全文HTML
--> --> --> 近年来,随着我国医疗、畜禽和水产养殖等行业快速发展,抗生素的用量日益增加。磺胺甲噁唑(sulfamethoxazole, SMX)作为一种人工合成的广谱抗菌药物,频繁被用作渔药、兽药等大量排入环境水体中[1-3]。有关磺胺甲噁唑在不同水体环境中对不同对象造成的影响已有相关报道[4-6]。长期摄入SMX会损伤机体免疫力、影响动物发育、对地表水和地下水产生不可修复的损坏,严重破坏生态环境。有研究表明,在废水处理中,传统的水处理技术如臭氧法[7]、离子交换法[8]对SMX的去除效果并不理想,光催化降解、生物降解等处理方法尽管已被证实能够有效去除SMX,但其价格昂贵且会生成中间产物[9-10]。吸附法是一种成熟的水处理方法,因吸附剂具有简单高效、可以重复利用等特点,故其应用较为广泛,被认为是能有效去除SMX的方法之一[11-12]。常见的吸附剂有活性炭、石墨烯、碳纳米管等,对污染物具有良好的吸附去除效果[13-14]。NAM等[15]用经过超声处理后的氧化石墨烯吸附磺胺甲噁唑,去除率约为30%。MOUNI等[16]用硫酸氧化改性杏壳活性炭吸附水溶液中Pb2+,最大吸附量为21.38 mg·g?1。但吸附剂的经济性、环保性是影响其广泛应用的首要条件,因此,将廉价易得的生物质制备成生物炭材料近年来广受关注。
松针作为生物炭材料的一种,具有四季均可采收、高产出量的特点,将废弃的松针用于磺胺甲恶唑的去除,既为水处理技术提供了一种新思路,又完成了废弃松针的资源化转化。AHMAD等[17]利用在300、500、700 ℃下制备得到的松针生物炭吸附三氯乙烯,结果表明,700 ℃制备得到的松针生物炭表面疏水性高、表面积大、有利于吸附,其对三氯乙烯的去除效果最好。改性会使生物炭的孔隙结构、比表面积等发生改变,合适的改性方法对废水中污染物的去除有实际应用意义。改性方法有许多,包括金属负载改性和氧化改性等[18]。常用的氧化剂有盐酸、硝酸、氢氧化钠、过氧化氢等。ZHANG等[19]用磷酸盐改性后的竹炭生物炭,其吸附Cd(Ⅱ)能力较原始竹炭提高近10倍,对Cd(Ⅱ)的去除率可达85.78%。张江等[20]使用海藻酸钠和氯化铁溶液制备改性石墨烯-生物炭复合材料吸附磺胺嘧啶,平衡时吸附量在20 mg·kg?1以上。本研究以常见的松针为原料,利用盐酸制备出松针生物炭,吸附去除水体中的磺胺甲噁唑,通过FT-IR、SEM、BET对其进行了微观表征,分别考察了PBC投加量、pH、阴离子等对SMX去除效果的影响,并采用吸附等温模型和吸附动力学模型进行分析研究,从而探讨了其吸附机制,为生物炭的实际应用提供参考。
1.1. 实验原料
松针收集于苏州某公园内,磺胺甲噁唑(C10H11N3O3S)购于Sigma-Aldrich,盐酸(HCl)、硫酸(H2SO4)、氢氧化钠(NaOH)均为分析纯,实验中用水为超纯水。1.2. 制备方法
将松针剪至3 cm左右,在已有研究[21]的基础上改进后,用超纯水将表面杂质洗净,至上清液出水澄清,在100 ℃烘箱中干燥后取出备用。将干燥后的松针经10% HCl浸泡12 h进行改性处理,并将其超声清洗15 min,再以超纯水反复清洗至中性,再将干燥的松针置于恒温鼓风烘箱中,200 ℃下预氧化2 h,最后于箱式气氛炉中700 ℃条件下活化60 min,活化时通入氮气加以保护。冷却至室温后,将其取出并研磨,筛选>100目粒径,将其密封保存,记为PBC。1.3. 实验方法
在进行吸附实验时,室温下,向100 mL浓度为0.04 mmol·L?1的SMX溶液中迅速加入0.04 g PBC,在磁力搅拌器上启动反应,并记为反应开始时间,在预定时间快速取样,经0.22 μm滤膜过滤。过滤液用高效液相色谱仪(HPLC)测定其浓度。采用NaOH和H2SO4调节溶液初始pH。在进行重复利用实验时,准确称取0.2 g PBC,加入浓度为0.2 mmol·L?1的SMX溶液中,实验结束后过滤材料,烘干称重。根据实验后PBC剩余量,按照PBC与SMX质量比4∶1确定下一次实验中SMX浓度,重复吸附实验,实验共进行5次。
1.4. 分析方法
SMX浓度利用美国Agilent公司1260型高效液相色谱仪测定。流动相为乙酸(1‰)和甲醇,具体配比为60∶40,流速为1.0 mL·min?1,色谱柱为C18柱(4.6 mm×250 mm,5 μm),检测波长为225 nm,进样量为40 μL。采用美国FEI Quanta 250扫描电子显微镜(SEM)测定材料表面形貌特征;用美国Micromeritics ASAP2020全自动比表面积测定仪(BET)分析材料比表面积及孔隙结构;采用美国Thermo公司Nicolet 6700型傅里叶变换红外光谱仪(FT-IR)测定PBC表面活性官能团。2.1. 松针生物炭的表征
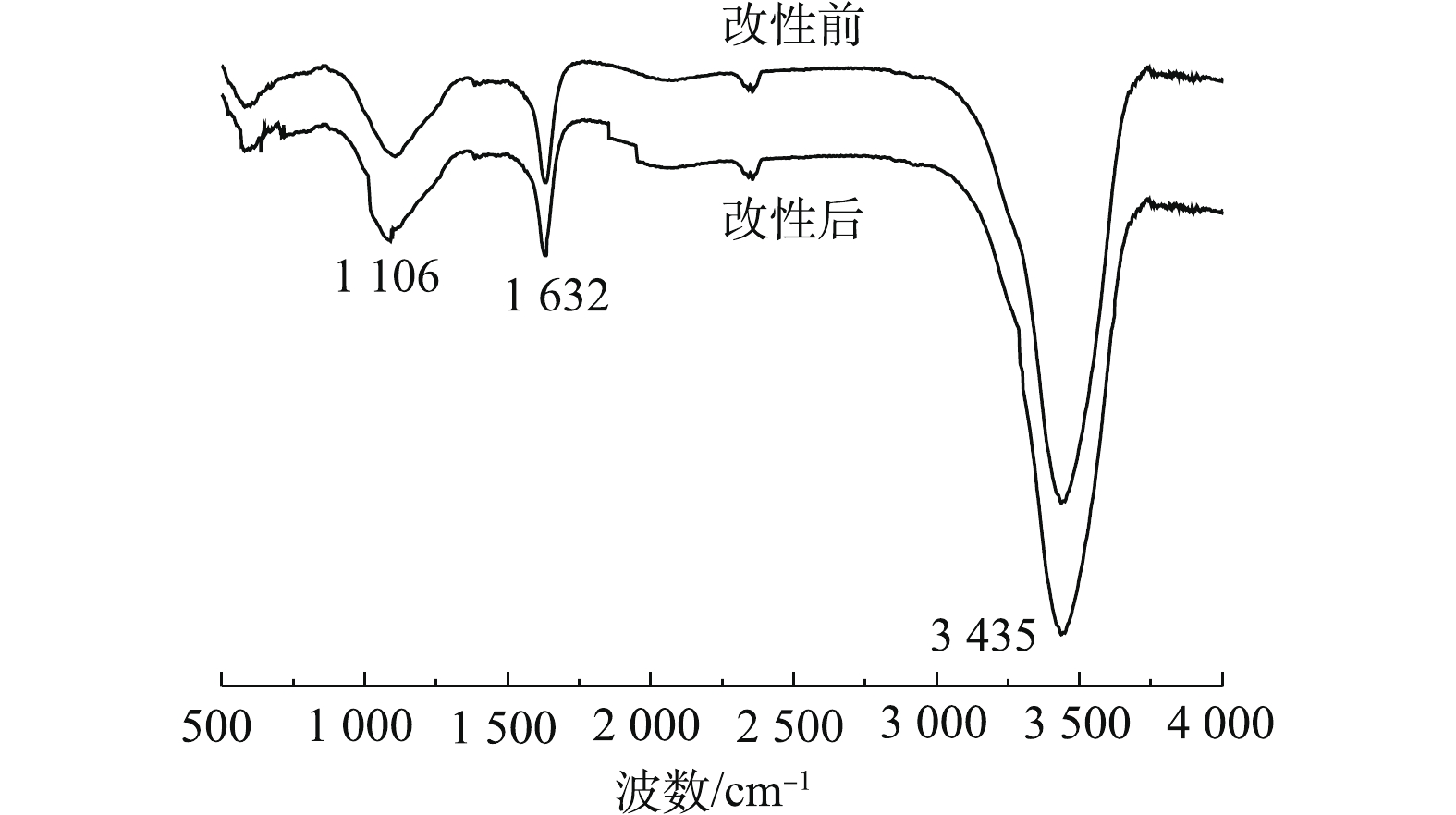
1)傅里叶红外光谱(FT-IR)分析。PBC改性前后的红外光谱如图1所示。可以看出,PBC在改性前后特征吸收峰的位置基本相同。在3 435 cm?1处的吸收峰为O—H的伸缩振动吸收峰;位于1 632 cm?1处的吸收峰可归于羧基的碳氧双键(C=O)产生的;在1 106 cm?1处为C—O对称伸缩振动峰。改性后在3 435、1 632和1 106 cm?1处吸收峰强度均有所增强,这说明改性有利于使PBC表面活性官能团含量增多。由此可见,PBC中主要含有羧基和羟基等含氧官能团,可以与SMX中的苯环电子形成氢键[22],有利于吸附的发生。2)扫描电镜(SEM)分析。图2(a)和图2(b)分别为PBC改性前后的SEM图。由图2(a)可见,改性前的松针生物炭表面光滑,无明显孔隙结构;改性后的松针生物炭表面更加疏松、凹凸不平(图2(b)),颗粒物破碎程度加深,孔隙结构更加复杂,这些特征均有利于吸附反应的进行。
3)比表面积(BET)分析。比表面积和孔径结构测定结果见表1。由表1可知,PBC的比表面积在吸附前为391.0 m2·g?1,吸附后较吸附前降低了30.1%,PBC吸附后的微孔体积及平均孔深较吸附前也均有所降低。原因可能是吸附后的SMX附着在PBC表面,占据了PBC的吸附点位,堵塞了PBC的一些微孔。
2.2. 吸附剂投加量对SMX去除效果的影响
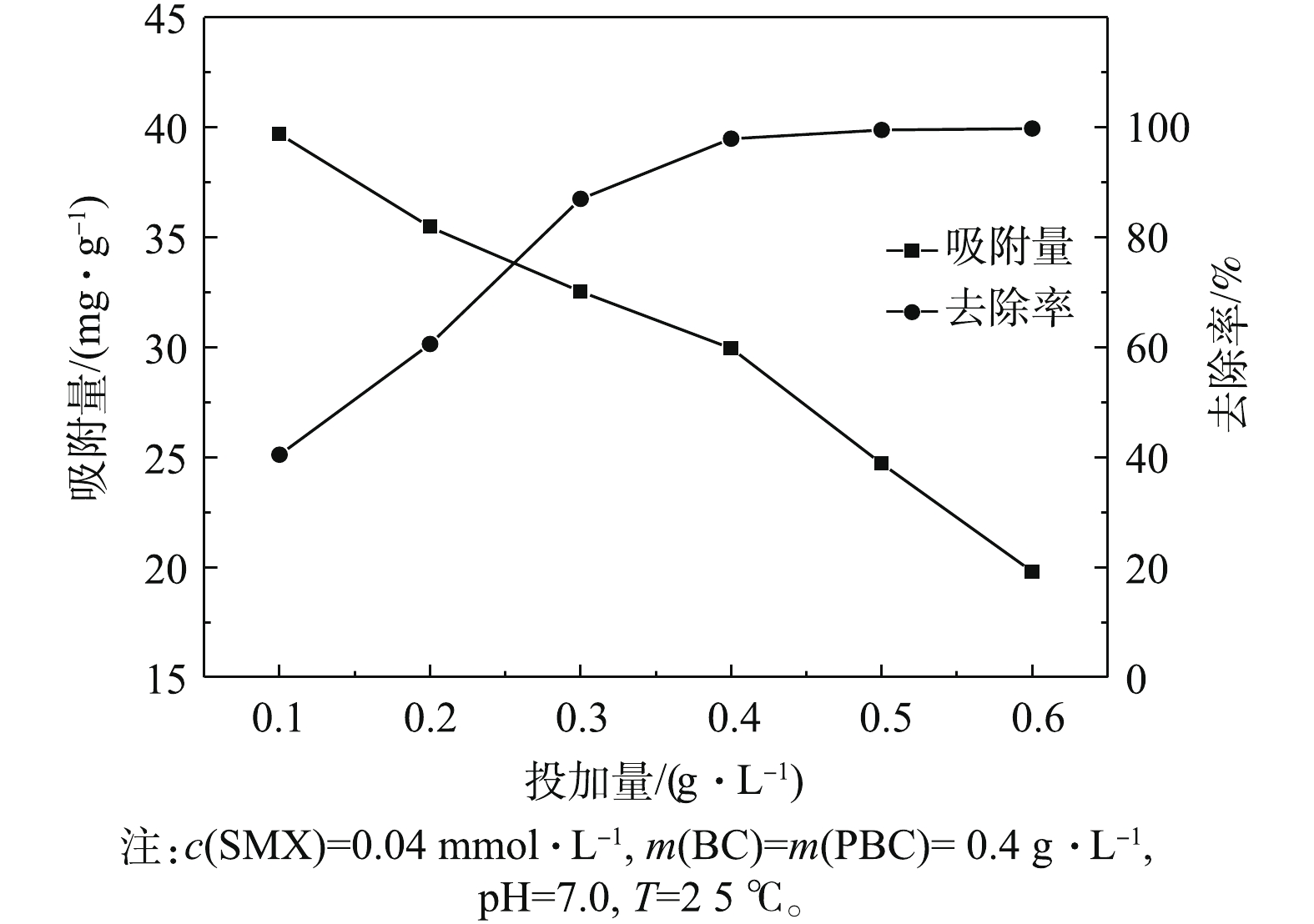
图3为PBC投加量对SMX去除效果的影响结果。分别选取0.1、0.2、0.3、0.4、0.5、0.6 g·L?1 PBC,反应达平衡后测得SMX平衡吸附量及SMX去除率。结果表明,在不同投加量条件下,PBC对SMX的去除率有所不同。随着PBC投加量的增加,对SMX的去除率呈先显著增大后逐渐趋于平衡的趋势。当投加量为0.5 g·L?1时,去除率可达到99.5%;当投加量继续增加到0.6 g·L?1时,去除率可高达100%,但在吸附过程中单位吸附量逐渐减少。其原因可能是,提高PBC投加量可降低吸附剂和吸附质之间的浓度梯度[23]。在PBC投加量较低时,PBC表面的吸附点位得到充分利用,PBC对SMX有较高的吸附量。随着投加量的增加,大量的吸附剂使SMX迅速吸附到PBC表面,导致溶液周边的SMX浓度降低,单位吸附容量下降。因此,选择0.4 g·L?1作为PBC最适投加量进行后续的实验。2.3. 初始pH对SMX去除效果的影响
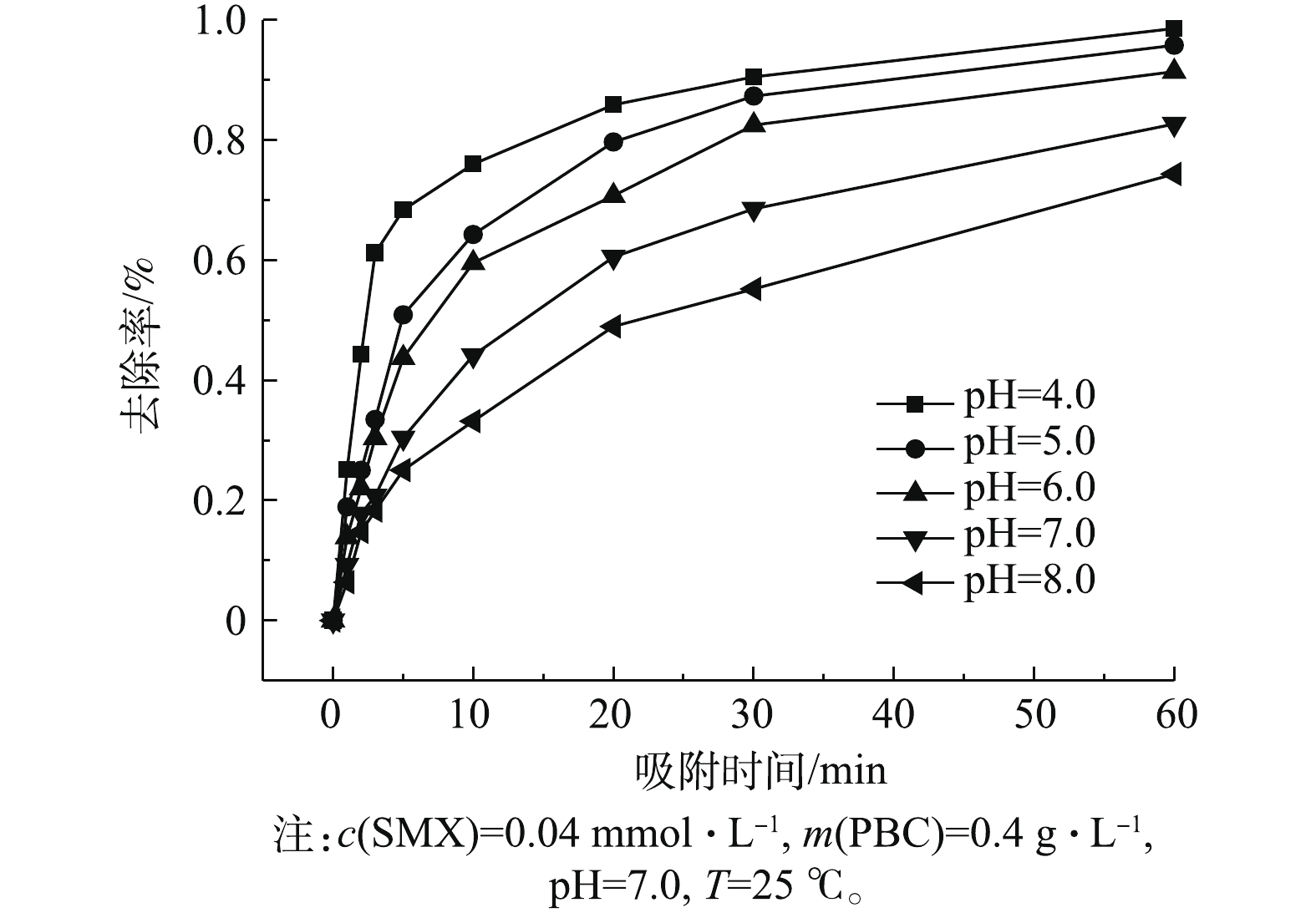
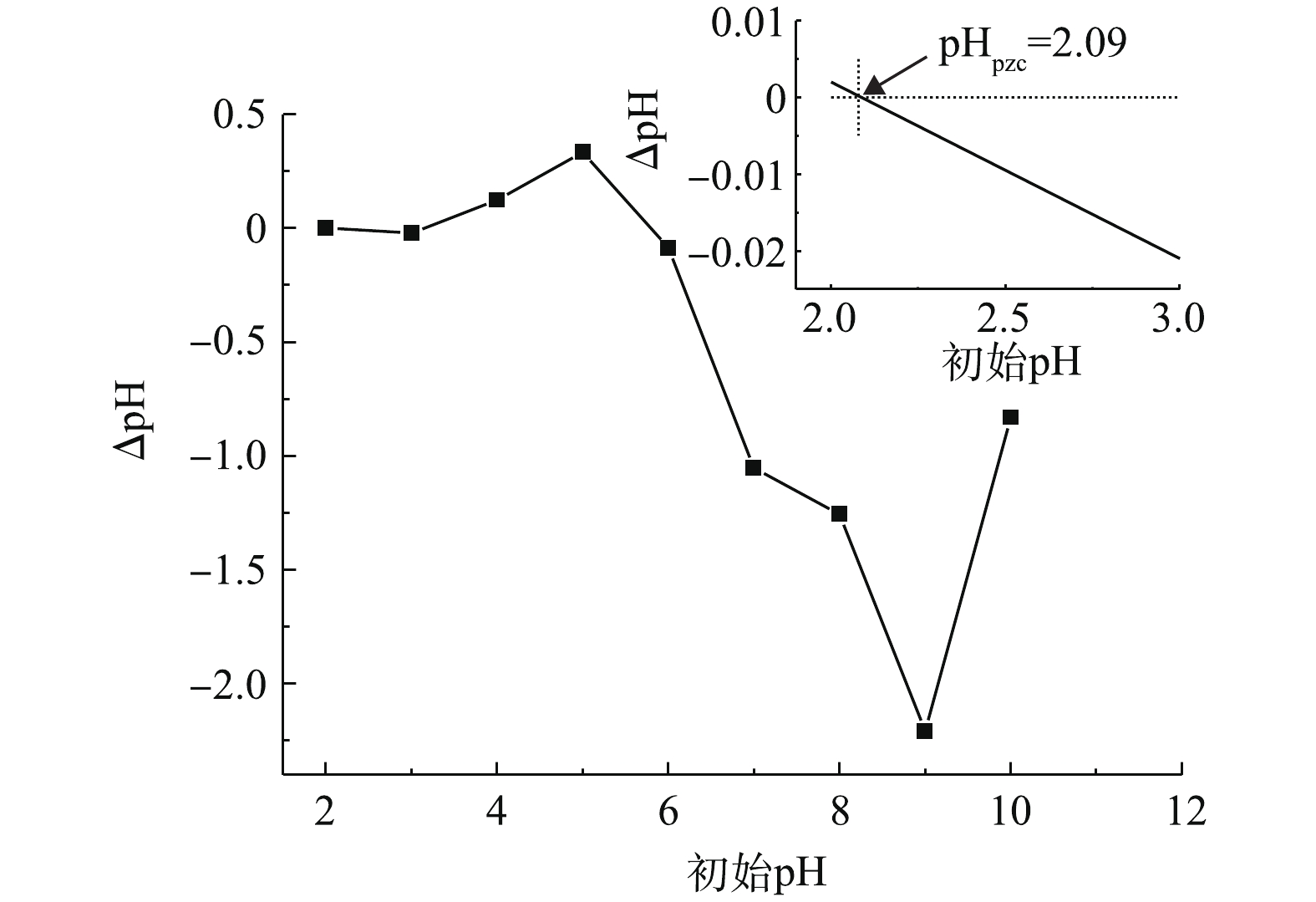
考察了初始pH(4.0~8.0)对PBC吸附SMX的影响,结果如图4所示。由图4可知:当pH为4.0~8.0时,随着pH升高,SMX去除率下降;当pH为4.0时,去除率达96.5%;当pH为8.0时,去除率降至76%。这可能与SMX的pKa有关。有研究[24]表明,SMX的pKa1为1.7,pKa2为5.6,当溶液pH小于pKa1时,SMX表面带正电荷;当pKa1<pH<pKa2时,SMX表面所带正电荷减少;当溶液pH>pKa2时,SMX以阴离子形态存在。PBC的零点电荷测定值为pHpzc=2.09(如图5所示),当溶液pH>pHpzc时,PBC表面带负电荷;当溶液pH为6.0、7.0和8.0时,pH>pKa2,此时SMX分子会与PBC发生静电排斥作用,故不利于吸附。因此,当溶液pH为4.0时,PBC对SMX的吸附效果最好。随着pH的增加,静电相互作用增强,吸附容量减弱,去除率也随之降低。有研究[25-26]也表明,溶液中pH的增加会降低黑碳对磺胺类抗生素的吸附能力。2.4. 吸附时间对SMX去除效果的影响
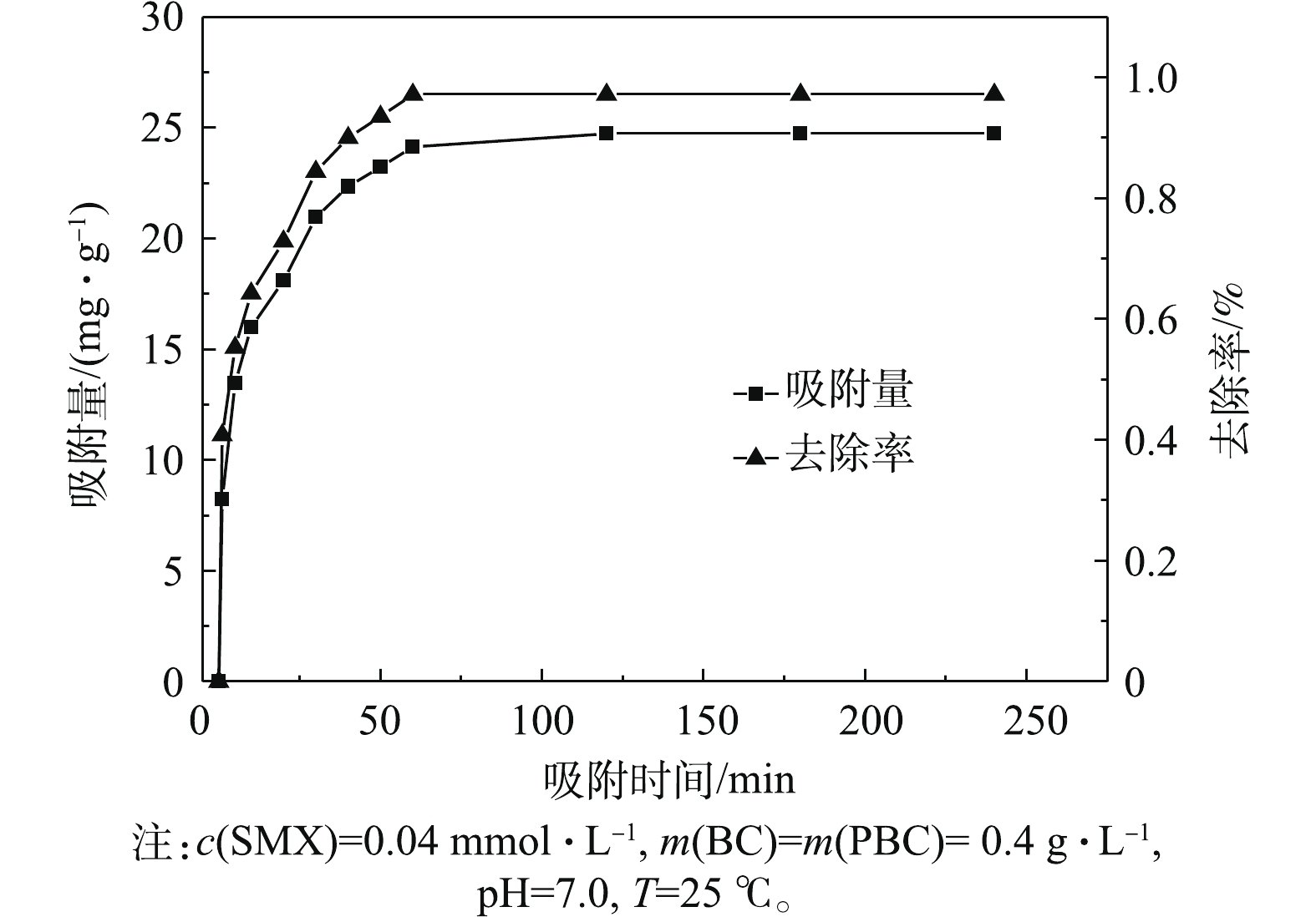
PBC对SMX的去除效果与吸附时间有关。为了使吸附反应彻底,实验须进行240 min,结果如图6所示,在0~60 min内,吸附容量和去除率都随时间增加明显增大,且吸附速率很快。当吸附60 min时,PBC对SMX去除率达97.1%,吸附容量为24.73 mg·g?1。当60 min以后,PBC去除率再无变化,证明吸附完全。当吸附时间为180 min时,吸附容量和去除率均无变化,说明吸附达平衡。可以解释为起初PBC表面的活性点位较多,使得SMX向PBC表面扩散速度很快。随着吸附时间的增加,吸附剂孔隙中的活性点位被SMX占据不断减少,吸附速率下降,从而使吸附过程趋于平衡。2.5. 阴离子对SMX去除效果的影响
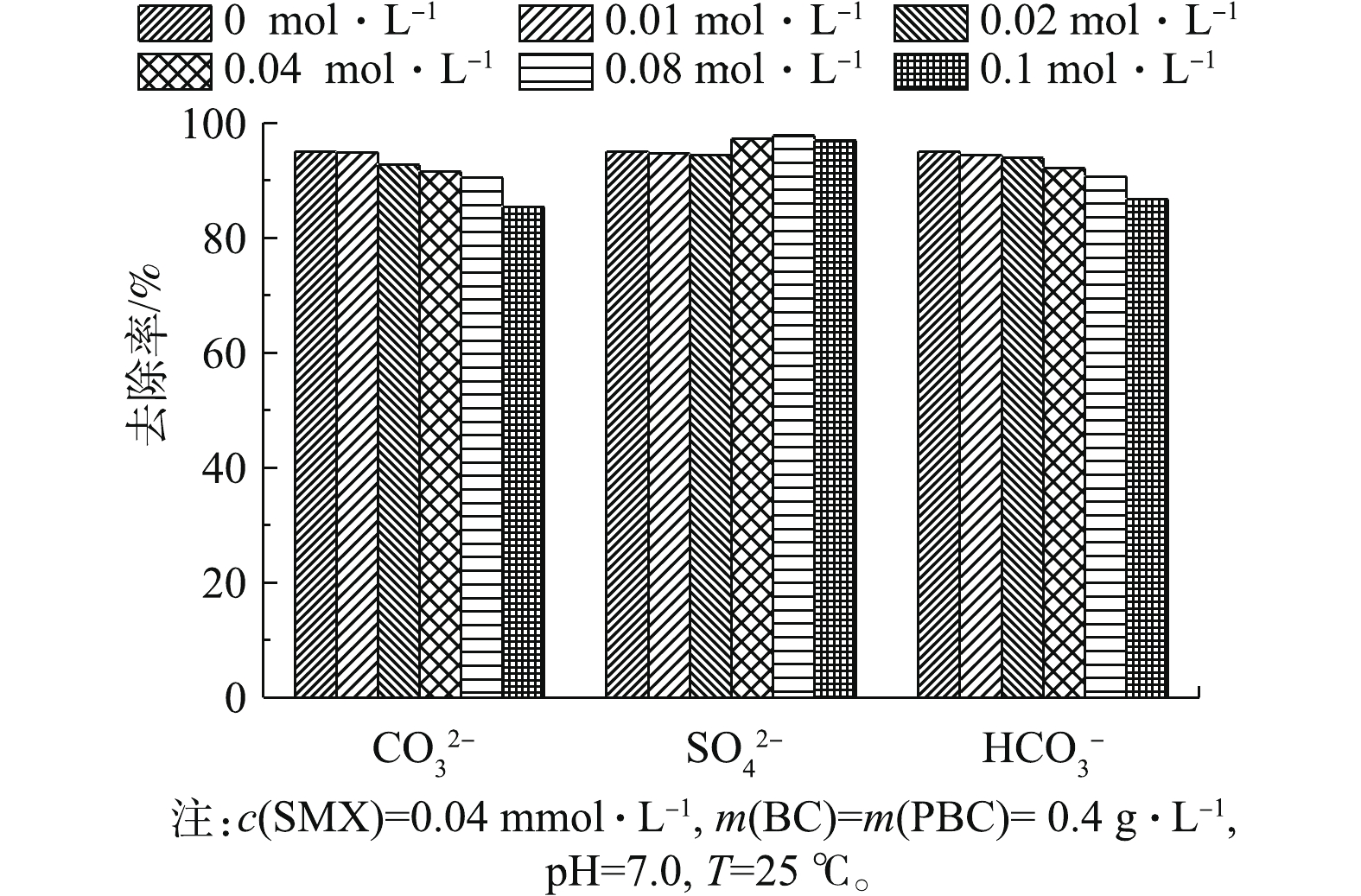
在实际应用中,天然水体中含有的2.6. 吸附动力学分析
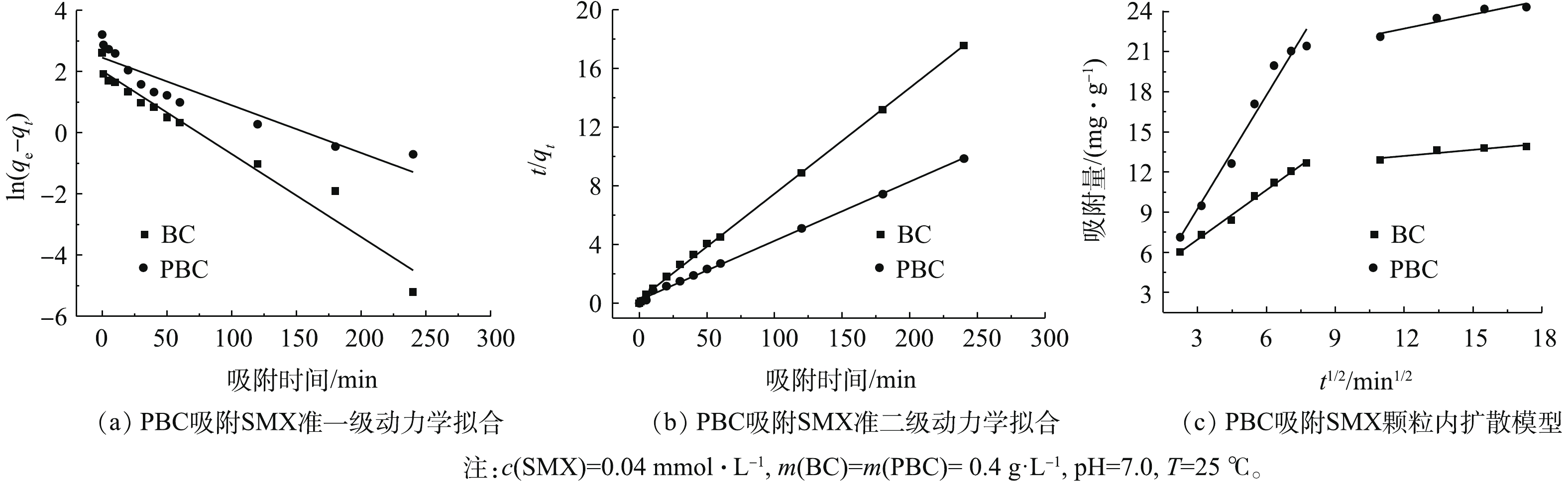
研究吸附动力学是了解吸附机制的重要途径。为了研究PBC对SMX吸附的动力学行为,分别利用准一级和准二级动力学模型对实验获得的吸附数据进行动力学拟合分析(图8)。准一级和准二级动力学模型拟合结果如图8(a)和图8(b)所示。表2列出了利用准一级和准二级动力学模型对实验数据进行拟合的结果,准二级动力学模型对SMX的实验数据拟合的可决系数较高(R2>0.99),且应用准二级动力学方程得出的qe的计算值与测得结果更为接近,这说明使用准二级动力学模型可以更好地描述此吸附过程。由表2可知,PBC对SMX的吸附量为24.79 mg·g?1,约为改性前的2倍。颗粒内扩散模型结果见图8(c)。由图8(c)可以看出,吸附过程分为2个阶段:第1阶段的斜率大,说明吸附速率快,原因是SMX分子直接在PBC表面占据其吸附位点;第2阶段直线缓慢上升,可能是由于PBC表面的吸附位点被占据,使得SMX分子只能在粒子内部扩散作用下进入到PBC内部占据其微孔点位[28]。拟合曲线不通过原点,表明表面扩散和颗粒内部扩散共同控制吸附进程。2.7. 吸附等温线拟合分析
吸附等温线模型反映了吸附平衡时吸附质分子在液相和固相间的分配情况,是描述吸附剂性能的重要指标。PBC对SMX的吸附过程可用2种吸附等温线模型描述。将等温吸附实验数据分别与Langmuir模型和Freundlish模型进行拟合,所得相关参数如表3所示。由表3可知,Freundlish模型的可决系数(R2>0.98)高于Langmuir模型的可决系数(R2>0.87),由此可见,PBC对SMX的吸附过程用Freundlish模型拟合效果更好。Freundlish模型描述了吸附质吸附于吸附剂表面的非均相多层吸附假设[29],模型参数n>1[30],说明PBC和SMX间有较强的吸附力。KF随温度升高而增加,说明此吸附过程为吸热过程。2.8. 吸附热力学分析
在不同温度下PBC对SMX的吸附热力学参数如表4所示。由表4可知,3个温度条件下标准吉布斯自由能变(ΔG)均小于零,且随温度的升高,ΔG的绝对值增大。这说明PBC对SMX的吸附过程是自发进行的,且温度升高,自发趋势增大。焓变(ΔH)大于零,表明PBC对SMX的吸附反应为吸热反应,且升高温度有利于吸附反应进行。熵变(ΔS)大于零,这说明吸附体系的混乱程度增加, 吸附反应发生在固液两相界面上。2.9. 重复利用性考察
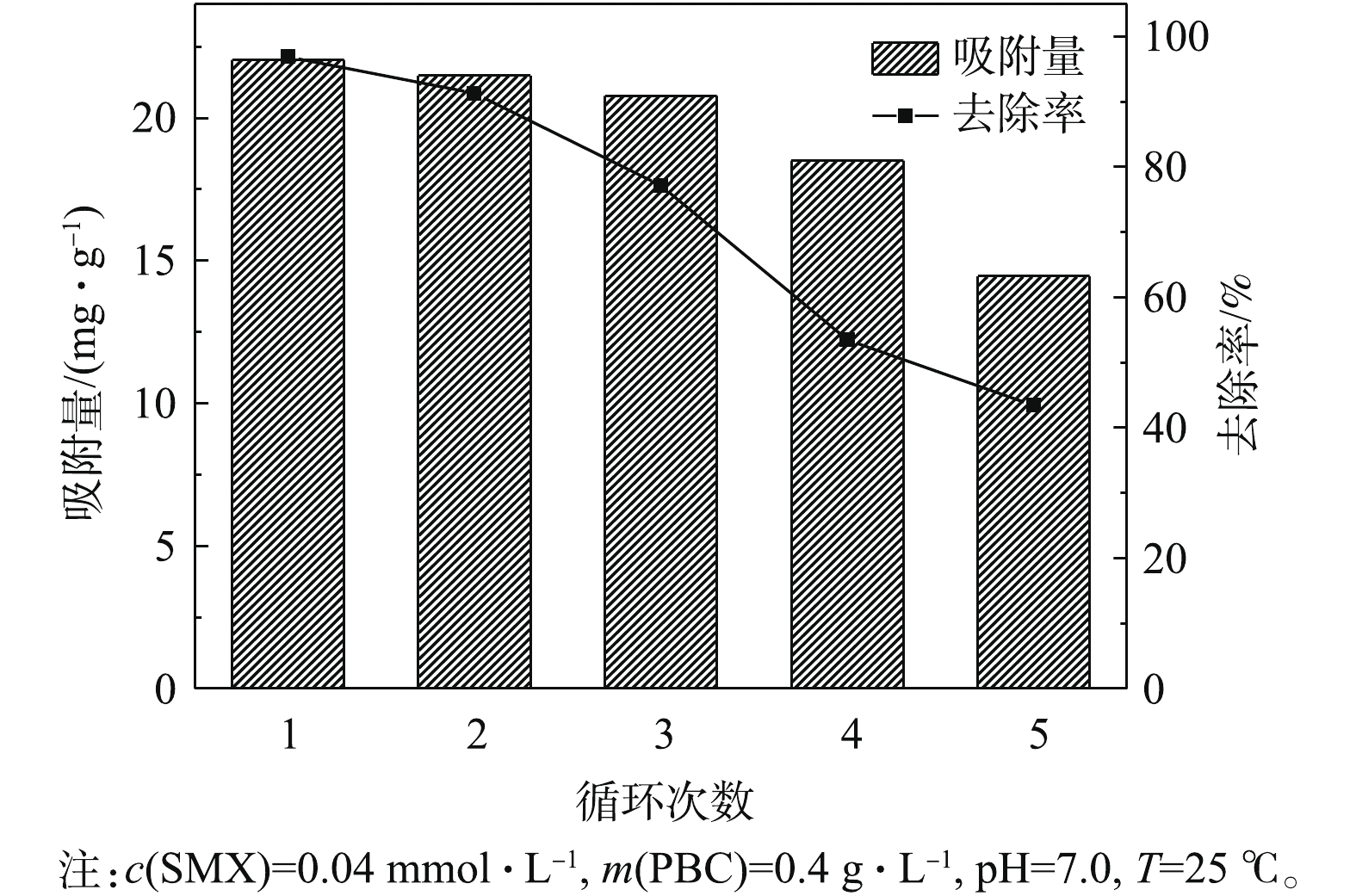
吸附剂的重复利用性能是衡量其经济价值的重要指标[31],通过5次实验考察了PBC的重复利用效果。由图9可知,PBC在第1、2和3次使用时,对SMX的去除率分别为96.9%、91.3%和77.1%。虽然PBC对SMX的吸附量和去除率均随着使用次数的增加在逐渐减少,但其仍具有较好的吸附效果。从第4次开始,PBC吸附性能明显有所下降,原因可能是PBC表面的吸附点位被SMX逐渐占据至饱和所致。但5次实验结束后,PBC吸附量仍为14.45 mg·g?1,去除率为43.4%,且保持稳定。由此可见,PBC是一种可回收、高效的吸附剂。2)在pH为4.0时,吸附剂对SMX的吸附效果最好;
3) PBC对SMX的吸附过程更符合准二级动力学模型学模型和Freundlish等温线吸附模型,吸附热力学参数表明PBC对SMX的吸附为自发的吸热反应。
4) PBC在循环使用5次后,对SMX的去除率仍在40%以上,依然维持着良好的吸附去除效果。因其具有吸附效果好、成本低、可回收等优点,因此,可以认为经盐酸活化后的松针炭可能是去除水中SMX最佳的吸附剂之一。
参考文献








 下载:
下载: 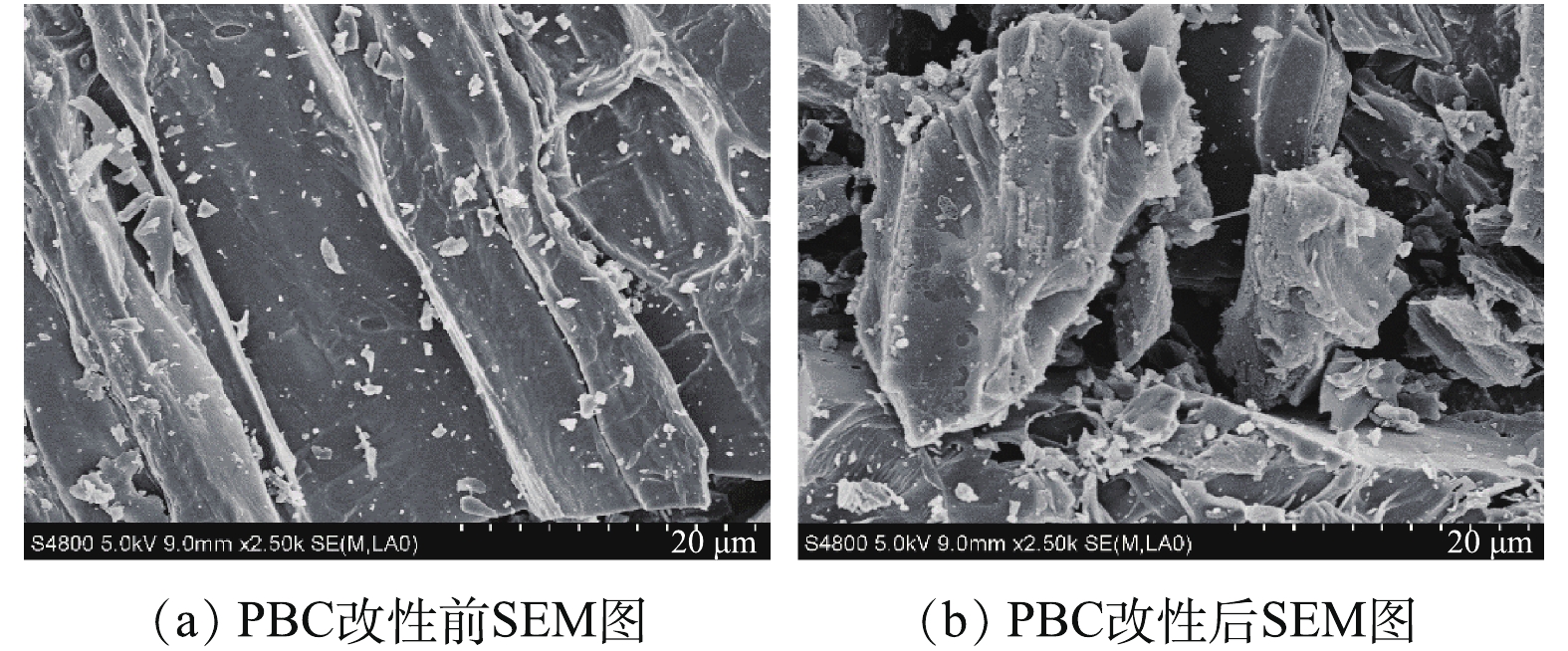
 点击查看大图
点击查看大图