2.湖南省灌溉水源水质污染净化技术工程研究中心,长沙 410128
1.College of Resources and Environment, Hunan Agricultural University, Changsha 410128, China
2.Hunan Engineering & Technology Research Center for Irrigation Water Purification, Changsha 410128, China
为了高效处理地下水中的As(Ⅲ),设计了一种流通式电芬顿处理系统,考察了电流密度、pH、曝气速率、流速、电解质浓度以及共存离子等关键因素对As(Ⅲ)去除率的影响。此外,对电芬顿体系中As(III)的去除机理进行了分析,并对该系统在连续运行下的处理效果进行了评估。结果表明:在最佳反应条件下(电流密度为7.6 mA·cm
),地下水中As(Ⅲ)的去除率接近100%,该系统可以在近中性的pH范围内发挥作用;在连续运行条件下,该系统能够保持良好的处理稳定性;在电芬顿反应体系中,·OH和HO
·能够共同促进As(III)的去除。水体中生成的As(Ⅴ)、Ni、Fe等能够在流通式系统中被过滤器有效地拦截,避免了二次污染的发生,污染水体得到净化。以上结果可为流通式电芬顿系统处理含As(Ⅲ)的地下水提供参考。
In order to provide a new solution for the efficient removal of As(Ⅲ) in groundwater, a flow-through electro-Fenton system was proposed in this study. The effects of key factors such as current density, pH, aeration rate, flow rate, electrolyte concentration, and coexisting ions on the removal efficiency of As(Ⅲ) were investigated. In addition, the As(Ⅲ) removal mechanism by the electro-Fenton system was analyzed, and the treatment effect of this system under continuous operation conditions was evaluated. The results showed that the removal efficiency of As(Ⅲ) in groundwater could reach nearly 100% under optimum conditions (current density of 7.6 mA·cm
). This system could have an important performance across a near-neutral pH range, and it could maintain good treatment stability under continuous running conditions. The mechanism studies indicated that ·OH and HO
· could promote As(Ⅲ) removal together by the electro-Fenton system. Moreover, the As(Ⅴ), Ni, Fe generated in this process could be effectively intercepted by the filter in the flow-through system, and the occurrence of secondary pollution was avoided, then the wastewater was purified. The above results could provide references for the efficient treatment of groundwater containing As(Ⅲ) by the flow-through electro-Fenton system.
.
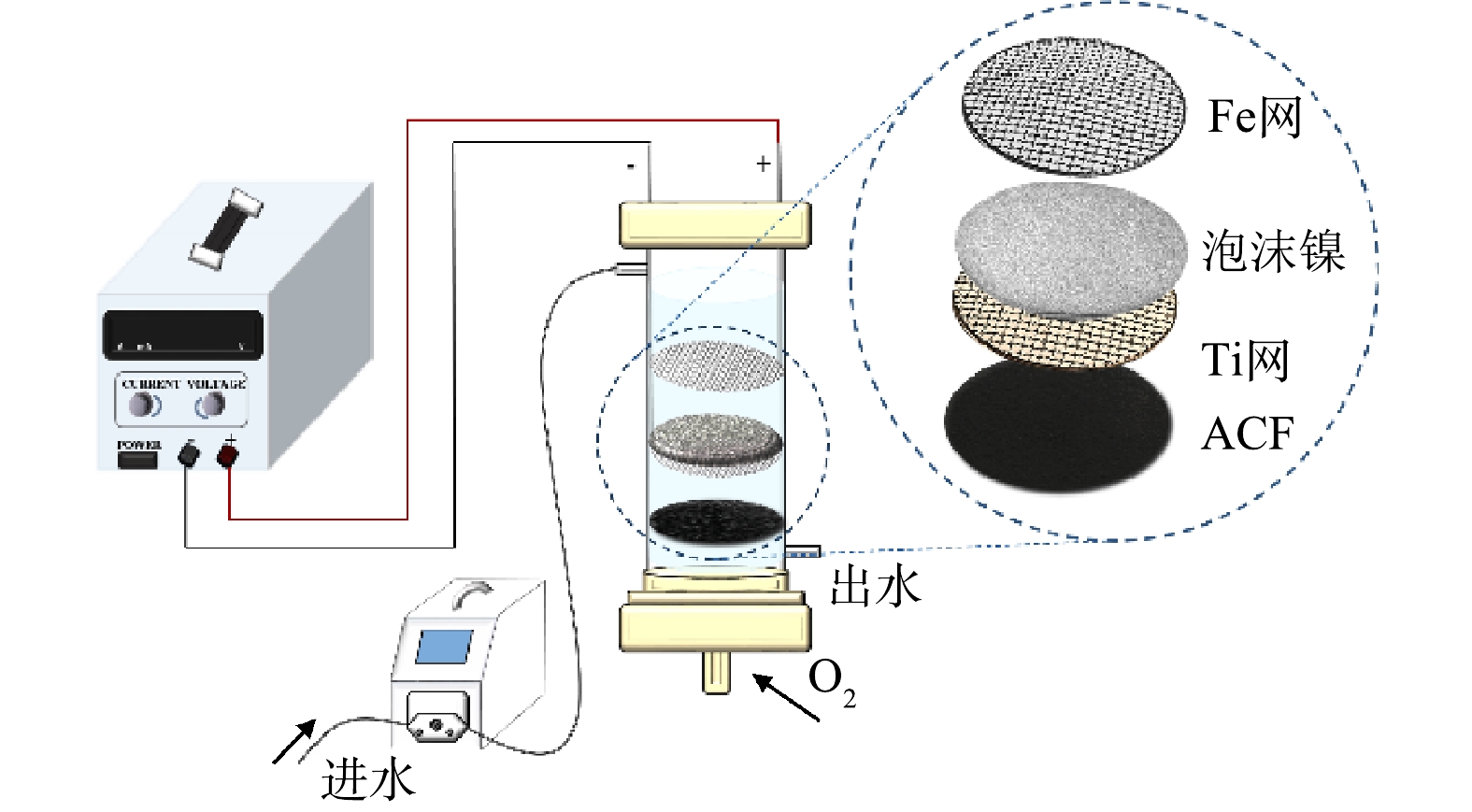
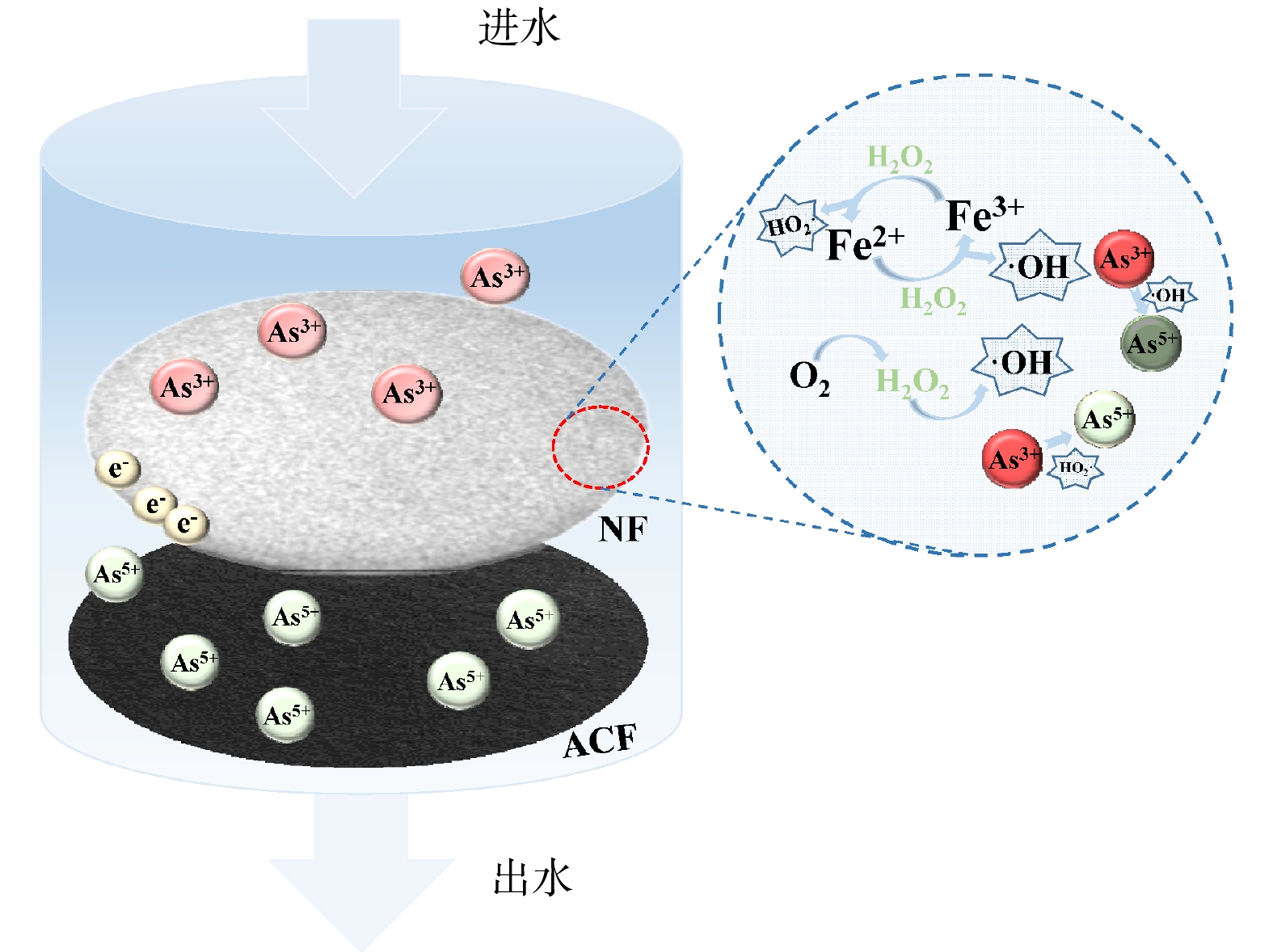
Device diagram of flow-through electro-Fenton reaction
Comparison of various performance of flow-through system
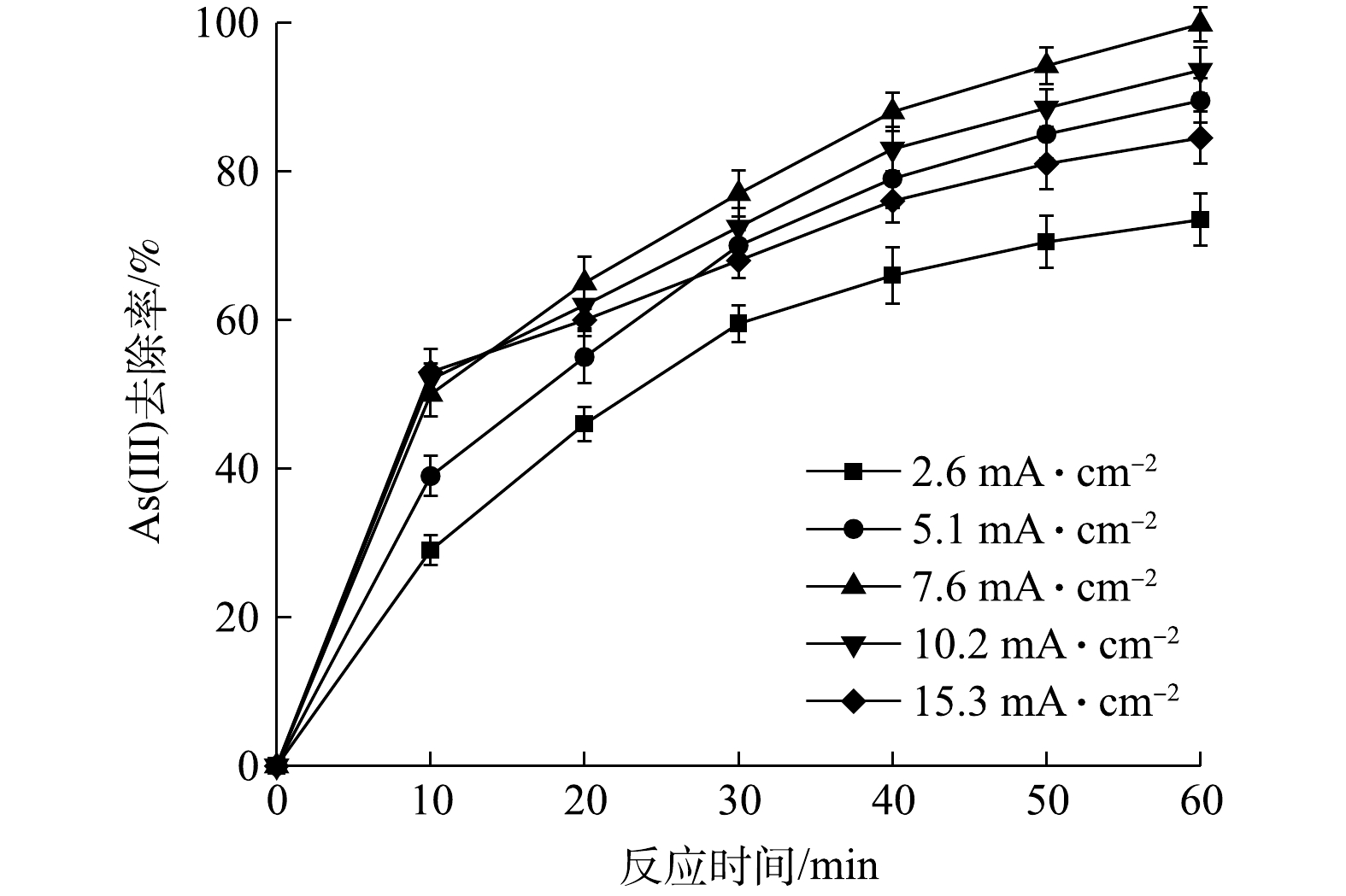
Effect of current density on removal efficiency of As(Ⅲ)
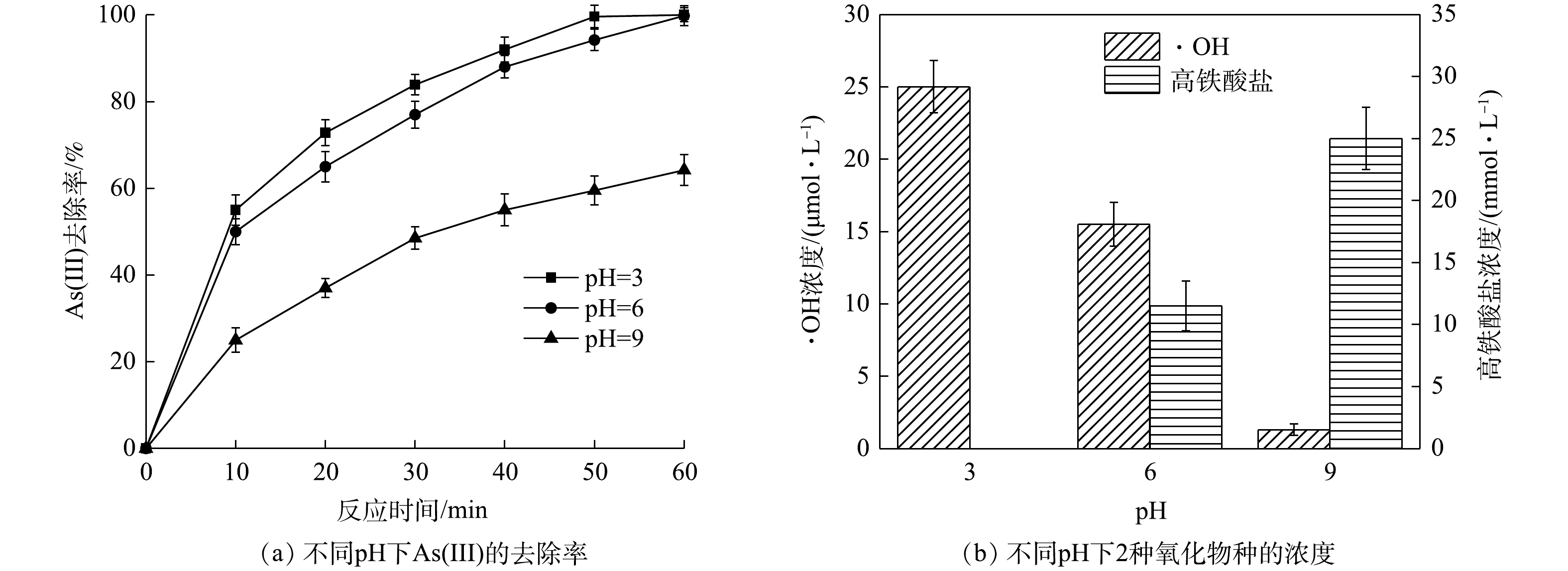
Effect of pH on As(Ⅲ) removal efficiency
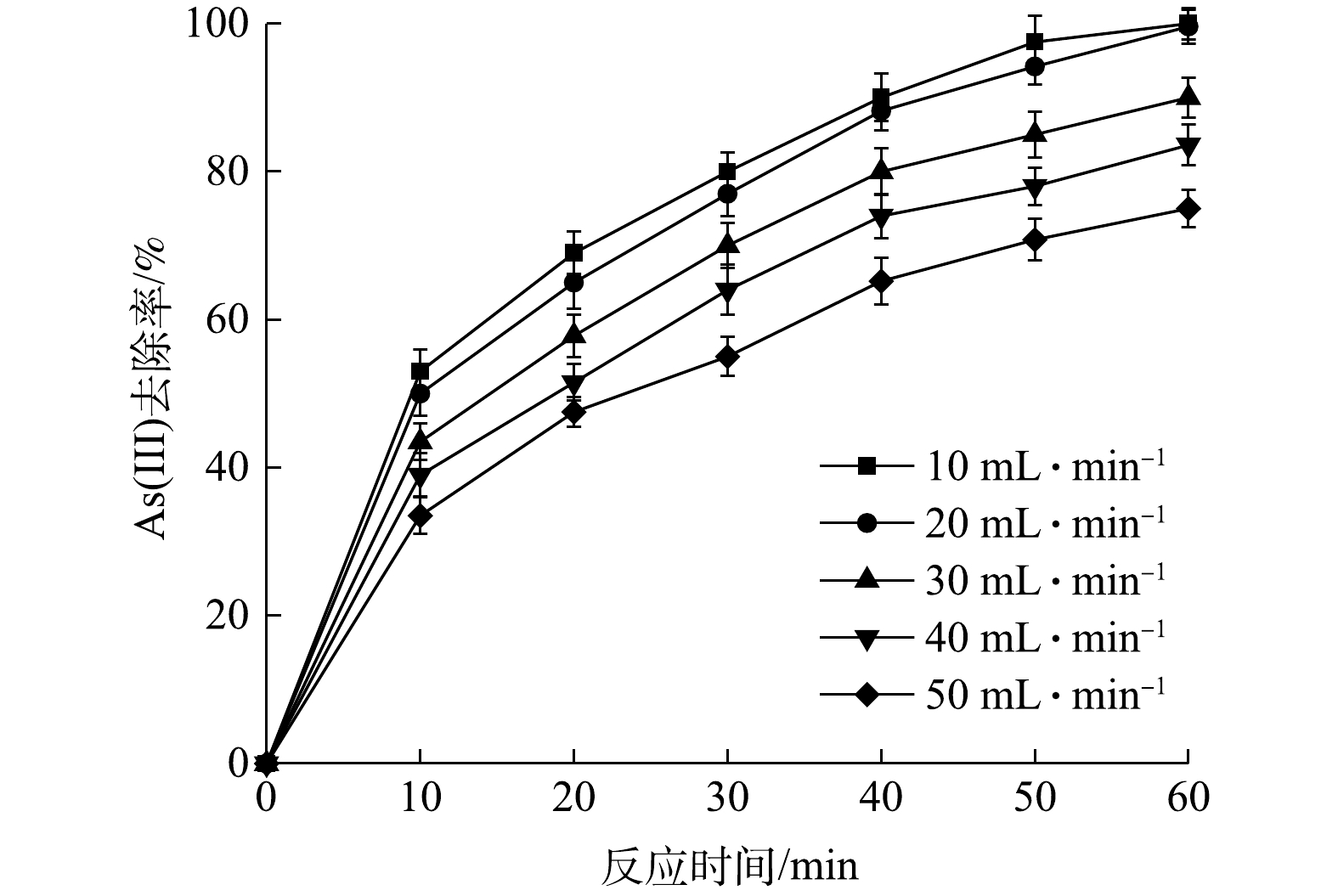
Effect of flow rate on As(Ⅲ) removal efficiency
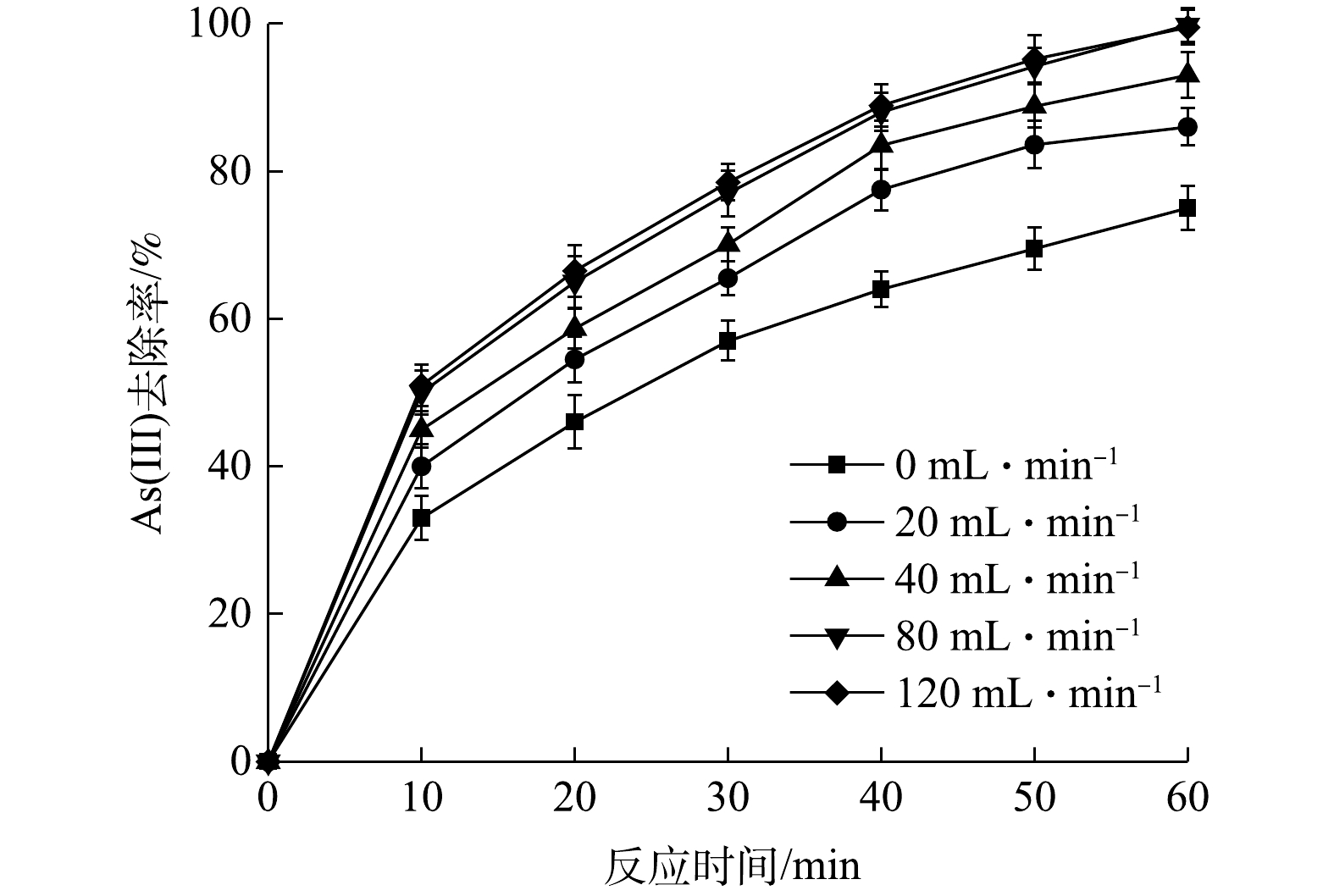
Effect of aeration rate on As(Ⅲ) removal efficiency
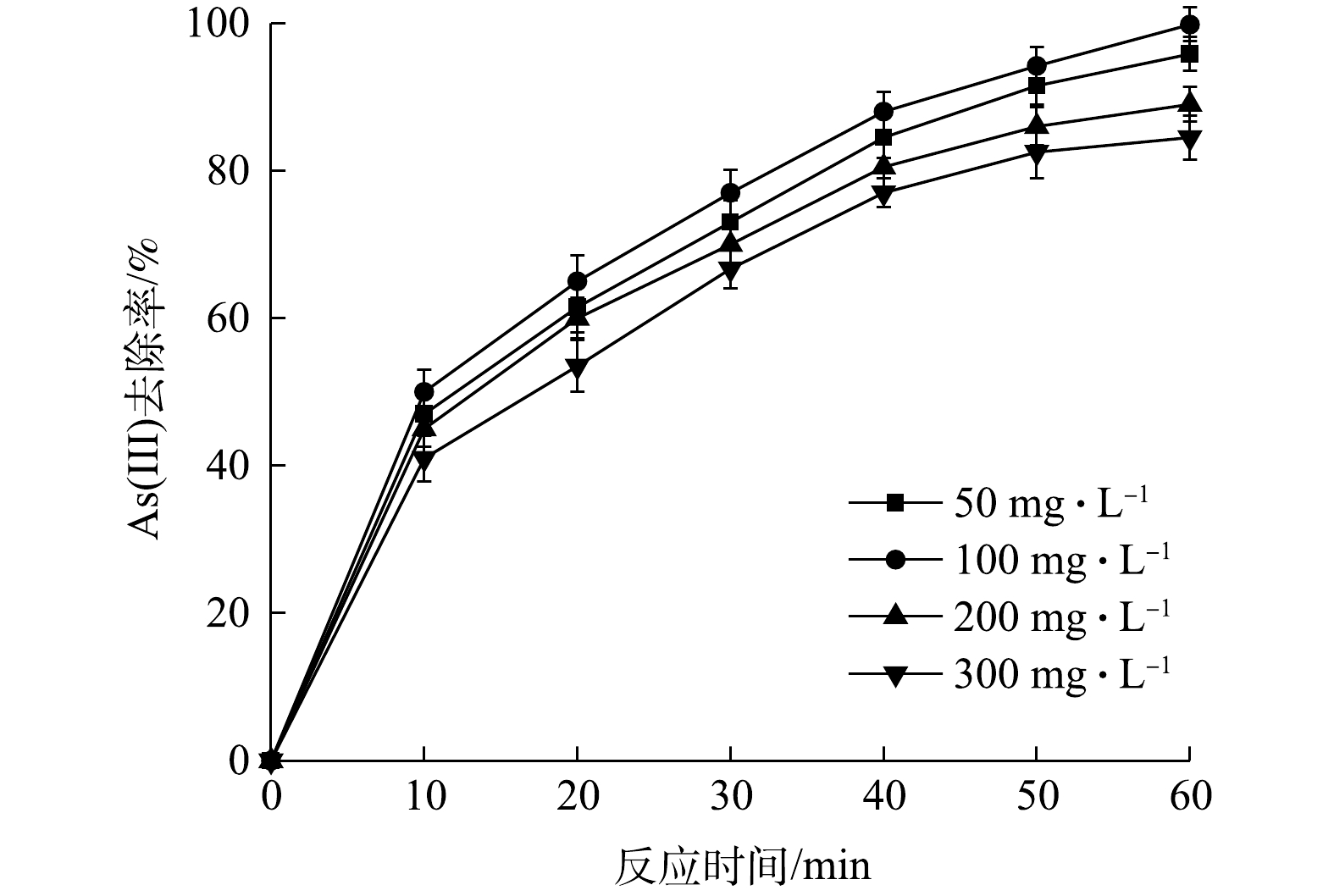
Effect of electrolyte mass concentration on As(Ⅲ) removal efficiency
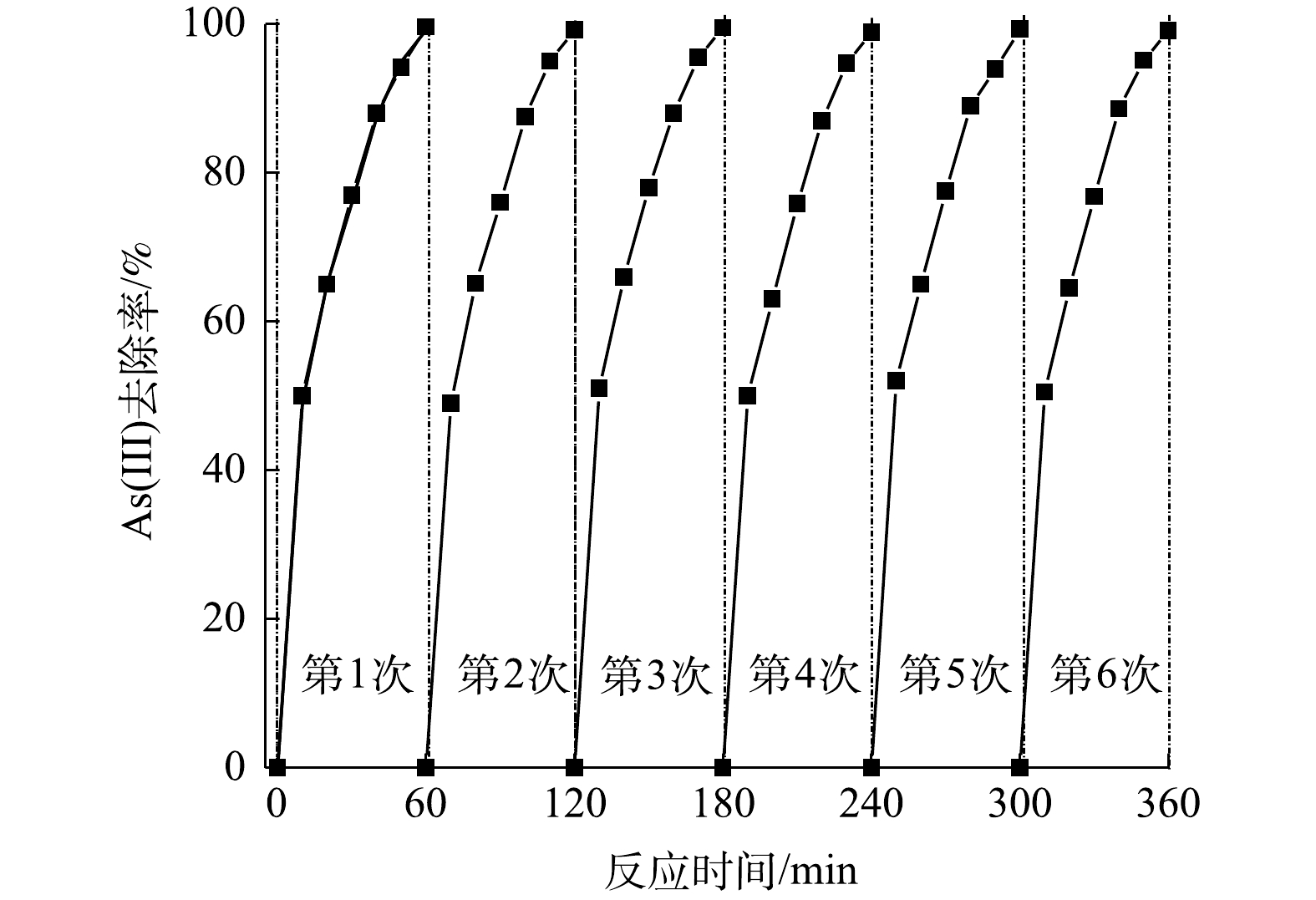
Change of As(Ⅲ) removal efficiency under continuous operation conditions
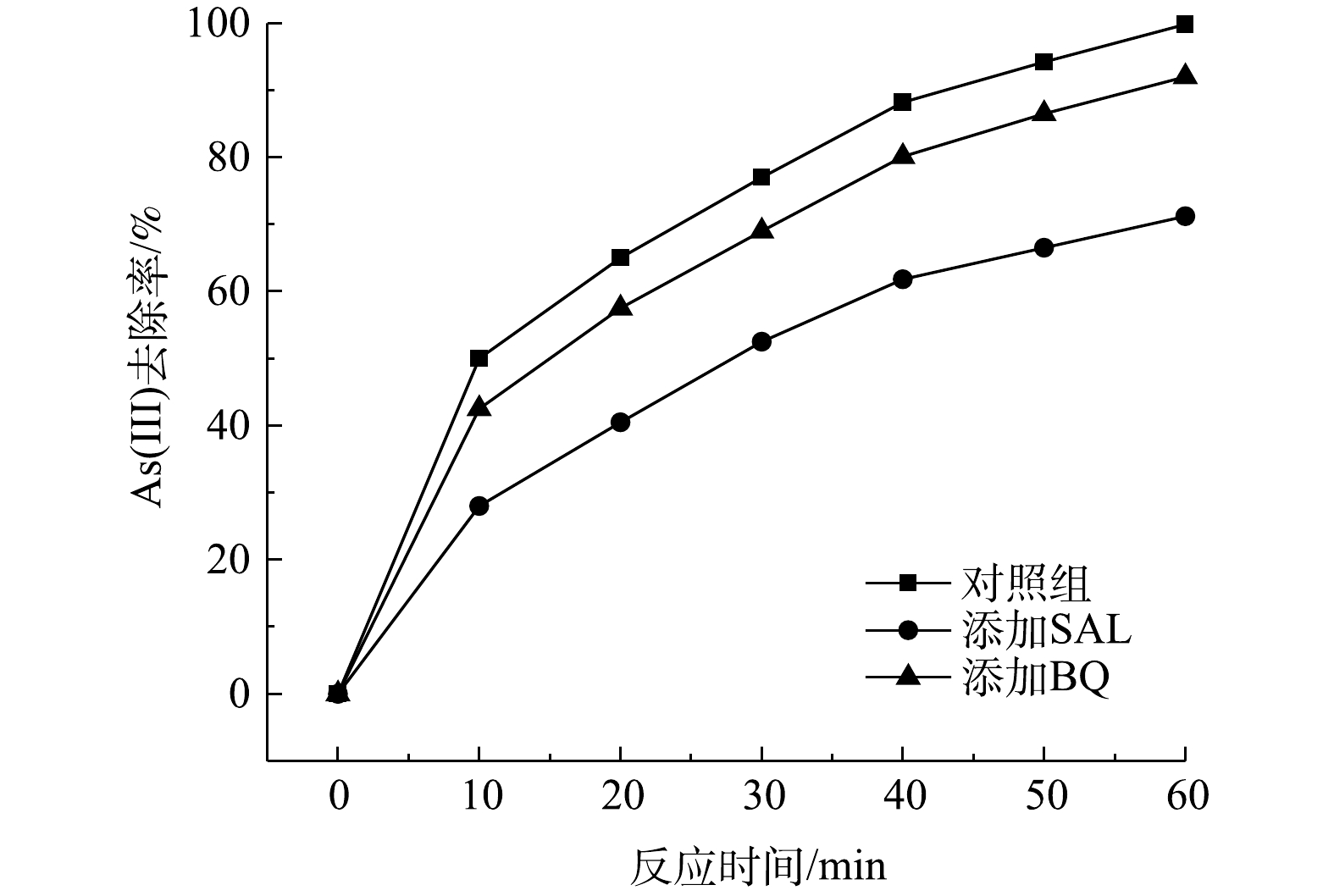
Effect of different capture agents on As(Ⅲ) removal efficiency
Removal mechanism of As(Ⅲ) in electro-Fenton system
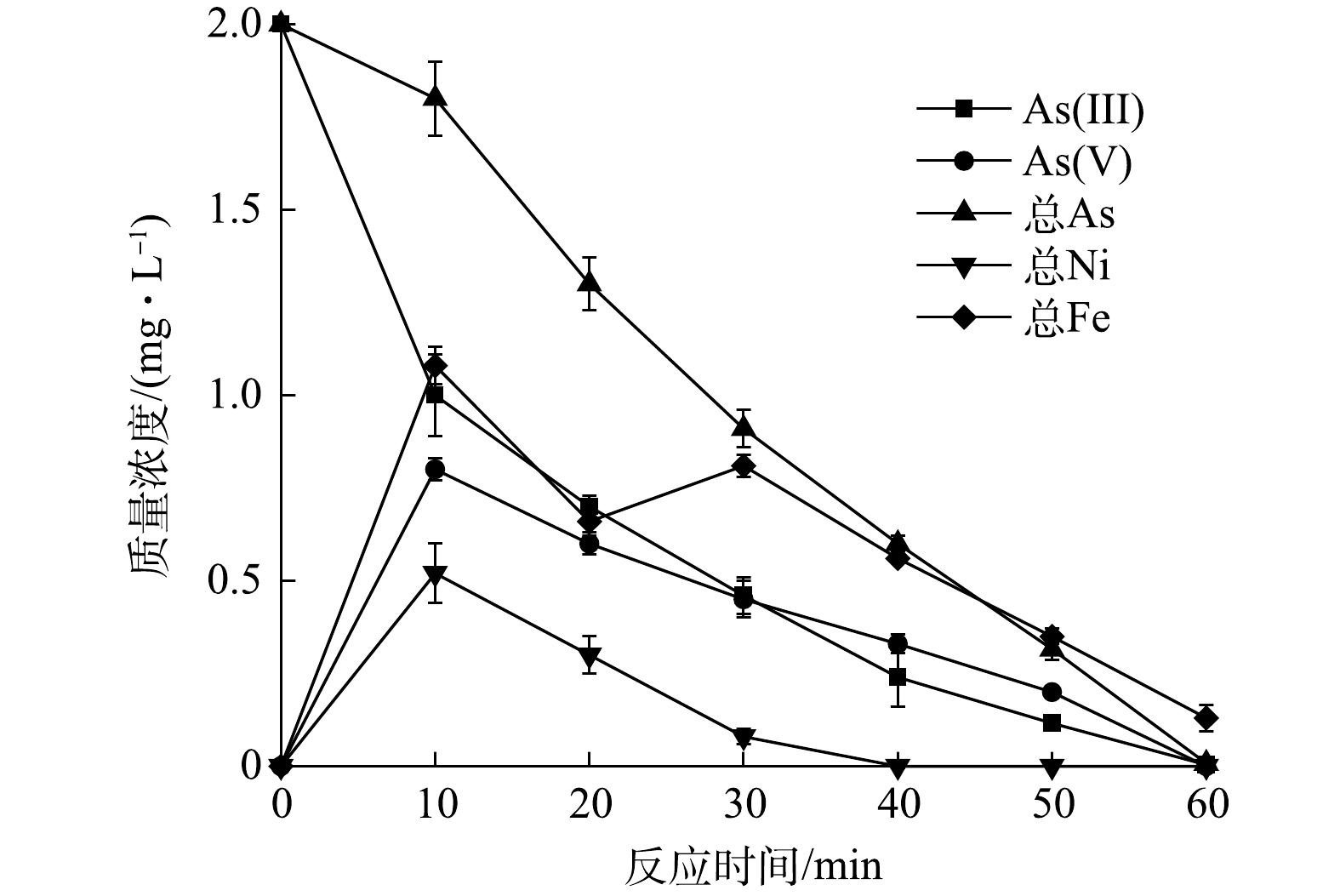
As(Ⅲ)、As(Ⅴ)、As、Ni、Fe的质量浓度变化
Mass concentration changes in As(Ⅲ), As(Ⅴ), As, Ni and Fe
| [1] | CHAKRABORTI D, RAHMAN M M, DAS B, et al. Groundwater arsenic contamination and its health effects in India[J]. Hydrogeology Journal, 2017, 25(4): 1165-1181. doi: 10.1007/s10040-017-1556-6 |
| [2] | GILHOTRA V, DAS L, SHARMA A, et al. Electrocoagulation technology for high strength arsenic wastewater: Process optimization and mechanistic study[J]. Journal of Cleaner Production, 2018, 198: 693-703. doi: 10.1016/j.jclepro.2018.07.023 |
| [3] | UNGUREANU G, SANTOS S, BOAVENTURA R, et al. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption[J]. Journal of Environmental Management, 2015, 151: 326-342. |
| [4] | NEPPOLIAN B, DORONILA A, ASHOKKUMAR M. Sonochemical oxidation of arsenic(III) to arsenic(V) using potassium peroxydisulfate as an oxidizing agent[J]. Water Research, 2010, 44(12): 3687-3695. doi: 10.1016/j.watres.2010.04.003 |
| [5] | 彭映林, 肖斌. 两级中和-铁盐沉淀法处理高砷废水[J]. 工业水处理, 2016, 36(6): 64-68. doi: 10.11894/1005-829x.2016.36(6).016 |
| [6] | CUI J, JING C, CHE D, et al. Groundwater arsenic removal by coagulation using ferric(III) sulfate and polyferric sulfate: A comparative and mechanistic study[J]. Journal Environmental Science (China), 2015, 32: 42-53. doi: 10.1016/j.jes.2014.10.020 |
| [7] | BORA A J, GOGOI S, BARUAH G, et al. Utilization of co-existing iron in arsenic removal from groundwater by oxidation-coagulation at optimized pH[J]. Journal of Environmental Chemical Engineering, 2016, 4(3): 2683-2691. doi: 10.1016/j.jece.2016.05.012 |
| [8] | CAO C Y, QU J, YAN W S, et al. Low-cost synthesis of flowerlike alpha-Fe2O3 nanostructures for heavy metal ion removal: Adsorption property and mechanism[J]. Langmuir, 2012, 28(9): 4573-4579. doi: 10.1021/la300097y |
| [9] | LI W G, GONG X J, WANG K, et al. Adsorption characteristics of arsenic from micro-polluted water by an innovative coal-based mesoporous activated carbon[J]. Bioresource Technology, 2014, 165: 166-173. doi: 10.1016/j.biortech.2014.02.069 |
| [10] | LEE C G, ALVAREZ P J J, NAM A, et al. Arsenic(V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies[J]. Journal of Hazardous Materials, 2017, 325: 223-229. doi: 10.1016/j.jhazmat.2016.12.003 |
| [11] | AN B, LIANG Q, ZHAO D. Removal of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles[J]. Water Research, 2011, 45(5): 1961-1972. doi: 10.1016/j.watres.2011.01.004 |
| [12] | MOHAPATRA B, SARKAR A, JOSHI S, et al. An arsenate-reducing and alkane-metabolizing novel bacterium, Rhizobium arsenicireducens sp. nov., isolated from arsenic-rich groundwater[J]. Archives of Microbiology, 2016, 199(2): 191-201. |
| [13] | HUANG A, TEPLITSKI M, RATHINASABAPATHI B, et al. Characterization of arsenic-resistant bacteria from the rhizosphere of arsenic hyperaccumulator Pteris vittata[J]. Canadian Journal of Microbiology, 2010, 56(3): 236-246. doi: 10.1139/W10-005 |
| [14] | LI L, VAN GENUCHTEN C M, ADDY S E, et al. Modeling As(III) oxidation and removal with iron electrocoagulation in groundwater[J]. Environmental Science & Technology, 2012, 46(21): 12038-12045. |
| [15] | QIAN A, YUAN S, ZHANG P, et al. A new mechanism in electrochemical process for arsenic oxidation: Production of H2O2 from anodic O2 reduction on the cathode under automatically developed alkaline conditions[J]. Environmental Science & Technology, 2015, 49(9): 5689-5696. |
| [16] | GUDE J C J, RIETVELD L C, VAN HALEM D. As(III) oxidation by MnO2 during groundwater treatment[J]. Water Research, 2017, 111: 41-51. doi: 10.1016/j.watres.2016.12.041 |
| [17] | SORLINI S, GIALDINI F. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine[J]. Water Research, 2010, 44(19): 5653-5659. doi: 10.1016/j.watres.2010.06.032 |
| [18] | 吕杰婵, 窦远明, 孙猛, 等. 感应电芬顿降解二甲基砷的效果与机理研究[J]. 环境科学学报, 2017, 37(6): 2152-2157. |
| [19] | LAN H, LI J, SUN M, et al. Efficient conversion of dimethylarsinate into arsenic and its simultaneous adsorption removal over FeCx/N-doped carbon fiber composite in an electro-Fenton process[J]. Water Research, 2016, 100: 57-64. doi: 10.1016/j.watres.2016.05.018 |
| [20] | ZHANG A Y, HUANG N H, ZHANG C, et al. Heterogeneous Fenton decontamination of organoarsenicals and simultaneous adsorption of released arsenic with reduced secondary pollution[J]. Chemical Engineering Journal, 2018, 344: 1-11. doi: 10.1016/j.cej.2018.03.072 |
| [21] | 张煜, 李明玉, 李善得, 等. 直接分光光度法测定高铁酸盐的含量[J]. 无机盐工业, 2011, 43(2): 59-62. doi: 10.3969/j.issn.1006-4990.2011.02.020 |
| [22] | ZHU R, YANG C, ZHOU M, et al. Industrial park wastewater deeply treated and reused by a novel electrochemical oxidation reactor[J]. Chemical Engineering Journal, 2015, 260: 427-433. doi: 10.1016/j.cej.2014.09.029 |
| [23] | REN G, ZHOU M, LIU M, et al. A novel vertical-flow electro-Fenton reactor for organic wastewater treatment[J]. Chemical Engineering Journal, 2016, 298: 55-67. doi: 10.1016/j.cej.2016.04.011 |
| [24] | ?ZCAN A, ATILIR ?ZCAN A, DEMIRCI Y. Evaluation of mineralization kinetics and pathway of norfloxacin removal from water by electro-Fenton treatment[J]. Chemical Engineering Journal, 2016, 304: 518-526. doi: 10.1016/j.cej.2016.06.105 |
| [25] | ZHOU W, RAJIC L, CHEN L, et al. Activated carbon as effective cathode material in iron-free electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption[J]. Electrochim Acta, 2019, 296: 317-326. doi: 10.1016/j.electacta.2018.11.052 |
| [26] | GAO G, ZHANG Q, HAO Z, et al. Carbon nanotube membrane stack for flow-through sequential regenerative electro-Fenton[J]. Environmental Science & Technology, 2015, 49(4): 2375-2383. |
| [27] | LI Z, SHEN C, LIU Y, et al. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton[J]. Applied Catalysis B: Environmental, 2020, 260: 118204. |
| [28] | MCBEATH S T, GRAHAM N J D. Simultaneous electrochemical oxidation and ferrate generation for the treatment of atrazine: A novel process for water treatment applications[J]. Journal of Hazardous Materials, 2021, 411: 125167. doi: 10.1016/j.jhazmat.2021.125167 |
| [29] | HE H, ZHOU Z. Electro-Fenton process for water and wastewater treatment[J]. Critical Reviews in Environmental Science & Technology, 2017, 47(21): 2100-2131. |
| [30] | GUAN W, ZHANG B, TIAN S, et al. The synergism between electro-Fenton and electrocoagulation process to remove Cu-EDTA[J]. Applied Catalysis B: Environmental, 2018, 227: 252-257. doi: 10.1016/j.apcatb.2017.12.036 |
| [31] | ZHANG Y, ZUO S, ZHOU M, et al. Removal of tetracycline by coupling of flow-through electro-Fenton and in-situ regenerative active carbon felt adsorption[J]. Chemical Engineering Journal, 2018, 335: 685-692. doi: 10.1016/j.cej.2017.11.012 |
| [32] | SANTANA-MARTíNEZ G, ROA-MORALES G, MARTIN DEL CAMPO E, et al. Electro-Fenton and electro-Fenton-like with in situ electrogeneration of H2O2 and catalyst applied to 4-chlorophenol mineralization[J]. Electrochimica Acta, 2016, 195: 246-256. doi: 10.1016/j.electacta.2016.02.093 |
| [33] | ZHANG H, WAN X, LI G, et al. A Three-electrode electro-Fenton system supplied by self-generated oxygen with automatic pH-regulation for groundwater remediation[J]. Electrochimica Acta, 2017, 250: 42-48. doi: 10.1016/j.electacta.2017.08.040 |
| [34] | LI X, JIN X, ZHAO N, et al. Novel bio-electro-Fenton technology for azo dye wastewater treatment using microbial reverse-electrodialysis electrolysis cell[J]. Bioresource Technology, 2017, 228: 322-329. doi: 10.1016/j.biortech.2016.12.114 |
| [35] | BOCOS E, GONZáLEZ-ROMERO E, PAZOS M, et al. Application of electro-Fenton treatment for the elimination of 1-Butyl-3-methylimidazolium triflate from polluted water[J]. Chemical Engineering Journal, 2017, 318: 19-28. doi: 10.1016/j.cej.2016.04.058 |
| [36] | KHANDEGAR V, SAROHA A K. Electrocoagulation for the treatment of textile industry effluent: A review[J]. Journal of Environmental Management, 2013, 128: 949-693. doi: 10.1016/j.jenvman.2013.06.043 |
| [37] | YOOSEFIAN M, AHMADZADEH S, AGHASI M, et al. Optimization of electrocoagulation process for efficient removal of ciprofloxacin antibiotic using iron electrode; kinetic and isotherm studies of adsorption[J]. Journal of Molecular Liquids, 2017, 225: 544-553. doi: 10.1016/j.molliq.2016.11.093 |
| [38] | AHMADZADEH S, DOLATABADI M. Removal of acetaminophen from hospital wastewater using electro-Fenton process[J]. Environmental Earth Sciences, 2018, 77(2): 1-11. |
| [39] | LIU Y, ZHANG J, LIU F, et al. Ultra-rapid detoxification of Sb(III) using a flow-through electro-Fenton system[J]. Chemosphere, 2019, 245: 125604. |
| [40] | 汤茜, 孙娟, 任小蕾, 等. 泡沫镍和泡沫铜阴极电类Fenton氧化降解对硝基酚的比较[J]. 化工进展, 2017, 36(7): 2653-2659. |
| [41] | DENG F, OLVERA-VARGAS H, GARCIA-RODRIGUEZ O, et al. The synergistic effect of nickel-iron-foam and tripolyphosphate for enhancing the electro-Fenton process at circum-neutral pH[J]. Chemosphere, 2018, 201: 687-696. doi: 10.1016/j.chemosphere.2018.02.186 |
| [42] | PRAMOD L, GANDHIMATHI R, LAVANYA A, et al. Heterogeneous Fenton process coupled with microfiltration for the treatment of water with higher arsenic content[J]. Chemical Engineering Communications, 2019, 207(12): 1-12. |





 下载:
下载: 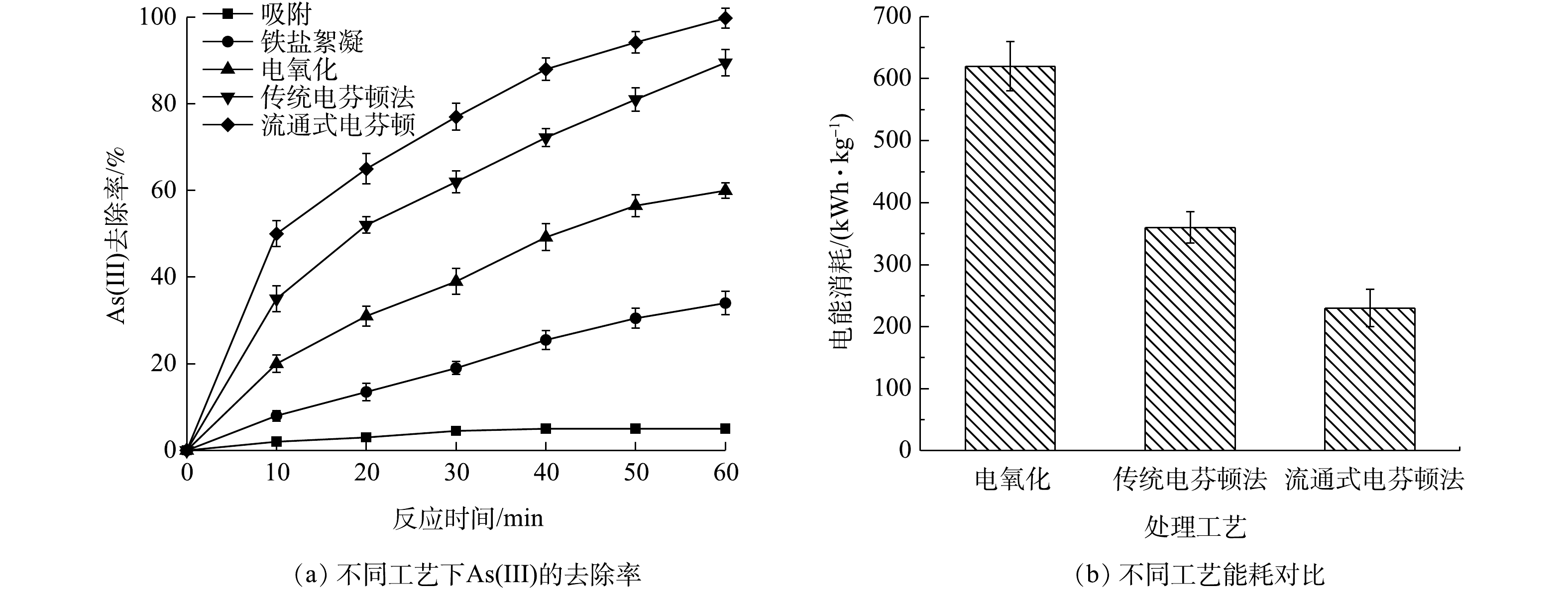
 点击查看大图
点击查看大图