-LC);另一种是主要在细胞质中的胞内受体LE (PGRP-LE)[29-30]。Imd信号通路通过PGRP-LC或PGRP-LE识别革兰氏阴性菌和一些革兰氏阳性菌(芽孢杆菌)的二氨基庚二酸型肽聚糖(DAP-type PGN)、肽聚糖单体(如气管毒素TCT)和肽聚糖聚合物而激活核酸转录因子Relish介导抗菌肽基因的表达(图 1)[31-33]。
在昆虫系统免疫反应中,Toll和Imd信号通路共同介导AMPs的表达,而在昆虫肠道免疫中,目前发现主要是由Imd信号通路介导AMPs的表达。已有研究表明肠道Imd信号通路在肠道免疫防御中起重要作用[34],Imd信号通路缺失型果蝇更容易被病原菌感染[35]。另外,在无菌品系果蝇肠道中研究发现AMPs表达水平显著低于正常虫体,说明宿主肠道共生微生物同样可以诱导Imd信号通路的启动[36]。然而Imd信号通路产生过多的AMPs对于宿主本身是不利的。因此,宿主昆虫存在多种机制以确保Imd途径的适度反应,以保证Imd信号通路在对病原菌免疫防御和肠道微生物菌群稳态调控作出适当的免疫反应的同时而避免过激的免疫反应。
首先Imd信号通路的激活需要肽聚糖识别受体与配体成功结合,而革兰氏阴性菌的细胞壁成分限制于细胞周质间隙间,外层是脂多糖,因此不易被宿主Imd免疫系统检测到[37],只有当细菌分裂时,细胞壁重建所释放大量肽聚糖片段而激活Imd免疫系统。正常条件下的宿主肠道细菌增殖速度低,密度远远低于病原菌感染时的菌体密度,所以正常条件下肠道细菌能够引起Imd信号通路免疫反应的肽聚糖浓度相对较低,这可能是正常条件下肠道免疫反应不是很强烈的原因之一[36]。另外,昆虫中肠是最易接触到细菌的部位,而中肠肽聚糖识别受体主要是识别肽聚糖单体的细胞内肽聚糖受体PGRP-LE,这种空间上的隔离可进一步保证在正常条件下低水平的免疫反应[29-30]。
此外,宿主昆虫采用精确复杂的负调控机制来调节Imd信号通路免疫系统,通过表达负调控因子下调Imd信号通路。这些负调控因子同时受Imd信号通路所调控,形成一种负反馈环来调整Imd信号通路对病原菌和共生菌不同程度的免疫反应[38]。这些负调控因子主要是一类具有酰胺酶活性的PGRPs家族蛋白(PGRP-LB,PGRP-SB1,PGRP-SB2,PGRP-SC1a,PGRP-SC1b,PGRP-SC2),它们通过裂解肽聚糖而降低肠道免疫刺激原[33, 39-42]。另外,还存在一些通过靶标Imd信号通路细胞内组件而实现负调控的负调控因子:Pirk (poor Imd response upon knock-in)基因通过编码产生蛋白与PGRP-LC结合,使其从细胞膜移位到细胞内,干扰其与Imd信号通路的联系从而限制Imd信号通路的启动[43];PGRP-LF拥有2个外部的PGRP结合域,通过与PGRP-LC结合形成二聚体隔绝PGRP-LC受体中的一部分而限制Imd信号通路的启动[44]。这些负调控作用保障Imd免疫信号通路对病原菌正常免疫反应的同时,避免其对共生菌产生过激的免疫反应(图 1)。
肠道抗菌肽区域化表达也是一种调控Imd信号通路的重要机制。尽管肠道各个区域均可作出对细菌的免疫反应,但肠道不同区域AMPs表达水平是不一样的。一些转录因子可以限制AMPs只在特定区域内表达,如后肠中的同源盒蛋白Caudal,它会阻碍AMPs基因在该区域的转录,但不干预PGRP-LB基因的表达[45]。因此Imd信号通路效应因子AMPs和负调控因子PGRP-LB在肠道不同区域有不同的调控模式,这说明肠道AMPs的表达同时受Imd信号和肠道区域调控。这些负调控机制和肠道AMPs区域化表达模式的存在保障了Imd免疫信号通路的正常工作,同时避免了过激的免疫反应对宿主产生伤害[35]。
1.3 Duox-ROS防御系统 氧分子过早和不彻底的还原过程中会形成一系列中间产物,即超氧化物,这些含氧自由基和易形成自由基的过氧化合物统称活性氧ROS。生物体内常见的ROS包括过氧化氢(H2O2)、羟自由基(HO·)、超氧阴离子(O2·–)、单线态氧(1O2)和臭氧(O3)等[46]。大量研究发现活性氧会对生物的各种大分子物质如蛋白、脂质和DNA等造成损伤[47],此过程即所谓的氧化胁迫(oxidative stress)。而抗氧化酶超氧化物歧化酶(SOD),谷胱甘肽过氧化物酶(GPx),硫氧还蛋白过氧化物酶(TPx)和过氧化氢酶(catalase)等可以清除细胞内的ROS而使细胞免受其毒害[48]。一直以来ROS被认为是有氧呼吸物种所产生的有害但又不能避免的副产品。但近年来研究发现,无论是线粒体还是NOX家族氧化酶所产生的ROS,在多种生理过程中扮演着信号分子的重要角色[49]。
Imd信号通路在昆虫系统免疫防御中发挥着重要作用,然而在肠道上皮细胞中,由Imd产生抗菌肽的防御方式就显得不那么重要了,因为缺乏抗菌肽产生的果蝇突变体完全可以抵抗大部分肠道外来菌的侵入,说明肠道中一定存在另一个抗菌系统来抵御外来微生物的入侵[50]。研究发现肠道上皮细胞产生的活性氧ROS具有杀菌活性,依赖Duox-ROS系统进行肠道免疫防御[14]。
已有研究表明ROS是很多昆虫防御细菌和真菌等病原微生物感染的重要免疫机制。例如在蟑螂(Blaberus discoidalis)、家蚕(Bombyx mori)、胭脂虫(Dactylopius coccus)、大蜡螟(Galleria mellonella)、长须罗玲(Lutzomyia longipalpis)、灯蛾(Parasemia plantaginis)、微小牛蜱(Rhipicephalus microplus)和冈比亚按蚊(Anopheles gambiae)等昆虫中已证明宿主可通过ROS来抵御细菌、真菌或疟原虫等病原微生物[51-60],蚊子肠道细胞内的线粒体和冈比亚按蚊肠道菌Enterobacter也可以提高ROS水平来击退疟原虫的侵染[6, 61],因此基于ROS的防御机制在昆虫中普遍存在。除了以上防御功能外,Duox还具有其他额外功能,在冈比亚按蚊中研究发现Duox与过氧化物酶共同作用在围食膜与肠道上皮细胞之间形成一种二酪氨酸网状结构,降低了免疫刺激原进入肠道黏膜的渗透性,阻断肠道过激免疫反应[62]。Duox在果蝇翅表面结构稳定中同样具有重要作用[63]。另外,ROS被报道参与调控肠道的创伤修复过程,表明ROS除了具有杀菌作用外,还可作为信号分子诱导修复反应或其他自我平衡信号通路的启动[36]。
人类基因组中还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶家族包含5个NOX 1–5和2个DUOX 1–2氧化酶[64-65]。其中DUOX家族区别于NOX家族的主要特征是位于细胞外N端有1个过氧化物酶同系物结合域PHD。在氯离子的存在下,该PHD能将NADPH氧化酶结合域生成的双氧水(H2O2)进一步转化成更具杀菌活力的次氯酸HOCl[10]。Duox基因在人结肠、直肠、唾腺管道和细支气管的黏膜等上皮细胞栅栏组织中均有表达[66-67]。但在果蝇基因组中只有1个NOX和1个DUOX氧化酶[15]。Ha等(2005)研究发现双氧化酶敲除型DUOX-KD果蝇而非氮氧化酶敲除型NOX-KD果蝇,在肠道感染条件下不能诱导产生ROS,DUOX-KD果蝇不能有效地清除外来微生物,对于肠道感染显示出高敏感性,说明在肠道防御上起作用的ROS是由DUOX介导产生的[14]。
肠道中DUOX介导产生ROS的过程主要受两条信号通路的调控:DUOX酶活主要由Gαq-PLCβ-Ca2+信号通路调控[68],而Duox基因的表达则由MEKK1-MKK3-p38-ATF2信号通路调控(图 1)[69-70]。然而有趣的是细菌肽聚糖PGN能够激活Duox基因表达信号通路,但并不能激活DUOX酶活,肽聚糖不能单独引起DUOX介导产生ROS[68-69, 71]。直到最近才研究发现,由细菌分泌的非肽聚糖配体尿嘧啶激活DUOX介导产生ROS[72]。尿嘧啶可能被G-蛋白偶联受体(GPCR)识别后,通过刺猬信号通路(hedgehog signaling pathway),介导依赖于钙粘蛋白qqc (Cadqqc)的核内体形成,核内体形成后依赖PLCβ的Ca2+流动而激活DUOX (图 1)[73]。值得注意的是只有外来病原菌才能够分泌尿嘧啶,而经过长期协同进化的宿主肠道共生菌丧失分泌尿嘧啶能力[72]。在细菌进化中,此种效应分子分泌方式的不同可能是宿主肠道上皮细胞能够很好区分肠道共生菌和病原菌的机制所在,从而能够有效地维持肠道微生物稳态与免疫平衡。
但当ROS产生的量超过一定阈值后,因其氧化应激会对宿主本身产生有害作用,伤害重要细胞的生命控制模块,如DNA、蛋白和脂类等[47]。因此宿主除了采用ROS作为自身防御的抗菌武器,必然伴随一个精密调控的清除机制。IRC-KD果蝇因为缺乏过氧化氢酶活性,导致肠道受到非致命性细菌的感染时产生过量ROS,宿主表现出很高的死亡率,而重新导入过氧化氢酶可以清除过量ROS,降低宿主死亡率,这种现象说明过氧化氢酶在肠道ROS清除上起着重要的作用[50]。总之,在肠道受到外来微生物入侵后,DUOX酶系统参与ROS的合成,清除外来微生物,然后再由过氧化氢酶清除机体产生的过量活性氧,恢复机体的氧化还原平衡。依靠DUOX产生ROS和依靠过氧化氢酶清除过量ROS的氧化还原平衡维持机制在宿主肠道免疫中至关重要。
2 昆虫免疫系统对肠道微生物稳态的调控机制 昆虫肠道微生物的群落结构组成受很多因素影响,如食物、生存环境、肠道理化因子以及免疫系统等[13]。但很多研究表明肠道免疫系统在宿主肠道微生物稳态维持中具有重要作用,宿主肠道免疫系统与肠道微生物之间存在多样性的互作关系[74]。从昆虫本身免疫系统而言,肠道不同的防御系统形成了宿主耐受和抵抗肠道微生物的不同特性。肠道耐受主要是降低细菌对于宿主的负面影响,而肠道抵抗主要是如何降低细菌载量,从而降低其对宿主健康的影响[75]。相比于消化道内微生物较少的昆虫,拥有大量肠道微生物的昆虫则表现出高水平的肠道耐受性和低水平的抵抗性[13]。大部分昆虫中肠肠道内存在围食膜,围食膜是半渗透性的,它可以允许营养物质、消化酶和防御因子的通过,但会阻止肠道上皮细胞与肠道微生物和有毒物质的直接接触[16]。在前后肠,肠道上皮细胞上的角质层行使着和围食膜同样的保护功能,这些在肠道上皮细胞和肠腔中的物理屏障是肠道耐受机制的很好例证,减少微生物的不利因子对于宿主的影响。而宿主昆虫肠道存在的溶菌酶以及免疫系统介导产生的效应物质如AMPs和ROS等,用来清除外来微生物的防御机制则是典型的抵抗机制[13]。
而从共生菌的角度而言,能够长期定殖于宿主肠道内的共生菌应至少具有以下三种特点之一:(1)能够有效抵抗肠道免疫效应分子;(2)缺乏肠道免疫系统诱导因子;(3)能够诱导表达免疫信号通路负调控因子[74]。因此,肠道共生微生物如何躲避免疫系统的攻击以及免疫系统如何耐受肠道共生微生物,也就成为了宿主肠道微生物群落稳态平衡维持及调控的重要节点。
2.1 Imd信号通路对肠道微生物的稳态调控机制 昆虫肠道与其肠道共生微生物持续不断地接触,然而肠道免疫系统如何区分肠道共生菌和病原菌,如何避免免疫效应因子AMPs和ROS的过量表达一直是一个难题。据报道,果蝇可以通过有效地调控肠道Imd信号通路和Duox-ROS系统来完成宿主肠道免疫系统对肠道共生菌的免疫耐受。Imd信号通路的缺失会导致肠道微生物增加10倍之多,表明肠道抗菌肽只是在压制而非清除肠道共生菌[36]。在Imd信号通路中,同源盒转录因子Caudal可以通过结合效应因子的启动区而特异性地抑制抗菌肽基因转录。果蝇Caudal基因的缺失会造成肠道抗菌肽基因的过量表达,最终导致肠道微生物群落结构变化,次要菌群(Gluconobacter morbifer,潜在致病菌)上升为主要菌群并引起肠道上皮细胞凋亡[45],表明Caudal基因能够有效抑制肠道共生菌对于肠道免疫系统的过度刺激。肠道上皮细胞内存在的具有酰胺酶活性的PGRPs也是宿主有效调控免疫反应进而维持肠道微生物群落稳定的一条途径,具有酰胺酶活性的PGRPs可以裂解Imd信号通路免疫刺激物细菌肽聚糖(PGN)为无免疫刺激物质,保障肠道低PGN水平,从而抑制Imd信号过激免疫反应[33, 40]。果蝇肠道PGRP-SC2基因表达受到抑制后会造成Imd信号通路长期处于激活状态,以及肠道细菌菌群密度显著增加[76]。另外,其他负调控因子如Pirk蛋白等可以隔绝细胞质内肽聚糖结合受体PGRP-LC,从而减少细胞表面PGRP-LC受体数量,阻断Imd信号通路的启动[43, 77]。而PGN结合受体PGRP-LE在肠道免疫调控中也具有重要作用,一方面,PGRP-LE诱导Relish介导的抗菌免疫反应;另一方面,PGRP-LE通过上调具有酰胺酶活性的PGRPs家族负调控基因和Pirk来保障宿主对于肠道共生菌的免疫耐受[30]。肠道内的肽聚糖主要来源于细菌裂解,肽聚糖密度是细菌细胞裂解的标志,肠道内短暂高载量的病原菌入侵会诱导Imd信号通路以及AMP的表达,而肠道共生菌的长期存在只会诱导Imd信号通路负调控基因的表达[30, 33]。这些负调控基因在有效阻断了Imd免疫信号通路过激反应的同时保护了宿主肠道共生菌。另外,Pang等(2016)在埃及伊蚊(Aedes aegypti)和尖音库蚊(Culex pipiens)中研究证明宿主通过表达C-型凝集素(C-type lectins)附着于细菌表面来抵消AMPs的活性,从而发现了一条宿主免疫系统保护肠道共生菌的新途径[78]。
2.2 Duox-ROS系统对肠道微生物的稳态调控机制 在肠道Duox免疫系统中,相似的免疫调控系统也同样有报道[68-69]。在肠道共生微生物存在时,肠道Duox基因的表达会被MKP3所抑制(图 1)。然而,非肽聚糖配体通过结合肠道上皮细胞可以改变细胞内钙离子浓度,从而诱导DUOX的活化,维持肠道内基础ROS的水平。基础ROS的维持对于肠道是很重要的,缺乏基础ROS后,宿主肠道内共生菌会过量生长[56, 79]。而Lee等(2013)研究发现没有灭活的食物酵母和特定的病原菌可以激活宿主DUOX活性,但宿主大部分肠道共生菌却不能激活DUOX,结果发现病原菌是通过分泌尿嘧啶激活信号通路的,而肠道共生菌却不能分泌尿嘧啶[72]。相较于果蝇肠道主要以厌氧菌醋杆菌属(Acetobacter)和乳杆菌属(Lactobacillus)存在的简单肠道微生物结构组成[80-82],橘小实蝇肠道内栖居着以肠杆菌科如克雷伯氏菌属(Klebsiella)、肠杆菌属(Enterobacter)、果胶杆菌属(Pectobacterium)和沙雷氏菌属(Serratia)等为主的复杂多样性微生物群落,为Duox-ROS免疫系统在宿主肠道微生物群落稳态调控中的作用提供了很好的研究材料[83-84]。研究发现Duox介导产生的ROS参与橘小实蝇肠道细菌群落结构组成的稳态调控:Duox-ROS系统耐受肠道共生菌的同时能够对外来微生物以及肠道次要菌群蜡样芽孢杆菌(Bacillus cereus)产生强烈免疫响应;肠道BdDuox基因的沉默导致肠道细菌量的显著增加,肠道细菌群落结构发生显著变化,致使肠道细菌群落稳态紊乱;菌群稳态紊乱反过来刺激宿主上调BdDuox基因表达水平,介导产生大量ROS抑制次要菌群的过量繁殖,最终恢复肠道微生物群落稳态平衡(图 1)[83]。Duox-ROS免疫系统能够耐受正常条件下宿主肠道微生物群落结构,但却能够对紊乱的肠道微生物群落结构作出高效调控策略,表明Duox-ROS免疫系统在宿主肠道微生物稳态中具有重要作用[84-85]。最近研究发现黑腹果蝇(Drosophila melanogaster)和埃及伊蚊在非感染条件下,细胞膜蛋白Mesh通过Arrestin介导的MAPK JNK/ERK磷酸化级联反应调控Duox基因的表达和肠道ROS水平变化,最终对肠道共生菌的增殖进行调控,进一步证明Duox-ROS免疫系统在宿主肠道微生物群落稳态调控中扮演着重要角色[86]。
另外,Duox-ROS系统除了直接参与肠道病原菌清除和调控肠道共生菌增殖外,还能够间接保障宿主肠道共生菌稳态平衡。在冈比亚按蚊中,DUOX系统与过氧化物酶(peroxidase)共同作用在围食膜和肠道上皮细胞间形成一层二酪氨酸网状结构,短时间内降低细胞外间质的渗透性。二酪氨酸网状结构的形成可以阻止宿主在吸血后血液中的细菌激发肠道过激的免疫反应,从而保护肠道共生菌[62]。
因此,Imd和Duox-ROS两条免疫信号通路的合理调控以及肠道共生菌在进化过程中所产生的适应性基因缺失保证了宿主耐受肠道共生菌的同时有效地杀灭外来微生物。
3 展望 肠道微生物与宿主之间存在着多样而复杂的关系,肠道共生微生物影响着宿主生命活动的方方面面。近几年在人类肠道微生物的研究中更是发现肠道微生物可以影响其行为,并能够改变大脑生理学和神经化学特征;肠道共生微生物群落的紊乱与很多疾病如炎症性肠病、肥胖症、糖尿病以及肠道慢性炎症和癌症等息息相关。因为肠道微生物与宿主生理、发育、行为等方面的多种相关性,维持一个健康而多样性的肠道微生物群落对于宿主昆虫进行各种生命活动是必需而又极具挑战的。在肠道与微生物长期的互作进程中,昆虫形成了自己独特的肠道免疫防御系统,但肠道免疫防御系统如何能够在有效清除外来病原物的同时而又保障肠道共生菌的存在,进而调控肠道微生物群落稳态平衡的分子机制还有待于进一步揭示。另外,具体由哪些外来微生物和肠道共生微生物通过分泌何种配体物质而激活相应的免疫信号通路的分子机制尚不清楚,了解这些微生物相关的配体物质形成过程和作用机制对微生物与免疫互作关系的完善有着重要意义。
References
| [1] | Thong-On A, Suzuki K, Noda S, Inoue JI, Kajiwara S, Ohkuma M. Isolation and characterization of anaerobic bacteria for symbiotic recycling of uric acid nitrogen in the gut of various termites. Microbes and Environments, 2012, 27(2): 186-192. DOI:10.1264/jsme2.ME11325 |
| [2] | Ceja-Navarro JA, Vega FE, Karaoz U, Hao Z, Jenkins S, Lim HC, Kosina P, Infante F, Northen TR, Brodie EL. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nature Communications, 2015, 6: 7618. DOI:10.1038/ncomms8618 |
| [3] | Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, Macpherson AJ, Strugnell R, von Mering C, Hardt WD. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathogens, 2010, 6(9): e1001097. DOI:10.1371/journal.ppat.1001097 |
| [4] | Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Current Opinion in Microbiology, 2011, 14(1): 82-91. DOI:10.1016/j.mib.2010.10.003 |
| [5] | Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. Animal behavior and the microbiome. Science, 2012, 338(6104): 198-199. DOI:10.1126/science.1227412 |
| [6] | Cirimotich CM, Dong YM, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, Dimopoulos G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science, 2011, 332(6031): 855-858. DOI:10.1126/science.1201618 |
| [7] | Cirimotich CM, Ramirez JL, Dimopoulos G. Native microbiota shape insect vector competence for human pathogens. Cell Host & Microbe, 2011, 10(4): 307-310. |
| [8] | Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(46): 20051-20056. DOI:10.1073/pnas.1009906107 |
| [9] | Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science, 2011, 334(6056): 670-674. DOI:10.1126/science.1212782 |
| [10] | Weiss BL, Wang JW, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biology, 2011, 9(5): e1000619. DOI:10.1371/journal.pbio.1000619 |
| [11] | Nyholm SV, Graf J. Knowing your friends:invertebrate innate immunity fosters beneficial bacterial symbioses. Nature Reviews Microbiology, 2012, 10(12): 815-827. DOI:10.1038/nrmicro2894 |
| [12] | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology, 2009, 9(5): 313-323. DOI:10.1038/nri2515 |
| [13] | Engel P, Moran NA. The gut microbiota of insects-diversity in structure and function. FEMS Microbiology Reviews, 2013, 37(5): 699-735. DOI:10.1111/1574-6976.12025 |
| [14] | Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science, 2005, 310(5749): 847-850. DOI:10.1126/science.1117311 |
| [15] | Ryu JH, Ha EM, Lee WJ. Innate immunity and gut-microbe mutualism in Drosophila. Developmental & Comparative Immunology, 2010, 34(4): 369-376. |
| [16] | Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annual Review of Entomology, 2009, 54: 285-302. DOI:10.1146/annurev.ento.54.110807.090559 |
| [17] | Tellam RL. The peritrophic matrix//Lehane MJ, Billingsley PF. Biology of the Insect Midgut. London: Chapman and Hall, 1996: 86-114. |
| [18] | Hayakawa T, Shitomi Y, Miyamoto K, Hori H. GalNAc pretreatment inhibits trapping of Bacillus thuringiensis Cry1Ac on the peritrophic membrane of Bombyx mori. FEBS Letters, 2004, 576(3): 331-335. DOI:10.1016/j.febslet.2004.09.029 |
| [19] | Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, Boccard F, Lemaitre B. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(32): 11414-11419. DOI:10.1073/pnas.0502240102 |
| [20] | Kuraishi T, Binggeli O, Opota O, Buchon N, Lemaitre B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(38): 15966-15971. DOI:10.1073/pnas.1105994108 |
| [21] | Opota O, Vallet-Gély I, Vincentelli R, Kellenberger C, Iacovache I, Gonzalez MR, Roussel A, van der Goot FG, Lemaitre B. Monalysin, a novel β-pore-forming toxin from the Drosophila pathogen Pseudomonas entomophila, contributes to host intestinal damage and lethality. PLoS Pathogens, 2011, 7(9): e1002259. DOI:10.1371/journal.ppat.1002259 |
| [22] | Blemont M, Vincentelli R, Kellenberger C, Opota O, Lemaitre B, Roussel A, Leone P. Crystallization and preliminary X-ray analysis of monalysin, a novel β-pore-forming toxin from the entomopathogen Pseudomonas entomophila. Acta Crystallographica Section F:Structural Biology and Crystallization Communications, 2013, 69(Pt 8): 930-933. |
| [23] | Syed ZA, H?rd T, Uv A, van Dijk-H?rd IF. A potential role for Drosophila mucins in development and physiology. PLoS One, 2008, 3(8): e3041. DOI:10.1371/journal.pone.0003041 |
| [24] | Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection:activation of host defense and stem cell proliferation. Cell Host & Microbe, 2009, 5(2): 200-211. |
| [25] | Bonnay F, Cohen-Berros E, Hoffmann M, Kim SY, Boulianne GL, Hoffmann JA, Matt N, Reichhart JM. Big bang gene modulates gut immune tolerance in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(8): 2957-2962. DOI:10.1073/pnas.1221910110 |
| [26] | Hegan PS, Mermall V, Tilney LG, Mooseker MS. Roles for Drosophila melanogaster myosin IB in maintenance of enterocyte brush-border structure and resistance to the bacterial pathogen Pseudomonas entomophila. Molecular Biology of the Cell, 2007, 18(11): 4625-4636. DOI:10.1091/mbc.e07-02-0191 |
| [27] | Lanan MC, Rodrigues PAP, Agellon A, Jansma P, Wheeler DE. A bacterial filter protects and structures the gut microbiome of an insect. The ISME Journal, 2016, 10(8): 1866-1876. DOI:10.1038/ismej.2015.264 |
| [28] | Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual Review of Immunology, 2007, 25: 697-743. DOI:10.1146/annurev.immunol.25.022106.141615 |
| [29] | Neyen C, Poidevin M, Roussel A, Lemaitre B. Tissue-and ligand-specific sensing of gram-negative infection in Drosophila by PGRP-LC isoforms and PGRP-LE. The Journal of Immunology, 2012, 189(4): 1886-1897. DOI:10.4049/jimmunol.1201022 |
| [30] | Bosco-Drayon V, Poidevin M, Boneca IG, Narbonne-Reveau K, Royet J, Charroux B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host & Microbe, 2012, 12(2): 153-165. |
| [31] | Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, Mengin-Lecreulx D, Lemaitre B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nature Immunology, 2003, 4(5): 478-484. DOI:10.1038/ni922 |
| [32] | Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N. Monomeric and polymeric Gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity, 2004, 20(5): 637-649. DOI:10.1016/S1074-7613(04)00104-9 |
| [33] | Zaidman-Rémy A, Hervé M, Poidevin M, Pili-Floury S, Kim MS, Blanot D, Oh BH, Ueda R, Mengin-Lecreulx D, Lemaitre B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity, 2006, 24(4): 463-473. DOI:10.1016/j.immuni.2006.02.012 |
| [34] | Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathogens, 2006, 2(6): e56. DOI:10.1371/journal.ppat.0020056 |
| [35] | Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world:insights from Drosophila melanogaster. Nature Reviews Microbiology, 2013, 11(9): 615-626. DOI:10.1038/nrmicro3074 |
| [36] | Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes & Development, 2009, 23(19): 2333-2344. |
| [37] | Johnson JW, Fisher JF, Mobashery S. Bacterial cell-wall recycling. Annals of the New York Academy of Sciences, 2013, 1277(1): 54-75. DOI:10.1111/j.1749-6632.2012.06813.x |
| [38] | Kuraishi T, Hori A, Kurata S. Host-microbe interactions in the gut of Drosophila melanogaster. Frontiers in Physiology, 2013, 4: 375. |
| [39] | Mellroth P, Steiner H. PGRP-SB1:an N-acetylmuramoyl L-alanine amidase with antibacterial activity. Biochemical and Biophysical Research Communications, 2006, 350(4): 994-999. DOI:10.1016/j.bbrc.2006.09.139 |
| [40] | Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathogens, 2006, 2(2): e14. DOI:10.1371/journal.ppat.0020014 |
| [41] | Zaidman-Rémy A, Poidevin M, Hervé M, Welchman DP, Paredes JC, Fahlander C, Steiner H, Mengin-Lecreulx D, Lemaitre B. Drosophila immunity:analysis of PGRP-SB1 expression, enzymatic activity and function. PLoS One, 2011, 6(2): e17231. DOI:10.1371/journal.pone.0017231 |
| [42] | Paredes JC, Welchman DP, Poidevin M, Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity, 2011, 35(5): 770-779. DOI:10.1016/j.immuni.2011.09.018 |
| [43] | Kleino A, Myllym?ki H, Kallio J, Vanha-Aho LM, Oksanen K, Ulvila J, Hultmark D, Valanne S, R?met M. Pirk is a negative regulator of the Drosophila Imd pathway. The Journal of Immunology, 2008, 180(8): 5413-5422. DOI:10.4049/jimmunol.180.8.5413 |
| [44] | Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J. The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host & Microbe, 2008, 3(5): 293-303. |
| [45] | Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science, 2008, 319(5864): 777-782. DOI:10.1126/science.1149357 |
| [46] | Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen:a double-edged sword revisited. Annual Review of Pathology:Mechanisms of Disease, 2014, 9: 119-145. DOI:10.1146/annurev-pathol-012513-104651 |
| [47] | Freeman BA, Crapo JD. Biology of disease:free radicals and tissue injury. Laboratory Investigation; A Journal of Technical Methods and Pathology, 1982, 47(5): 412-426. |
| [48] | Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology, 2004, 4(3): 181-189. DOI:10.1038/nri1312 |
| [49] | Zug R, Hammerstein P. Wolbachia and the insect immune system:what reactive oxygen species can tell us about the mechanisms of Wolbachia-host interactions. Frontiers in Microbiology, 2015, 6: 1201. |
| [50] | Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, Jang IH, Brey PT, Lee WJ. An antioxidant system required for host protection against gut infection in Drosophila. Developmental Cell, 2005, 8(1): 125-132. DOI:10.1016/j.devcel.2004.11.007 |
| [51] | Whitten MMA, Ratcliffe NA. In vitro superoxide activity in the haemolymph of the West Indian leaf cockroach, Blaberus discoidalis. Journal of Insect Physiology, 1999, 45(7): 667-675. DOI:10.1016/S0022-1910(99)00039-6 |
| [52] | Ishii K, Hamamoto H, Kamimura M, Sekimizu K. Activation of the silkworm cytokine by bacterial and fungal cell wall components via a reactive oxygen species-triggered mechanism. Journal of Biological Chemistry, 2008, 283(4): 2185-2191. DOI:10.1074/jbc.M705480200 |
| [53] | Hu XL, Yang R, Zhang X, Chen L, Xiang XW, Gong CL, Wu XF. Molecular cloning and functional characterization of the dual oxidase (BmDuox) gene from the silkworm Bombyx mori. PLoS One, 2013, 8(8): e70118. DOI:10.1371/journal.pone.0070118 |
| [54] | de Mu?oz FGG, Lanz-Mendoza H, Hernández-Hernández FC. Free radical generation during the activation of hemolymph prepared from the homopteran Dactylopius coccus. Archives of Insect Biochemistry and Physiology, 2007, 65(1): 20-28. DOI:10.1002/(ISSN)1520-6327 |
| [55] | Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes:identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infection and Immunity, 2005, 73(7): 4161-4170. DOI:10.1128/IAI.73.7.4161-4170.2005 |
| [56] | Diaz-Albiter H, Sant'Anna MRV, Genta FA, Dillon RJ. Reactive oxygen species-mediated immunity against Leishmania mexicana and Serratia marcescens in the phlebotomine sand fly Lutzomyia longipalpis. Journal of Biological Chemistry, 2012, 287(28): 23995-24003. DOI:10.1074/jbc.M112.376095 |
| [57] | Mikonranta L, Mappes J, Kaukoniitty M, Freitak D. Insect immunity:oral exposure to a bacterial pathogen elicits free radical response and protects from a recurring infection. Frontiers in Zoology, 2014, 11: 23. DOI:10.1186/1742-9994-11-23 |
| [58] | Pereira LS, Oliveira PL, Barja-Fidalgo C, Daffre S. Production of reactive oxygen species by hemocytes from the cattle tick Boophilus microplus. Experimental Parasitology, 2001, 99(2): 66-72. DOI:10.1006/expr.2001.4657 |
| [59] | Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(24): 14139-14144. DOI:10.1073/pnas.2036262100 |
| [60] | Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Gutierrez G, Barillas-Mury C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. Journal of Biological Chemistry, 2008, 283(6): 3217-3223. DOI:10.1074/jbc.M705873200 |
| [61] | Gon?alves RLS, Oliveira JHM, Oliveira GA, Andersen JF, Oliveira MF, Oliveira PL, Barillas-Mury C. Mitochondrial reactive oxygen species modulate mosquito susceptibility to Plasmodium infection. PLoS One, 2012, 7(7): e41083. DOI:10.1371/journal.pone.0041083 |
| [62] | Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science, 2010, 327(5973): 1644-1648. DOI:10.1126/science.1184008 |
| [63] | Anh NTT, Nishitani M, Harada S, Yamaguchi M, Kamei K. Essential role of Duox in stabilization of Drosophila wing. Journal of Biological Chemistry, 2011, 286(38): 33244-33251. DOI:10.1074/jbc.M111.263178 |
| [64] | Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radical Biology and Medicine, 2007, 43(3): 319-331. DOI:10.1016/j.freeradbiomed.2007.03.028 |
| [65] | Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases:host defense and beyond. Journal of Biological Chemistry, 2004, 279(50): 51715-51718. DOI:10.1074/jbc.R400024200 |
| [66] | Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. The FASEB Journal, 2003, 17(11): 1502-1504. DOI:10.1096/fj.02-1104fje |
| [67] | Forteza R, Salathe M, Miot F, Forteza R, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. American Journal of Respiratory Cell and Molecular Biology, 2005, 32(5): 462-469. DOI:10.1165/rcmb.2004-0302OC |
| [68] | Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang DM, Lee WJ. Regulation of DUOX by the Gαq-phospholipase Cβ-Ca2+ pathway in Drosophila gut immunity. Developmental Cell, 2009, 16(3): 386-397. DOI:10.1016/j.devcel.2008.12.015 |
| [69] | Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nature Immunology, 2009, 10(9): 949-957. DOI:10.1038/ni.1765 |
| [70] | Chakrabarti S, Poidevin M, Lemaitre B. The Drosophila MAPK p38c regulates oxidative stress and lipid homeostasis in the intestine. PLoS Genetics, 2014, 10(9): e1004659. DOI:10.1371/journal.pgen.1004659 |
| [71] | Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends in Immunology, 2010, 31(7): 278-287. DOI:10.1016/j.it.2010.05.003 |
| [72] | Lee KA, Kim SH, Kim EK, Ha EM, You H, Kim B, Kim MJ, Kwon Y, Ryu JH, Lee WJ. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell, 2013, 153(4): 797-811. DOI:10.1016/j.cell.2013.04.009 |
| [73] | Lee KA, Kim B, Bhin J, Kim DH, You H, Kim EK, Kim SH, Ryu JH, Hwang D, Lee WJ. Bacterial uracil modulates Drosophila DUOX-dependent gut immunity via Hedgehog-induced signaling endosomes. Cell Host & Microbe, 2015, 17(2): 191-204. |
| [74] | Douglas AE. The molecular basis of bacterial-insect symbiosis. Journal of Molecular Biology, 2014, 426(23): 3830-3837. DOI:10.1016/j.jmb.2014.04.005 |
| [75] | Schneider DS, Ayres JS. Two ways to survive infection:what resistance and tolerance can teach us about treating infectious diseases. Nature Reviews Immunology, 2008, 8(11): 889-895. DOI:10.1038/nri2432 |
| [76] | Guo LL, Karpac J, Tran SL, Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell, 2014, 156(1/2): 109-122. |
| [77] | Lhocine N, Ribeiro PS, Buchon N, Wepf A, Wilson R, Tenev T, Lemaitre B, Gstaiger M, Meier P, Leulier F. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host & Microbe, 2008, 4(2): 147-158. |
| [78] | Pang XJ, Xiao XP, Liu Y, Zhang RD, Liu JY, Liu QY, Wang PH, Cheng G. Mosquito C-type lectins maintain gut microbiome homeostasis. Nature Microbiology, 2016, 1: 16023. DOI:10.1038/nmicrobiol.2016.23 |
| [79] | Oliveira JHM, Gon?alves RLS, Lara FA, Dias FA, Gandara ACP, Menna-Barreto RFS, Edwards MC, Laurindo FRM, Silva-Neto MAC, Sorgine MHF, Oliveira PL. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathogens, 2011, 7(3): e1001320. DOI:10.1371/journal.ppat.1001320 |
| [80] | Staubach F, Baines J F, Künzel S, Bik EM, Petrov DA. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS One, 2013, 8(8): e70749. DOI:10.1371/journal.pone.0070749 |
| [81] | Wong CNA, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environmental Microbiology, 2011, 13(7): 1889-1900. DOI:10.1111/j.1462-2920.2011.02511.x |
| [82] | Broderick NA, Buchon N, Lemaitre B. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology. mBio, 2014, 5(3): e01117-14. |
| [83] | Wang H, Jin L, Zhang H. Comparison of the diversity of the bacterial communities in the intestinal tract of adult Bactrocera dorsalis from three different populations. Journal of Applied Microbiology, 2011, 110(6): 1390-1401. DOI:10.1111/jam.2011.110.issue-6 |
| [84] | Yao ZC, Wang AL, Li YS, Cai ZH, Lemaitre B, Zhang HY. The dual oxidase gene BdDuox regulates the intestinal bacterial community homeostasis of Bactrocera dorsalis. The ISME Journal, 2016, 10(5): 1037-1050. DOI:10.1038/ismej.2015.202 |
| [85] | Champion CJ, Xu JN. The impact of metagenomic interplay on the mosquito redox homeostasis. Free Radical Biology and Medicine, 2017, 105: 79-85. DOI:10.1016/j.freeradbiomed.2016.11.031 |
| [86] | Xiao XP, Yang LJ, Pang XJ, Zhang RD, Zhu YB, Wang PH, Gao GJ, Cheng G. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nature Microbiology, 2017, 2: 17020. DOI:10.1038/nmicrobiol.2017.20 |
