张永杰1

 , 赵宇翔1, 张姝1, 陈里2, 刘杏忠3
, 赵宇翔1, 张姝1, 陈里2, 刘杏忠3 1.山西大学生命科学学院, 山西 太原 030006;
2.美国宾夕法尼亚大学细胞与发育生物学系, 宾夕法尼亚州 费城 19104;
3.中国科学院微生物研究所真菌学国家重点实验室, 北京 100101
收稿日期:2016-10-15;修回日期:2016-12-02;网络出版日期:2016-12-21
基金项目:国家自然科学基金 (81102759);山西省自然科学基金 (2014021030-2, 201601D011065);山西省留学回国人员科技活动择优资助项目; 山西省大型科学仪器设备专项
*通信作者:Yongjie Zhang, E-mail:zhangyj2008@sxu.edu.cn
摘要: [目的]Glarea lozoyensis是抗真菌药物卡泊芬净的产生菌, 其突变菌株ATCC 74030的线粒体基因组已被报道.我们此前的研究发现诱变剂能引起该菌某些细胞核基因的突变, 但诱变剂是否也能引起线粒体DNA序列的改变并不清楚.[方法]组装野生型菌株ATCC 20868的线粒体基因组, 并与发表的突变型菌株ATCC 74030的线粒体基因组进行比较.通过PCR验证野生和突变菌株线粒体基因组间表现差异之处, 并利用正确的线粒体基因组序列进行新的分析.[结果]我们成功组装出野生型菌株ATCC 20868的线粒体基因组, 通过比较其与发表的ATCC 74030的线粒体基因组序列, 发现存在6处单核苷酸变异位点和2处具有长度差异的区域.然而, 随后的PCR验证和序列比较并没有发现2个菌株间存在这些差异.最初观察到的碱基差异是因为发表的ATCC 74030线粒体基因组存在序列错误.有趣的是, 在Glarea lozoyensis的线粒体基因组中, 我们发现存在3个具有内含子的tRNA基因和1个rnpB基因.同时, 该菌线粒体基因组中存在多种重复序列, 在其线粒体和细胞核基因组间也存在明显的DNA片段重复事件.[结论]诱变剂没有引起G. lozoyensis线粒体DNA的任何改变; 发表的ATCC 74030的线粒体基因组存在序列错误.我们报道G. lozoyensis正确的线粒体基因组序列, 并且发现该菌线粒体和细胞核基因组间频繁的基因交流.
关键词: 基因转移 Glarea lozoyensis 内含子tRNA 线粒体基因组 诱变剂
Reanalysis of the mitochondrial genome of the pneumocandin-producing fungus Glarea lozoyensis
Yongjie Zhang1

 , Yuxiang Zhao1, Shu Zhang1, Li Chen2, Xingzhong Liu3
, Yuxiang Zhao1, Shu Zhang1, Li Chen2, Xingzhong Liu3 1.School of Life Sciences, Shanxi University, Taiyuan 030006, Shanxi Province, China;
2.Department of Cell and Developmental Biology, University of Pennsylvania, Philadelphia, Pennsylvania 19104, U. S. A;
3.State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
Received 15 October 2016; Revised 02 December 2016; Published online 21 December 2016
*Corresponding author: Yongjie Zhang, E-mail:zhangyj2008@sxu.edu.cn
Supported by the National Natural Science Foundation of China (81102759), by the Natural Science Foundation of Shanxi Province (2014021030-2, 201601D011065), by the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province and by the Special Fund for Large Scientific Instruments and Equipments in Shanxi Province
Abstract: [Objective]Glarea lozoyensis is a filamentous fungus used for industrial production of the antifungal drug caspofungin. Previously, the mitochondrial genome (mitogenome) of a mutant strain ATCC 74030 was reported. The purpose of the current study is to test if mutagen treatments have caused changes on the mitogenome of the fungus.[Methods]The mitogenome of the wild strain ATCC 20868 was assembled and compared with the published mitogenome of ATCC 74030. PCR assays were done for both strains. Additional analyses were done using correct mitogenome sequences.[Results]We successfully assembled the mitogenome of the wild strain ATCC 20868. Initial comparison of the mitogenomes of the wild and mutant strains indicated six variable nucleotide sites and two regions with length variations. PCR assays and subsequent sequencing, however, showed no difference between the two strains. The differences observed from initial comparison were due to sequence errors present in the published mitogenome of ATCC 74030. Interestingly, three intron-containing tRNAs and a rnpB gene were detected in the mitogenome of the fungus. Obvious repetitive elements were identified within the G. lozoyensis mitogenome, and duplication events were identified between its mitochondrial and nuclear genomes.[Conclusion]We verified that there existed erroneous sequences in the published mitogenome of ATCC 74030; mutagens did not cause variations on the mitogenome of G. lozoyensis. We reported the authentic mitogenome sequence of G. lozoyensis and found frequent gene transfer between mitochondrial and nuclear genomes in the fungus.
Key words: gene transfer Glarea lozoyensis intron-containing tRNA mitochondrial genome mutagen
Glarea lozoyensis Bills & F. Peláez, formerly classified as Zalerion arboricola Buczacki, is an anamorphic fungus belonging to the Leotiales order of ascomycetes[1]. It produces lipopeptides with antifungal activities called pneumocandins which belong to the group of the echinocandin antibiotics[2]. The fungus was originally discovered by plating filtrates of pond water near Madrid, Spain in 1985[1]. The wild-type strain ATCC 20868 produces pneumocandin A0 predominantly[3]. From the parent wild strain, several mutant strains were obtained during development of pneumocandin B0 as a product candidate[4]. Pneumocandin B0 is chemically converted into caspofungin (CANCISASTM), a potent antibiotic against clinically important fungal pathogens[5-6]. Among those mutant strains, ATCC 74030, which was obtained after two cycles of mutagenesis, is an overproducer of pneumocandin B0[4]. During two dozen years after the description of the medicinally important fungus, only one wild strain (i.e., ATCC 20868 = CBS 492.88) is known. Until very recently, new G. lozoyensis strains were reported from soil or leaf litter in Argentina and the USA, indicating a wide distribution of this fungus[7].
As an antibiotic-producing fungus, researches regarding G.lozoyensis have traditionally focused on new potent metabolite exploration[2], strategies for increasing target metabolite yield[8-10], and understanding molecular bases underlying its metabolite production[11-12]. To elucidate the biosynthetic pathway to pneumocandins, genome sequences of the wild strain ATCC 20868 and the mutant strain ATCC 74030 were recently sequenced[13-14]. About 50 secondary metabolite biosynthesis gene clusters were predicted from the genome[13]. The gene cluster responsible for pneumocandin production was predicted in silico and identified by deletion of the core genes glpks4 and glnrps4 and bioassay experiments[13]. Genetic manipulations of the biosynthetic pathway changed it metabolic profile[15-16].
In the scope of a whole genome sequencing of ATCC 20868, the complete mitochondrial genome (mitogenome) of G. lozoyensis ATCC 20868 was assembled by our research group. During the implementation of our project, the mitogenome of the mutant strain ATCC 74030, which is generated from ATCC 20868, was published[17]. Comparison of the two mitogenomes showed several nucleotide differences and the translocation of a large fragment. In one of our recent investigations, we found that chemical mutagenesis disrupted the GLOXY4 gene function of G. lozoyensis by introducing two amino acid mutations in GLOXY4[15]. We suspected whether the variations observed between the two mitogenomes also resulted from mutagen treatments used for generating ATCC 74030. To test our hypothesis, we performed PCR assays with genomic DNAs extracted from the two G. lozoyensis strains as templates. Different from our expectations, however, no difference was detected mitogenomesbetween the parental and mutant strains. In this study, we provided authentic mitogenome sequences of G. lozoyensis, re-annotated the mitogenome, and performed additional analysis.
1 Materials and methods 1.1 Fungal material, cultivation and DNA extraction Two G. lozoyensis strains ATCC 20868 (wild-type) and ATCC 74030 (mutant strain) were grown for 20 days at 25 ℃ on potato dextrose agar plates. They were then subcultured for additional 20 days on the same medium but with a piece of sterile cellophane paper covering the medium surface. Fresh mycelia were scraped off the plate and then used for extracting total genomic DNA using the cetyltrimethylammonium bromide method[18].
1.2 Assembly of the complete mitogenome of G. lozoyensis ATCC 20868 When we initiated the current project, the mitogenome of ATCC 74030 has not been released. To assemble the mitogenome of ATCC 20868, we performed local BLAST searches against the raw genome data of G. lozoyensis ATCC 20868 using the Phialocephala subalpina mitogenome sequence (NC_015789) as the query. Two scaffolds harboring mitochondrial genes were identified, but they were incomplete due to existence of 25 gap regions. To fill the gaps and to join the two scaffolds, multiple primer pairs were designed based on known sequences using the online program Primer3 (http://bioinfo.ut.ee/primer3/). PCRs were performed using a high-fidelity DNA polymerase KOD FX (TOYOBO Bio-Technology Co. LTD, Japan), and sequences of amplicons were determined by Sanger sequencing at SinoGenoMax Co. Ltd. (Beijing, China).
1.3 Comparison between the mitogenomes of ATCC 74030 and 20868 The mitogenome of G. lozoyensis ATCC 20868 assembled in this study was aligned with the published mitogenome of ATCC 74030 using the online alignment program MAFFT (http://mafft.cbrc.jp/alignment/server/). Nucleotide differences from the alignment were illustrated by Mauve 2.3.1[19].For regions showing difference between the two mitogenomes, PCR primers were designed according to flanking sequences in order to verify if the observed difference was true (Table 1). PCR amplification and sequence determination referred to those described above.
Table 1. Primers used in this study
| Locus | Primer names | Primer sequences (5′→3′) | Direction | Annealing temp./℃ | Expected size/bp |
| VG1 a | VG1-F1 | CAATATACTCAGATTCATCAAGCGT | F | 51 | 940 |
| VG1-R1 | TGTGTGAAGGCTGAAGAGATATAA | R | |||
| VG2 a | VG2-F1 | GCTATCCCTAGTACGCAGCT | F | 52 | 1700 |
| VG2-R1 | ACCCGTTGTAATTCCTAGTATACCT | R | |||
| R3937_mt b | 3937mt-F1 | GGCCACCTAAGAACTACCCG | F | 51 | 4402 |
| 3937mt-R1 | TCTTGAAAATAGAGTCCCCAGG | R | |||
| R3937_nr b | 3937nr-F2 | AATAGGCACAAGAGATATAGGACC | F | 51 | 4212 |
| 3937nr-R2 | ATCCGCGCTATAAATACTAGAATTC | R | |||
| R585_mt b | 585mt-F1 | ACCCAAAACTAACCCAGGTC | F | 54-51 | 1053 |
| 585mt-R1 | ACACACATCGCTTGGAAGGC | R | |||
| R585_nr b | 585nr-F1 | TTACCGGAATGTTGCTGCTG | F | 52 | 1551 |
| 585nr-R1 | TTTCATGGGCACTGTCTGAC | R | |||
| R418_mt b | 418mt-F1 | CCAGCTATGTCAGTATTAGCCG | F | 52 | 958 |
| 418mt-R1 | TCAACCTGTACCTGCACCAT | R | |||
| R418_nr b | 418nr-F1 | GTGCCTGAGAAGTGAACGATT | F | 52 | 731 |
| 418nr-R1 | ACTAGCCAACCTCTCTGAGTG | R | |||
| R388_mt b | 388mt-F1 | GACAAGTTTAACCGTTCGCCT | F | 51 | 845 |
| 388mt-R1 | ACTTACCGATTAGTCCACAACAT | R | |||
| R388_nr b | 388nr-F1 | AACTTCAACCTCGGGAGCTT | F | 52 | 1052 |
| 388nr-R1 | ATCGCTGGTGTATTTGGAGC | R | |||
| a These fragments were amplified to verify if there is indeed length variation between ATCC 20868 and ATCC 74030. Refer to Figure 1 for positions of VG1 and VG2. b These fragments were amplified to confirm sequence authenticity of corresponding mitochondrial (mt) and nuclear (nr) fragments generated by blasting the mitogenome against the nuclear genome. Primers anchored at regions flanking the target fragment. For the mitochondrial and nuclear partners of each alignment, different expected sizes were considered when designing primers to facilitate the judging of correct amplification. | |||||
表选项
1.4 Annotation of the mitogenome of ATCC 20868 Since there are sequence errors in the mitogenome of ATCC 74030 published by Youssar et al. (2013), we provided a new annotation of the mitogenome of G.lozoyensis ATCC 20868. The mitogenome of G. lozoyensis ATCC 20868 was first annotated automatically using the MFannot tool (http://megasun.bch.umontreal.ca/cgi-bin/mfannot/mfannotInterface.pl) based on the Mold/Protozoan/ Coelenterate mitochondrial genetic code (i.e., genetic code 4), and then manual corrections were performed. First, the boundaries of rRNA genes (especially for the 5′ and 3′ termini of rnl) were determined by aligning with rRNAs from other Leotiomycetes whose mitogenomes were reported. Second, tRNA genes were predicted by RNAweasel (http://megasun.bch.umontreal.ca/cgi-bin/RNAweasel/RNAweaselInterface.pl), tRNAscan-SE 1.3.1[20], ARAGORN 1.2.36[21], and ARWIN 1.2.3[22]using genetic code 4. A potential tRNA was considered as proven when it was found by at least two of these tools. Third, introns within rRNA genes and protein-coding genes were identified b y aligning with corresponding intronless genes from a related species and for protein-coding genes also by BLASTX searches of remaining sequences after removing putative introns. Fourth, open reading frames (ORFs) within introns and intergenic regions were identified using ORF Finder (http://www. ncbi.nlm.nih.gov), BLASTX searches against NCBI databases, HMM searches against the protein families in the Pfam 28.0 database[23], and ExPASy translation tool (http://web.expasy.org/translate/). Those having no significant similarity to known genes were annotated as hypothetical proteins. Only ORFs > 300 bp were considered.
1.5 Identification of repetitive elements To know if there is intra-genomic duplication of large fragments and to detect interspersed repeats, a local BLASTn search of the whole mitogenome against itself was performed; matches with E value < 10-5were taken into account. These hits were clustered as a function of similarity in CD-HIT Suite[24], and the h-cd-hit-est algorithm was run with three consecutivecut-off values of 1.00, 0.90 and 0.80, where repetitive sequences were first clustered at a higher identity and then non-redundant sequences were further clustered at a lower identity.
In addition, tandem repeats were analyzed by the online program Tandem Repeat Finder (http:// tandem.bu.edu/trf/trf.basic.submit.html) using default parameters. Simple sequence repeats (SSRs) were detected by SSR Finder (http://www.csufresno.edu/ssrfinder/) under default parameters. Repeated sequences were also searched by REPuter (https://bibiserv2.cebitec.uni-bielefeld.de/reputer), which identifies and locates forward (direct), reverse, complement, and palindromic (revere complement) repeats. Default settings of REPuter were used, but we filtered to use only repeats with E value < 10-5.
1.6 Gene transfer between the mitochondrial and nuclear genomes To know if there is gene transfer between mitochondrial and nuclear genomes of the fungus, we performed BLAST searches of the G. lozoyensis ATCC 20868 mitogenome against its nuclear genome (GenBank accession ALVE00000000) with the same strategy as described above. Sequence authenticity of corresponding mitochondrial and nuclear fragments related to some large alignments was assayed by PCR amplification using designed primers (Table 1). Origins of these fragments were deduced by performing online BLAST searches.
1.7 GenBank accession number The mitogenome sequence of G. lozoyensis ATCC 20868 was deposited in GenBank under the accession number KX450332.
2 Results and analyses 2.1 Confirmation of the mitogenome sequence of G. lozoyensis The mitogenome of the wild-type strain ATCC 20868 was successfully assembled as a circular molecule of 45501 bp in length. It was 463 bp longer than the published mitogenome of the mutant strain ATCC 74030, which is 45038 bp in length (GenBank accession no. KF169905). Sequence alignment indicated six variable nucleotide sites and two insertion/deletion (indel) regions between the two mitogenomes (Figure 1). At the first indel region (VG1), ATCC 74030 was 354 bp longer than ATCC 20868; at the second indel region (VG2), ATCC 20868 was 817 bp longer than ATCC 74030. A fragment seemed to jump between the two regions of both strains (Figure 1). PCR assays performed in this study, however, did not find length variation at any of the two indel regions between the two strains (Figure 2). Neither other nucleotide differences were confirmed in this study. Actually, the mitogenomes of ATCC 20868 and ATCC 74030 are quite identical without any nucleotide difference.
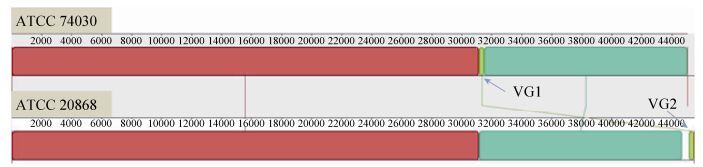 |
| Figure 1. Alignment between the published mitogenome of ATCC 74030 and that of ATCC 20868 assembled in this study. Each locally collinear block was indicated by a unique color. The alignment revealed two major varying regions (VG1 and VG2) plus six variable nucleotide sites (all adjacent to 5′ VG1). The two varying regions showed length variations between the two mitogenomes and involved the jumping of a fragment from one region to another. |
| 图选项 |
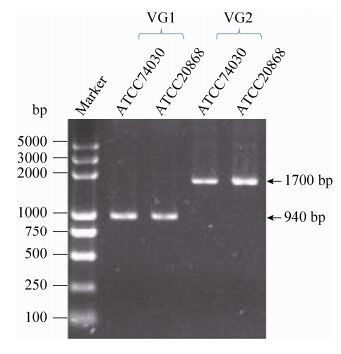 |
| Figure 2. PCR assays of the two varying regions. Primers were designed to amplify the two varying regions observed in Figure 1. Inconsistent to the alignment shown in Figure 1, mitogenomes of ATCC 74030 and 20868 are actually identical without any nucleotide variation. |
| 图选项 |
2.2 Annotation of the mitogenome of G.lozoyensis The mitogenome of G. lozoyensis contained two ribosomal RNAs (rnl and rns), 33 tRNAs, 14 standard proteins of the oxidative phosphorylation system, and five free-standing ORFs (Table 2, Figure 3). The 33 tRNAs coded for all 20 standard amino acids, and three (trnN_2, trnI_2 and trnI_3) contained an intron of 6-23 bp. For the five free-standing ORFs, four (orf291, orf254, orf309 and orf132) are in the forward strand, and one (orf104) is in the reverse strand. Two ORFs, orf132 and orf104, located on opposite strands but overlapped by 248 bp. An rnpB gene (RNase P RNA) was identified at the trnC/nad1 intergenic region.
Table 2. General features of the mitogenome of G. lozoyensis
| Gene a | Start | End | Length/bp | Start codon | Stop codon | Anti-codon | Notes |
| nad4 | 299 | 1783 | 1485 | ATG | TAA | ||
| rns | 2048 | 3844 | 1797 | ||||
| trnY | 4728 | 4812 | 85 | GTA | |||
| trnN_1 | 4894 | 4964 | 71 | GTT | |||
| trnR_1 | 4973 | 5044 | 72 | TCG | |||
| nad6 | 5332 | 5988 | 657 | ATG | TAA | ||
| trnV | 6063 | 6134 | 72 | TAC | |||
| cox3 | 6586 | 8072 | 1487 | GTG | TAA | With an intron | |
| trnK_1 | 8329 | 8400 | 72 | TTT | |||
| trnG_1 | 8483 | 8553 | 71 | TCC | |||
| atp9 | 8790 | 9014 | 225 | ATG | TAA | ||
| trnD | 9065 | 9136 | 72 | GTC | |||
| trnS | 9210 | 9289 | 80 | GCT | |||
| trnW | 9332 | 9402 | 71 | TCA | |||
| trnI_1 | 9558 | 9629 | 72 | GAT | |||
| trnS | 9743 | 9828 | 86 | TGA | |||
| trnP | 9915 | 9987 | 73 | TGG | |||
| rnl | 10095 | 15989 | 5895 | With an intron | |||
| trnT | 16151 | 16221 | 71 | TGT | |||
| trnE | 16225 | 16296 | 72 | TTC | |||
| trnM_1 | 16299 | 16369 | 71 | CAT | |||
| trnM_2 | 16503 | 16575 | 73 | CAT | |||
| trnL | 16617 | 16699 | 83 | TAA | |||
| trnA | 17546 | 17618 | 73 | TGC | |||
| trnF | 17674 | 17746 | 73 | GAA | |||
| trnL | 17751 | 17835 | 85 | TAG | |||
| trnQ | 17866 | 17938 | 73 | TTG | |||
| trnH | 18017 | 18089 | 73 | GTG | |||
| trnM_3 | 18168 | 18240 | 73 | CAT | |||
| orf291 | 18809 | 19684 | 876 | ATG | TAA | ||
| trnR | 20018 | 20088 | 71 | TCT | |||
| trnK_2 | 20163 | 20234 | 72 | TTT | |||
| trnG_2 | 20317 | 20387 | 71 | TCC | |||
| trnN_2 | 20928 | 21021 | 94 | GTT | With an intron | ||
| trnR_2 | 21030 | 21101 | 72 | TCG | |||
| orf254 | 21424 | 22188 | 765 | ATG | TAA | ||
| cob | 22532 | 23698 | 1167 | ATG | TAG | ||
| orf309 | 23781 | 24710 | 930 | ATG | TAG | ||
| orf104 | 26011 | 25697 | 315 | ATG | TAG | At reverse strand | |
| orf132 | 25764 | 26162 | 399 | ATG | TAG | ||
| trnF_1 | 26274 | 26354 | 81 | AAA | |||
| nad4L | 26748 | 27017 | 270 | ATG | TAA | ||
| nad5 | 27017 | 29551 | 2535 | ATG | TAG | ||
| trnF_2 | 29903 | 29981 | 79 | AAA | |||
| cox2_1 | 30683 | 31165 | 483 | TTA | TAA | Incomplete cox2 copy | |
| cox1 | 31455 | 36881 | 5427 | ATG | TAA | With 3 introns | |
| trnI_2 | 37115 | 37193 | 79 | GAT | With an intron | ||
| nad2 | 37195 | 39066 | 1872 | GTG | TAA | ||
| nad3 | 39067 | 39549 | 483 | ATG | TAG | ||
| trnI_3 | 39806 | 39884 | 79 | GAT | With an intron | ||
| atp8 | 40546 | 40692 | 147 | ATG | TAA | ||
| atp6 | 40868 | 41644 | 777 | ATG | TAG | ||
| trnC | 41657 | 41728 | 72 | GCA | |||
| rnpB | 41835 | 42071 | 237 | ||||
| nad1 | 43041 | 44123 | 1083 | ATG | TAA | ||
| cox2_2 | 44661 | 45416 | 756 | ATG | TAA | Complete cox2 copy | |
| aA total 33 tRNA genes are identified and they code for all 20 standard amino acids. Most of them are clustered at regions flanking rnl (6 before rnl and 16 after rnl) and rns (3 after rns). For methionine, three tRNA genes are found with the same anticodon. Three tRNAs are identified for phenylalanine, isoleucine and arginine, but each with two different anticodons. For glycine, lysine and asparagine, there are two tRNA genes with identical anticodons. For leucine and serine, there are two tRNA genes with different anticodons. Three intron-containing tRNAs (trnN_2, trnI_2, and trnI_3) were also identified and they harbored an intron of 6-23 bp. | |||||||
表选项
 |
| Figure 3. Circular map of the G.lozoyensis mitogenome. The mitogenome of G. lozoyensis contained two ribosomal RNAs (rnl and rns), 33 tRNAs, 14 standard proteins of the oxidative phosphorylation system, and five free-standing ORFs. An rnpB gene and an additional incomplete copy of cox2 (cox2_1) were also identified. The precise positions of genes are listed in Table 2. Blue ribbons indicate repetitive elements occurring between different mitochondrial regions. |
| 图选项 |
Five introns were found in ribosomal RNA/protein-encoding genes, including one each in rnl and cox3, and three in cox1 (Table 2). Three others introns (one each in nad2 and nad5, and an additional intron in cox1) identified by Youssar et al. (2013) were not recognized by us. Within the five introns that we identified, the cox3 intron and cox1-i3 harbored no intronic ORFs, but other three introns harbored an intronic ORF encoding for RPS3 (in rnl), a LAGLIDADG homing endonuclease (in cox1-i1), and a GIY-YIG homing endonuclease (in cox1-i2). Degeneration of the intronic ORF in cox1-i2 was obvious because of the detection of frame shifts and multiple stop codon mutations.
Intra-genomic duplication events were detected. For example, partial sequences of orf132/orf104, which located at around 5.7 kb upstream of cox1, showed some similarity (85%, 81/95) to the cox1 gene. Two copies of cox2, one being incomplete (cox2_1, 483 bp) and one being complete (cox2_2, 756 bp) were identified, and they showed a similarity of 98% (467/476). Locations of cox2_1 and cox2_2 corresponded to the two above-mentioned indel regions (VG1 and VG2), and are separated by about 13.5 kb in the mitogenome. In the published mitogenome of ATCC 74030, cox2_1 and cox2_2 erroneously hybridized as one gene. This is also the main sequence difference between the mitogenome reported by Youssar et al. (2013) and that reported in the present study.
2.3 Repetitive sequences in the mitogenome of G. lozoyensis Since we identified intra-genomic duplication events in the above analyses, it is interesting to know if there are other repetitive elements in the G.lozoyensis mitogenome. BLASTn analysis of the whole mitogenome against itself revealed 69 repeat alignments (Figure 3). These repeat alignments showed 76.1%-100% identities at an alignment length of 30-559 bp. Of these repeats, 60 were direct repeats (plus/plus matches), and 9 were inverted repeats (plus/minus matches). The largest direct repeat (a 559 bp alignment) occurred between cox2_1 and cox2_2; the largest inverted repeat (a 111 bp alignment) occurred between the trnG_1/atp9 intergenic region and the orf309/orf104 intergenic region. After clustering analysis (21 repeats left), repeated sequences accounted for about 7.3% (3323 bp) of the G. lozoyensis mitogenome.
In addition, we found 12 tandem repeats in a total of 726 bp, accounting for 1.6% of the mitogenome, ranging from 14 to 43 bp in the period size. Total length of SSRs identified by SSR Finder was 804 bp ( < 1.8% of the mitogenome) with only dimers and trimers detected. REPuter was used to identify 40 forward (in total 2621 bp), one reverse (23 bp) and three palindromic (76 bp) repeats, accounting for 5.9% of the mitogenome and targeting either intergenic or coding regions. No complement repeat was identified by REPuter.
2.4 Gene transfer between the mitochondrial and nuclear genomes of G. lozoyensis BLASTN analyses of the mitogenome of G. lozoyensis ATCC 20868 against its nuclear genome revealed 27 alignments with 75%-100% identities at the lengths of 33-3937 bp (Table 3). These mitochondrial fragments mainly located at either the coding or intergenic regions, and their nuclear partners distributed in 13 of the total 22 scaffolds of the nuclear genome of G.lozoyensis ATCC 20868 (Table 3; Figure 4-A). After clustering analysis, 6399 bp (14.1%) of the mitogenome sequences (21 alignments after de-replication) showed similarities with nuclear genome sequences of G. lozoyensis. Mitochondrial and nuclear fragments related to the four largest alignments were all confirmed by PCR assays (Figure 4-B), excluding the possibility of sequence errors/contaminations. Corresponding nuclear/mitochondrial fragments all had best hits with mitochondrial sequences from fungi as revealed by online BLASTn searches, suggesting their gene transfer from mitochondrion to the nucleus.
Table 3. Local BLAST analysis of the ATCC 20868 mitochondrial genome against its nuclear genome
| Query start | Query end | Subject sequence ID | Subject scaffolds | Subject sequence length/bp | Subject start | Subject end | Subject strand | Alignment length/bp | Percent identity /% | Bit score | E value |
| 44843 | 44899 | KE145353 | GLAREA10 | 2366044 | 500534 | 500478 | minus | 57 | 84.21 | 56.5 | 1.00E-06 |
| 30865 | 30921 | KE145353 | GLAREA10 | 2366044 | 500534 | 500478 | minus | 57 | 82.46 | 51.0 | 5.00E-05 |
| 31220 | 31631 | KE145354 | GLAREA11 | 1492152 | 926036 | 926447 | plus | 418 | 77.99 | 252.0 | 8.00E-66 |
| 43704 | 43742 | KE145355 | GLAREA12 | 1502965 | 1484370 | 1484408 | plus | 39 | 100.00 | 73.1 | 7.00E-12 |
| 35098 | 35157 | KE145357 | GLAREA14 | 2105285 | 1475927 | 1475987 | plus | 61 | 90.16 | 78.7 | 2.00E-13 |
| 37291 | 37340 | KE145360 | GLAREA17 | 1175165 | 481697 | 481648 | minus | 50 | 92.00 | 71.3 | 2.00E-11 |
| 15056 | 15105 | KE145361 | GLAREA18 | 842895 | 774071 | 774120 | plus | 50 | 86.00 | 54.7 | 1.00E-06 |
| 3266 | 3359 | KE145362 | GLAREA19 | 791833 | 63474 | 63567 | plus | 94 | 93.62 | 141.0 | 9.00E-33 |
| 40524 | 44458 | KE145365 | GLAREA21 | 575289 | 245429 | 249364 | plus | 3937 | 91.90 | 5500.0 | 0 |
| 30199 | 30348 | KE145365 | GLAREA21 | 575289 | 249212 | 249361 | plus | 150 | 94.00 | 228.0 | 5.00E-59 |
| 42141 | 42208 | KE145365 | GLAREA21 | 575289 | 247090 | 247155 | plus | 69 | 86.96 | 75.0 | 7.00E-13 |
| 42182 | 42251 | KE145365 | GLAREA21 | 575289 | 247041 | 247112 | plus | 72 | 80.56 | 54.7 | 9.00E-07 |
| 42092 | 42124 | KE145365 | GLAREA21 | 575289 | 247027 | 246995 | minus | 33 | 93.94 | 51.0 | 1.00E-05 |
| 19398 | 19970 | KE145367 | GLAREA3 | 3594336 | 3038249 | 3037670 | minus | 585 | 85.47 | 593.0 | 3.00E-168 |
| 580 | 798 | KE145367 | GLAREA3 | 3594336 | 3037962 | 3037741 | minus | 222 | 90.99 | 296.0 | 9.00E-79 |
| 26760 | 26868 | KE145367 | GLAREA3 | 3594336 | 2890209 | 2890313 | plus | 110 | 86.36 | 115.0 | 3.00E-24 |
| 36109 | 36162 | KE145367 | GLAREA3 | 3594336 | 1410134 | 1410187 | plus | 54 | 92.59 | 78.7 | 3.00E-13 |
| 19971 | 20013 | KE145367 | GLAREA3 | 3594336 | 3037663 | 3037705 | plus | 43 | 93.02 | 63.9 | 9.00E-09 |
| 36432 | 36567 | KE145368 | GLAREA4 | 1824436 | 1206355 | 1206218 | minus | 139 | 79.86 | 99.0 | 1.00E-19 |
| 36608 | 36719 | KE145368 | GLAREA4 | 1824436 | 1206163 | 1206053 | minus | 112 | 75.00 | 51.0 | 4.00E-05 |
| 10829 | 10902 | KE145369 | GLAREA5 | 2448679 | 1459303 | 1459379 | plus | 78 | 84.62 | 73.1 | 1.00E-11 |
| 392 | 461 | KE145369 | GLAREA5 | 2448679 | 1459455 | 1459385 | minus | 71 | 84.51 | 69.4 | 1.00E-10 |
| 17278 | 17647 | KE145372 | GLAREA8 | 1378442 | 472599 | 472986 | plus | 388 | 92.78 | 545.0 | 3.00E-154 |
| 36749 | 36789 | KE145372 | GLAREA8 | 1378442 | 1263988 | 1263948 | minus | 41 | 95.12 | 65.8 | 1.00E-09 |
| 21218 | 21272 | KE145373 | GLAREA9 | 2086076 | 1540686 | 1540632 | minus | 55 | 100.00 | 102.0 | 1.00E-20 |
| 5170 | 5215 | KE145373 | GLAREA9 | 2086076 | 1540677 | 1540632 | minus | 46 | 93.48 | 69.4 | 1.00E-10 |
| 35202 | 35305 | KE145373 | GLAREA9 | 2086076 | 1340852 | 1340750 | minus | 107 | 78.50 | 63.9 | 5.00E-09 |
| The four alignments that are chosen to be confirmed by PCR assays are shown in bold (see Figure 4). | |||||||||||
表选项
 |
| Figure 4. Gene transfer between mitochondrial and nuclear genomes. A: blue ribbons linked regions of the mitogenome (shown in orange) with regions of the nuclear genome (shown in black) with significant similarities. Numbers in the mitogenome indicate positions in kb. The nuclear genome is composed of 22 scaffolds as indicated in numbers (1-22). B: corresponding mitochondrial and nuclear fragments related to the four largest alignments were all confirmed by PCR assays. The DNA ladder is as in Figure 2. |
| 图选项 |
Discussion In this study, we assembled the mitogenome of the pneumocandin-producing fungus G.lozoyensis using a wild-type strain ATCC 20868. We initially suspected that mutagen treatments might have caused mutations in the mitogenome of the mutant strain ATCC 74030. PCR assays and subsequent Sanger sequencing, however, revealed that the mitogenomes of ATCC 20868 and ATCC 74030 were exactly identical without any nucleotide difference. The two chemical mutagens (N-nitroso-N-methylurethane and N-methyl-N-nitro-N-nitrosoguanidine) used for generating ATCC 74030 obviously caused mutations on nuclear DNA, as reported previously[15]. Since only one mutant strain was tested in this study, we cannot tell that the two chemical mutagens cannot mutate the mitogenome of G. lozoyensis until additional mutant strains are examined. Variation of mitochondrial DNA of an organism has been reported on exposure to factors such as arsenic, smoking and chemical mutagens[25-27].
The presence of erroneous sequence in the published mitogenome of ATCC 74030 is reasonable considering that it was assembled directly by combination of data from Illumina mate-pair and pair-end sequencing[17]. The assembly errors mainly resulted from the presence of two copies of cox2. The two cox2 copies erroneously hybridized together in the published mitogenome of ATCC 74030 although they are actually separated by about 13.5 kb (Table 2).In this study, we provided the correct mitogenome of G. lozoyensis using the wild-type strain ATCC 20868.
Due to the presence of sequence errors in the published mitogenome of ATCC 74030, we re-annotated the G. lozoyensis mitogenome using ATCC 20868. One interesting finding is the presence of three tRNAs with introns (i.e., trnN_2, trnI_2, and trnI_3), where the introns all located 1 base 3′ to the anticodon. As much as we know, intron-containing tRNAs have not been reported from mitogenomes of eukaryotes but were seen from archaeal, bacterial and eukaryotic nuclear genomes as well as plant plastid genomes[28-29]. Whether the three intron-containing tRNAs are functional and how their introns are removed during tRNA maturation remain unknown. The second interesting finding is thepresence of a mitochondrial rnpB gene, which encodes the RNA subunit of RNase P (P-RNA), the enzymatically active part of an endonuclease (ribonucleoprotein) responsible for tRNA maturation[30]. The rnpB gene has so far only been recognized in the mitogenomes of a few fungal species[30-32], including the fungus Sclerotinia borealis[33], who belongs to the same order (i.e., Helotiales) as G. lozoyensis. It is interesting to know whether rnpB accounts for the maturation of intron-containing tRNAs found in the mitogenome of G. lozoyensis.
Another major finding is the detection of gene duplication events. On the one hand, some DNA fragment duplications are detected within the mitogenome of G.lozoyensis, such as partial sequences of orf132 or orf104 showing similarity to the cox1 gene, an additional incomplete copy of the cox2 gene, and more than two copies of some tRNA genes (Table 2). The phenomena of DNA duplicationwithin mitogenomes are frequently reported from fungi, especially those species with a large mitogenome[33-34]. On the other hand, evidence for DNA fragment duplications between mitochondrialand nuclear genomes of G. lozoyensis is also obvious (Table 3, Figure 4-A). The natural transfer of DNA from mitochondria to the nucleus that generates nuclear copies of mitochondrialDNA (numts) is an ongoing evolutionary process and is estimated in a wide variety of eukaryotes[35]. Exceptions, however, do exist, such as in the ascomycetous fungus Cordyceps militaris, where no nuclear scaffolds with significant sequence similarities to mitochondrial sequences were found[36].
参考文献
| [1] | Bills GF, Platas G, Peláez F, Masurekar P. Reclassification of a pneumocandin-producing anamorph, Glarea lozoyensis gen. et sp. nov., previously identified as Zalerion arboricola.Mycological Research, 1999, 103(2): 179–192DOI:10.1017/S095375629800687X. |
| [2] | Schwartz RE, Sesin DF, Joshua H, Wilson KE, Kempf AJ, Goklen KA, Kuehner D, Gailliot P, Gleason C, White R, Inamine E, Bills G, Salmon P, Zitano L. Pneumocandins from Zalerion arboricola. I. Discovery and isolation.The Journal of Antibiotics, 1992, 45(12): 1853–1866DOI:10.7164/antibiotics.45.1853. |
| [3] | Schwartz RE, Giacobbe RA, Bland JA, Monaghan RL. L-671, 329, a new antifungal agent. I. Fermentation and isolation.The Journal of Antibiotics, 1989, 42(2): 163–167DOI:10.7164/antibiotics.42.163. |
| [4] | Masurekar PS, Fountoulakis JM, Hallada TC, Sosa MS, Kaplan L. Pneumocandins from Zalerion arboricola. Ⅱ. Modification of product spectrum by mutation and medium manipulation.The Journal of Antibiotics, 1992, 45(12): 1867–1874DOI:10.7164/antibiotics.45.1867. |
| [5] | McCormack PL, Perry CM. Caspofungin:a review of its use in the treatment of fungal infections.Drugs, 2005, 65(14): 2049–2068DOI:10.2165/00003495-200565140-00009. |
| [6] | Leonard Jr WR, Belyk KM, Conlon DA, Bender DR, DiMichele LM, Liu J, Hughes DL. Synthesis of the antifungal β-1, 3-glucan synthase inhibitor CANCIDAS (caspofungin acetate) from pneumocandin B0.Journal of Organic Chemistry, 2007, 72(7): 2335–2343DOI:10.1021/jo062008i. |
| [7] | Peláez F, Collado J, Platas G, Overy DP, Martín J, Vicente F, del Val AG, Basilio A, De la Cruz M, Tormo JR, Fillola A, Arenal F, Villareal M, Rubio V, Baral HO, Galán R, Bills GF. Phylogeny and intercontinental distribution of the pneumocandin-producing anamorphic fungus Glarea lozoyensis.Mycology, 2011, 2(1): 1–17DOI:10.1080/21501203.2010.544334. |
| [8] | Connors N, Petersen L, Hughes R, Saini K, Olewinski R, Salmon P. Residual fructose and osmolality affect the levels of pneumocandins B0 and C0 produced by Glarea lozoyensis.Applied Microbiology and Biotechnology, 2000, 54(6): 814–818DOI:10.1007/s002530000447. |
| [9] | Connors N, Pollard D. Pneumocandin B0 production by fermentation of the fungus Glarea lozoyensis//An ZQ. Handbook of Industrial Mycology.New York: Marcel Dekker, 2004: 515-538. |
| [10] | Petersen LA, Hughes DL, Hughes R, DiMichele L, Salmon P, Connors N. Effects of amino acid and trace element supplementation on pneumocandin production by Glarea lozoyensis:impact on titer, analogue levels, and the identification of new analogues of pneumocandin B0.Journal of Industrial Microbiology and Biotechnology, 2001, 26(4): 216–221DOI:10.1038/sj.jim.7000115. |
| [11] | Lu P, Zhang A, Dennis LM, Dahl-Roshak AM, Xia YQ, Arison B, An Z, Tkacz JS. A gene (pks2) encoding a putative 6-methylsalicylic acid synthase from Glarea lozoyensis.Molecular Genetics and Genomics, 2005, 273(2): 207–216DOI:10.1007/s00438-005-1132-y. |
| [12] | Zhang A, Lu P, Dahl-Roshak AM, Paress PS, Kennedy S, Tkacz JS, An Z. Efficient disruption of a polyketide synthase gene (pks1) required for melanin synthesis through Agrobacterium-mediated transformation of Glarea lozoyensis.Molecular Genetics and Genomics, 2003, 268(5): 645–655. |
| [13] | Chen L, Yue Q, Zhang XY, Xiang MC, Wang CS, Li SJ, Che YS, Ortiz-López FJ, Bills GF, Liu XZ, An ZQ. Genomics-driven discovery of the pneumocandin biosynthetic gene cluster in the fungus Glarea lozoyensis.BMC Genomics, 2013, 14: 339DOI:10.1186/1471-2164-14-339. |
| [14] | Youssar L, Grüning BA, Erxleben A, Günther S, Hüttel W. Genome sequence of the fungus Glarea lozoyensis:the first genome sequence of a species from the Helotiaceae family.Eukaryotic Cell, 2012, 11(2): 250DOI:10.1128/EC.05302-11. |
| [15] | Chen L, Yue Q, Li Y, Niu XM, Xiang MC, Wang WZ, Bills GF, Liu XZ, An ZQ. Engineering of Glarea lozoyensis for exclusive production of the pneumocandin B0 precursor of the antifungal drug caspofungin acetate.Applied and Environmental Microbiology, 2015, 81(5): 1550–1558DOI:10.1128/AEM.03256-14. |
| [16] | Li Y, Chen L, Yue Q, Liu XZ, An ZQ, Bills GF. Genetic manipulation of the pneumocandin biosynthetic pathway for generation of analogues and evaluation of their antifungal activity.ACS Chemical Biology, 2015, 10(7): 1702–1710DOI:10.1021/acschembio.5b00013. |
| [17] | Youssar L, Grüning BA, Günther S, Hüttel W. Characterization and phylogenetic analysis of the mitochondrial genome of Glarea lozoyensis indicates high diversity within the order Helotiales.PLoS One, 2013, 8(9): e74792DOI:10.1371/journal.pone.0074792. |
| [18] | Zhang YJ, Zhang S, Liu XZ, Wen HA, Wang M. A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains.Letters in Applied Microbiology, 2010, 51(1): 114–118. |
| [19] | Darling AE, Mau B, Perna NT. Progressive-mauve:multiple genome alignment with gene gain, loss and rearrangement.PLoS One, 2010, 5(6): e11147DOI:10.1371/journal.pone.0011147. |
| [20] | Lowe TM, Eddy SR. tRNAscan-SE:a program for improved detection of transfer RNA genes in genomic sequence.Nucleic Acids Research, 1997, 25(5): 955–964DOI:10.1093/nar/25.5.0955. |
| [21] | Laslett D, Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences.Nucleic Acids Research, 2004, 32(1): 11–16DOI:10.1093/nar/gkh152. |
| [22] | Laslett D, Canb?ck B. ARWEN:a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences.Bioinformatics, 2008, 24(2): 172–175DOI:10.1093/bioinformatics/btm573. |
| [23] | Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer ELL, Tate J, Punta M. Pfam:the protein families database.Nucleic Acids Research, 2014, 42(D1): D222–230DOI:10.1093/nar/gkt1223. |
| [24] | Huang Y, Niu B, Gao Y, Fu L, Li W. CD-HIT Suite:a web server for clustering and comparing biological sequences.Bioinformatics, 2010, 26(5): 680–682DOI:10.1093/bioinformatics/btq003. |
| [25] | Bodhicharla R, Ryde IT, Prasad GL, Meyer JN. The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces mitochondrial and nuclear DNA damage in Caenorhabditis elegans.Environmental and Molecular Mutagenesis, 2014, 55(1): 43–50DOI:10.1002/em.v55.1. |
| [26] | Liu CS, Chen HW, Lii CK, Tsai CS, Kuo CL, Wei YH. Alterations of plasma antioxidants and mitochondrial DNA mutation in hair follicles of smokers.Environmental and Molecular Mutagenesis, 2002, 40(3): 168–174DOI:10.1002/(ISSN)1098-2280. |
| [27] | Sinha S, Giri AK, Chowdhury R, Ray K. Mitochondrial genome variations among arsenic exposed individuals and potential correlation with apoptotic parameters.Environmental and Molecular Mutagenesis, 2014, 55(1): 70–76DOI:10.1002/em.v55.1. |
| [28] | Fujishima K, Kanai A. tRNA gene diversity in the three domains of life.Frontiers in Genetics, 2014, 5: 142. |
| [29] | Yoshihisa T. Handling tRNA introns, archaeal way and eukaryotic way.Frontiers in Genetics, 2014, 5: 213. |
| [30] | Seif E, Leigh J, Liu Y, Roewer I, Forget L, Lang BF. Comparative mitochondrial genomics in zygomycetes:bacteria-like RNase P RNAs, mobile elements and a close source of the group I intron invasion in angiosperms.Nucleic Acids Research, 2005, 33(2): 734–744DOI:10.1093/nar/gki199. |
| [31] | Aguileta G, de Vienne DM, Ross ON, Hood ME, Giraud T, Petit E, Gabaldon T. High variability of mitochondrial gene order among fungi.Genome Biology and Evolution, 2014, 6(2): 451–465DOI:10.1093/gbe/evu028. |
| [32] | Seif ER, Forget L, Martin NC, Lang BF. Mitochondrial RNase P RNAs in ascomycete fungi:lineage-specific variations in RNA secondary structure.RNA, 2003, 9(9): 1073–1083DOI:10.1261/rna.5880403. |
| [33] | Mardanov AV, Beletsky AV, Kadnikov VV, Ignatov AN, Ravin NV. The 203 kb mitochondrial genome of the phytopathogenic fungus Sclerotinia borealis reveals multiple invasions of introns and genomic duplications.PLoS One, 2014, 9(9): e107536DOI:10.1371/journal.pone.0107536. |
| [34] | Férandon C, Xu JP, Barroso G. The 135 kb mitochondrial genome of Agaricus bisporus is the largest known eukaryotic reservoir of group I introns and plasmid-related sequences.Fungal Genetics and Biology, 2013, 55: 85–91DOI:10.1016/j.fgb.2013.01.009. |
| [35] | Hazkani-Covo E, Zeller RM, Martin W. Molecular poltergeists:mitochondrial DNA copies (numts) in sequenced nuclear genomes.PLoS Genetics, 2010, 6(2): e1000834DOI:10.1371/journal.pgen.1000834. |
| [36] | Zhang YJ, Zhang S, Zhang GZ, Liu XZ, Wang CS, Xu JP. Comparison of mitochondrial genomes provides insights into intron dynamics and evolution in the caterpillar fungus Cordyceps militaris.Fungal Genetics and Biology, 2015, 77: 95–107DOI:10.1016/j.fgb.2015.04.009. |
