全文HTML
--> --> --> 水是地球上含量最多的一种化合物,地球上接近3/4的面积覆盖着水,水的总体积约为13.8×108 km3[1]。随着电子科技产业和传统重工业的发展,对采矿、冶炼、金属电镀、颜料、电池制造、冶金工业的需求也越来越多[2-4]。加工这些产品的过程中产生了许多的重金属废水,企业废水的排放造成了水环境的污染,威胁到人类和生态系统的健康和稳定[5]。其中,铅、镉是日常工业生产中产生为数较多的重金属污染物[6],世界卫生组织对这几种污染物的毒性和生态毒性进行了定义。例如,铅是一种可以在较低浓度下对人类和环境具有较高毒性和毒副作用的物质[7],对于人类健康会产生各种短期和长期的毒副作用,包括对神经、心血管、肾脏、肠胃消化、血液循环和生殖系统的影响[8]。如果铜的摄取过量,会引起肝硬化、肠胃疾病、运动功能受阻和神经系统障碍[9-10]。镉暴露产生的污染经过肾脏器官的吸收积累会对人类产生各种各样的急慢性肾脏疾病[11]。不明原因慢性肾病(CKDu)是斯里兰卡北中部旱区高发的慢性疾病,目前有逐渐向周边地区蔓延的趋势,是影响斯里兰卡社会安定的民生问题,已受到世界卫生组织(WHO)等国际机构的高度关注。有关研究发现镉污染是斯里兰卡CKDu发病的一种潜在致病因[12]。因此,重金属离子污染物进入环境,不能进行自然或生物降解,其在环境中通过各形态之间的相互转化,在人、动物、植物中富集,从而对环境和人的健康造成严重危害。目前,重金属离子污染处理技术有离子交换法[13],电化学方法[14],膜技术[15]等,但这些方法能耗高,技术要求高,操作过程复杂,从而限制了这些技术在实际废水处理中的应用。相比较其他技术,吸附法具有操作简单、处理成本低、应用范围广等特点,在废水处理中应用最为广泛[16]。在废水处理中使用的吸附剂可以分为矿物吸附剂、有机吸附剂、生物吸附剂和纳米碳质吸附剂[17]。纳米技术是近几年发展迅速的废水新技术[18],纳米材料具有大比表面积和高表面活性位点等特点,对于吸附去除水中的重金属离子污染物有独特的优势,在废水处理方面取得了一些效果[19]。纳米二维碳质材料因其独特的物理性质在水处理领域显示了较大的应用潜力,二维纳米材料的长度没有限制,远大于纳米级的厚度,因此,其具有更好的电子流动性和更多的吸附活性位点[20]。SHEN等[21]成功合成了类石墨烯g-C3N4二维材料,且将其应用于水中重金属离子的吸附和去除。然而,对于废水中重金属离子的处理,如何大量地制备成本低廉、合成方法简单、吸附量高的二维碳纳米材料还存在很多的挑战。
本研究利用尿素和乙二胺四乙酸作为前躯体,通过高温固相一步合成法,大量地合成二维氮掺杂的碳基多层纳米材料(2-D CNx);通过研究材料的结构特点,考察材料对废水中重金属Cd2+、Cu2+和Pb2+的吸附性能,包括等温吸附过程,时间对吸附的影响,溶液pH对材料吸附容量的影响;为进一步提高材料的实用化潜力,对材料进行动态吸附滤柱穿透实验,并对材料的吸附机理进行讨论,研究可为开发高效去除斯里兰卡地下水中氟、重金属等污染物的吸附剂提供参考。
1.1. 实验试剂
硝酸铅、四水硝酸镉、三水硝酸铜、尿素、盐酸、乙醇、氢氧化钠、乙二胺四乙酸钠均为分析纯化学试剂。此外,实验中所用水均由实验室Milli-Q (18.2 MΩ·cm)超纯水净化装置提供。1.2. 材料制备
先将尿素分散充分,然后置于60 ℃干燥箱中脱水;乙二胺四乙酸二钠(EDTA)研磨充分,置于60 ℃下脱水干燥;将干燥后的尿素和EDTA混合均匀后,放置耐高温容器中封闭,通入氮气作为保护气。干燥后的尿素和EDTA按比例进行混合,在550 ℃下反应6 h,反应完成后,冷却至室温。样品经去离子水洗涤多次,干燥后,密封保存,得到二维多孔氮掺杂活性碳,将其命名为2-D CNx。1.3. 材料表征与分析
所制备的2-D CNx的晶型结构通过X射线衍射仪(X’Pert ProMPD)测定;比表面积及孔隙结构由比表面积及孔隙分析仪(型号Ommishop 100CX)测定,样品比表面积是由N2气氛下的吸附-脱附等温线(77 K)计算得出的,孔隙尺寸分布是由Barrett-joyner-Halenda(BJH)脱附等温线得出的;样品材料的形貌是通过加速电压为10 kV的FEI sirion 200 FEG以及型号为FEI Tecnai G2 F20’S-TWIN加速电压为200 kV的透射电子显微镜观察得到的;Zeta电势使用Microtrac Zeta电位仪测定;pH由pH电极(pHb-8)测定;普析原子吸收光谱仪用来测定Pb2+的浓度;吸附剂对Cd2+吸附前后的红外光谱由型号为NEXUS-870的傅里叶变换红外光谱仪(FT-IR)测得;XPS采用以Al Kα作为射线源,型号为Themo ESCALAB 250的X射线光电子能谱仪进行测试分析。1.4. 吸附实验
分别称取一定量的Pb(NO3)2、Cd(NO3)2·4H2O和Cu(NO3)2·3H2O,加入到100 mL去离子水中,得到1 000 mg·L?1的Cd2+、Cu2+和Pb2+母液,备用。为研究吸附材料对污染物的去除性能,分别考察了等温吸附过程、吸附平衡时间、溶液的pH与吸附量的关系。吸附动力学实验中重金属离子的浓度均为40 mg·L?1,吸附剂投加量为0.5 g·L?1,以150 r·min?1的转速在25 ℃下振荡反应。在一定间隔时间内取出5 mL,用0.22 μm的一次性针头将其分离,测定溶液中Cd2+浓度。
在吸附等温线实验的测定过程中,吸附剂投量为0.5 mg·L?1,pH为3。具体操作步骤如下:分别称取5 mg合成的材料,加入到装有10 mL含有Cd2+、Cu2+和Pb2+离子溶液(20~150 mg·L?1)的15 mL离心管中。将离心管置于摇床中,在150 r·min?1和25 ℃下振荡24 h,而后在离心机上以5 000 r·min?1的速度离心3 min,取上清液并将其过滤。
称取5 mg合成的2-D CNx,加入到装有10 mL初始浓度为40 mg·L?1重金属离子溶液的15 mL离心管中,探究pH对2-D CNx去除Pb2+、Cd2+、Cu2+的影响。分别调节溶液pH至2~7,将离心管放到摇床中,在25 ℃和150 r·min?1下振荡2 h,以达到吸附平衡,将上清液离心并分离,测定溶液中的重金属离子浓度。
在动态吸附柱透过实验的测定中,使用在柱径为1.9 cm,长度为20 cm的玻璃虑柱,2-D CNx的填充高度为2 cm,填充体积为5.8 cm3,吸附剂质量为3 g。重金属溶液采用上流式的流液方式,流速设定为1.5 mL·min?1。重金属离子的初始浓度均为40 mg·L?1,在实验中,测定不同时间出水的Cd2+、Cu2+和Pb2+的即时浓度和重金属溶液母液的平均浓度。
2.1. 材料的表征
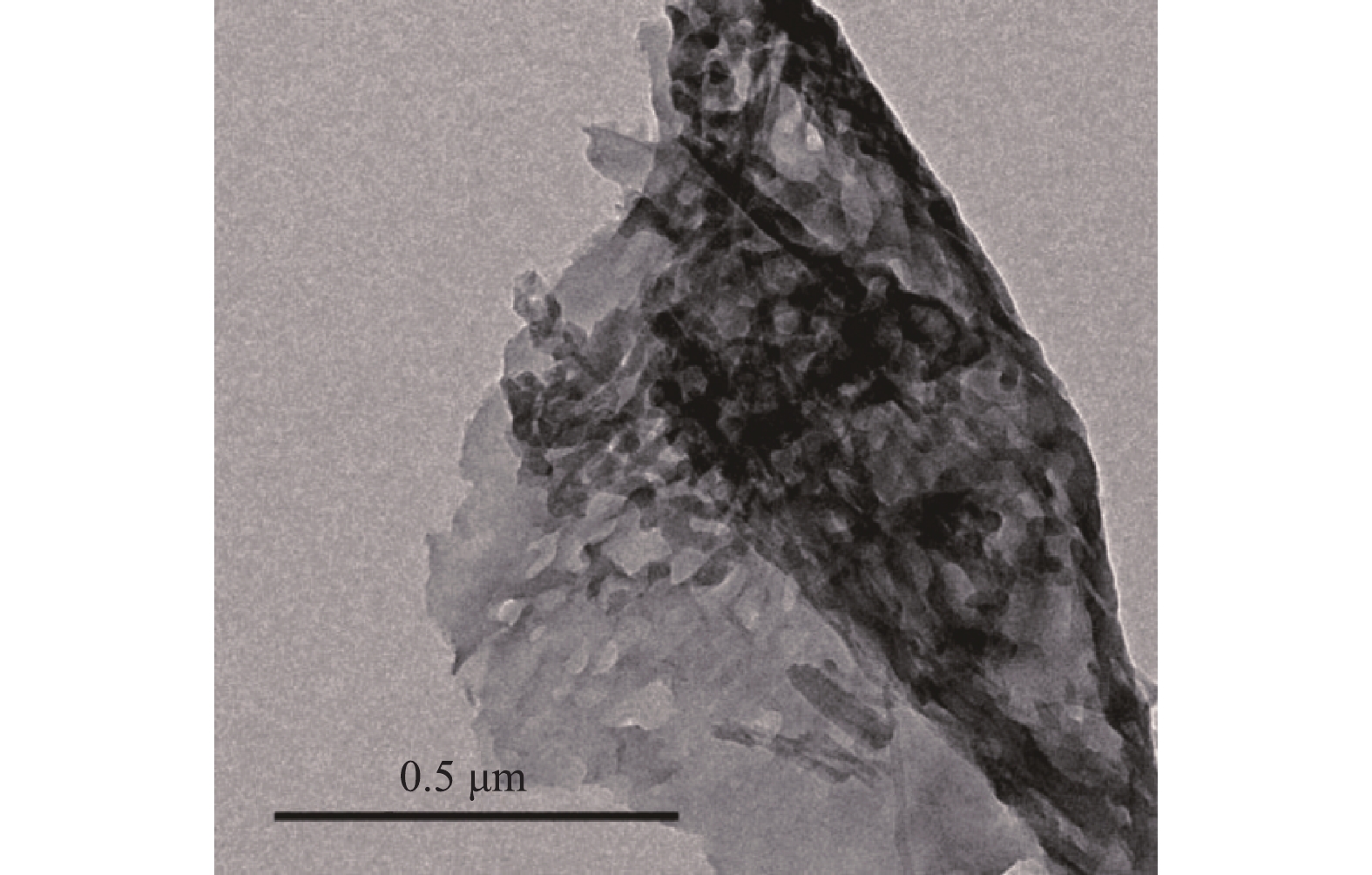
实验制备的二维多孔氮掺杂活性碳(2-D CNx)的形貌如图1所示。由图1可见,在高温下,2-D CNx产生了大量的孔洞,存在较多的平面卷曲。通过高分辨电子透射显微镜可以观察到2-D CNx存在石墨微晶相和多孔结构,且具有明显的无定形结晶结构和无序的石墨缎带结构[22]。通过BET对合成的2-D CNx比表面积和孔隙率进行表征,结果如图2所示。图2(a)为2-D CNx吸附-脱附等温线,属于第Ⅳ种类型,并且有明显的滞后循环,表明2-D CNx存在介孔结构[23]。2-D CNx材料具有较高的比表面积(11.5 m2·g?1)。BJH孔隙分布同样也说明了介孔结构的存在,材料的平均孔隙为23 nm。
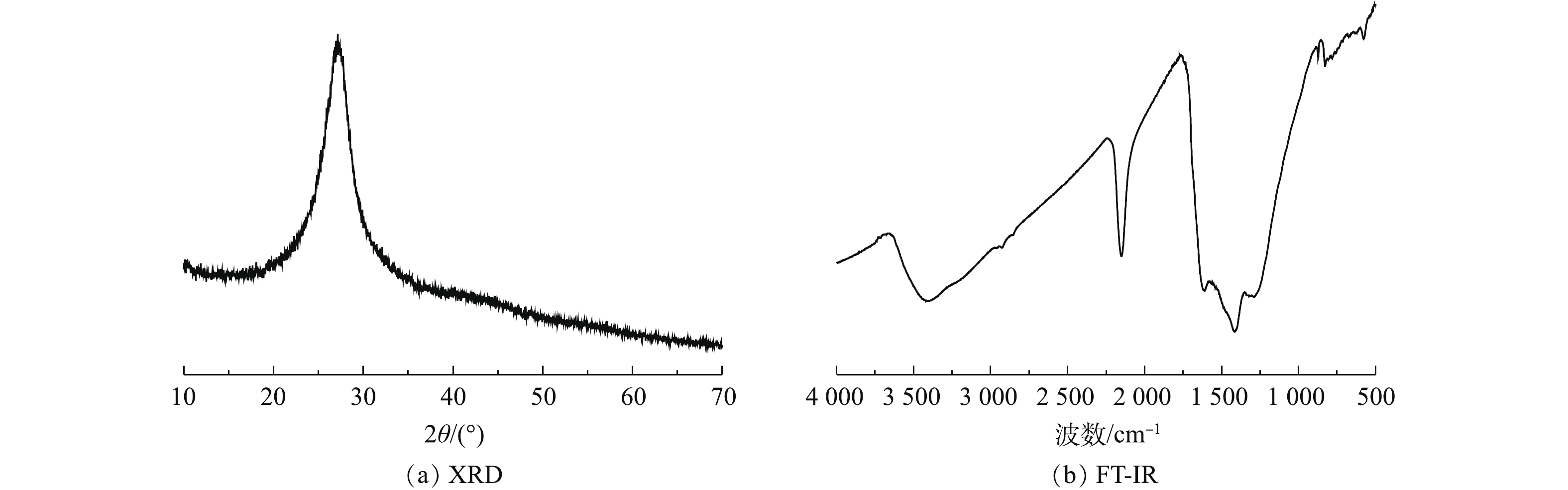
通过XRD和FT-IR光谱对2-D CNx的晶体结构和表面基团进行表征,结果如图3所示。由图3(a)可知,2-D CNx只在27.2o位置处存在1个较宽的衍射峰,此处对应着石墨相氮化碳的(002)晶面,揭示该材料为无定形的石墨结构[24]。电子透射显微镜的形貌表征结果发现,2-D CNx 存在着层状堆叠的无定形石墨结构,这与前面的XRD分析结果一致。由图3(b)可见,3 000~3 650 cm?1处峰对应着材料上的—NH2和H2O基团的伸缩振动峰[25];位于619 cm?1处的峰对应着2-D CNx的—COOH伸缩振动的指纹峰;位于1 700 cm?1处的峰对应着2-D CNx的—COOH的伸缩振动峰,位于2 150 cm?1处的峰对应着2-D CNx上—C=N的伸缩振动峰[26]。
2.2. 重金属离子初始浓度的影响
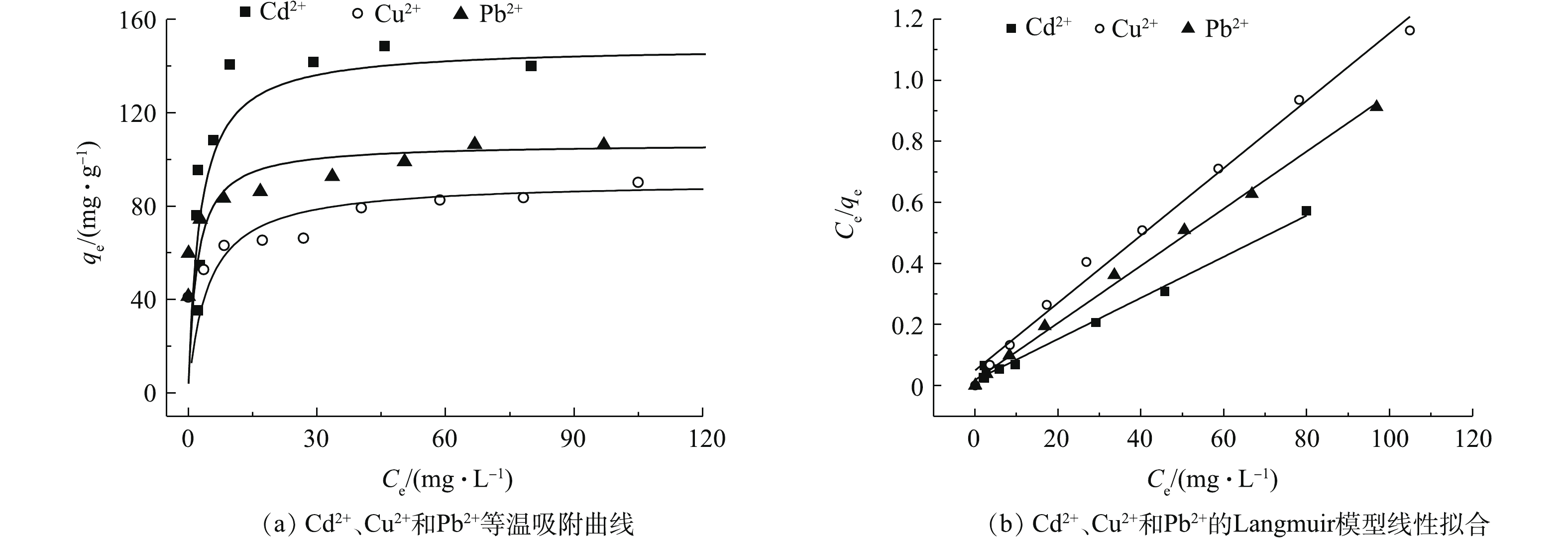
研究不同初始浓度的重金属离子对吸附材料的吸附性能影响以及吸附材料对不同重金属离子的最大吸附效果,对吸附剂的工业化应用具有明显的实际意义[27-29]。在本研究中,重金属离子的浓度为0~150 mg·L?1。图4为2-D CNx材料对Cd2+、Cu2+和Pb2+吸附量随着溶液初始浓度的变化结果。可以看出,随着污染物初始浓度的增加,2-D CNx对污染物的吸附量呈升高的趋势,当重金属离子浓度从20 mg·L?1增加到60 mg·L?1时,材料2-D CNx对重金属Cd2+、Cu2+和Pb2+离子的吸附量从35、40、40 mg·g?1分别增加到108、66、82 mg·g?1。随着重金属离子溶液浓度的继续增加,吸附材料对污染物的吸附量变化趋势趋于平衡。由此可知,此吸附材料适合应用于低浓度的重金属废水的处理。Langmuir吸附等温模型为单分子层吸附的经验公式;Freundlich吸附等温模型为多分子层吸附的经验公式。图4(a)为平衡吸附量与Pb2+离子平衡时浓度的吸附等温曲线。通过Langmuir和Freundlich等温模型对实验数据进行拟合分析。结果表明,吸附过程是由多相表面控制的,计算后的结果汇总在表1中。可以看出,材料去除重金属污染物的等温过程符合Langmuir等温吸附模型。根据Langmuir等温吸附模型,计算材料2-D CNx对重金属Cd2+、Cu2+和Pb2+离子的吸附量分别为148.4、90.6、106.8 mg·g?1,其吸附容量与目前成熟的离子交换树脂和活性炭相比(表2),2-D CNx对3种金属离子的吸附容量较大。
2.3. 吸附动力学
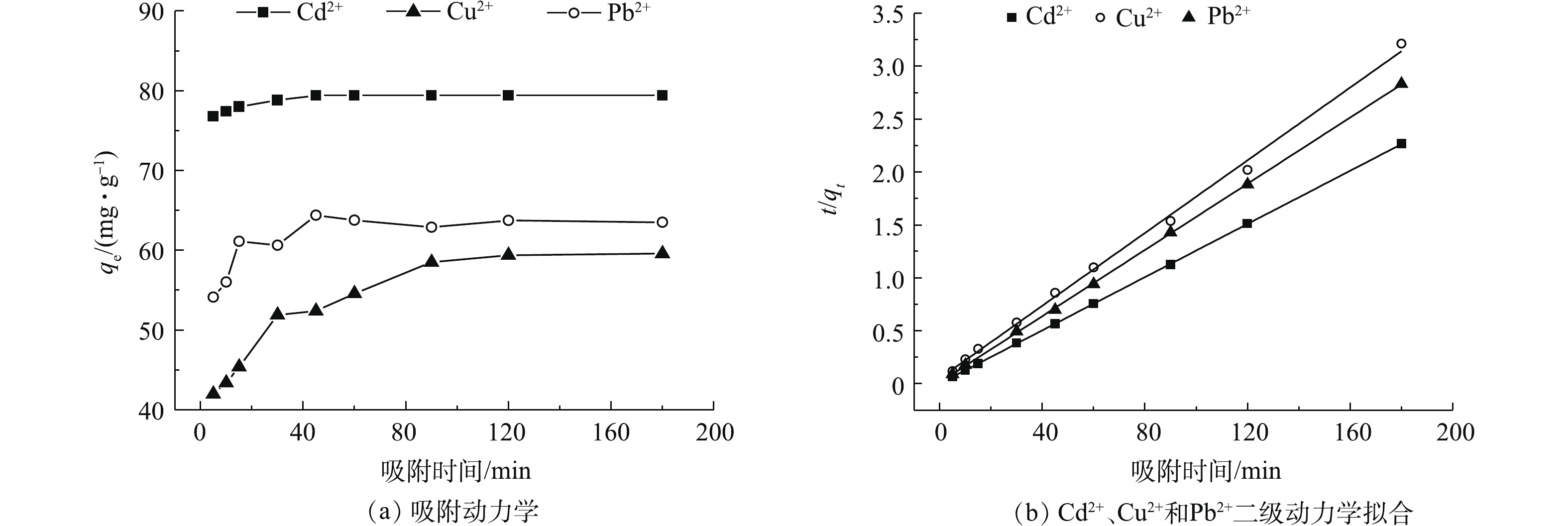
材料对污染物的吸附是在一定时间内完成的,在吸附材料与污染物的有效接触时间内,污染物需和吸附材料进行充分的接触,从而提高两者之间的相互作用[36]。对实际污染物处理过程中的吸附性能优化、设备工艺参数设定、节能减排均有重要的意义[37-38]。图5(a)为Cd2+、Cu2+和Pb2+吸附量随吸附时间的变化结果(具体吸附动力学参数见表3),图5(b)为t/qt对时间的拟合线性关系。由图5(a)可知,随着吸附时间的延长,材料对污染物的吸附量呈现先增加后趋于平衡的趋势。在5~10 min内,材料的吸附速率增加较快,吸附量增大;在10~30 min内,吸附量的增加变得比较缓和;在60 min后,趋于稳定。在Cd2+、Cu2+和Pb2+的初始浓度为40 mg·L?1时,吸附剂用量为0.5 g·L?1。在25 ℃下吸附平衡时,Cd2+、Cu2+和Pb2+的吸附量分别达到了79.4、56.0、63.5 mg·g?1。材料对污染物的吸附量大小顺序为Cd2+>Pb2+>Cu2+。导致以上结果的主要原因可能与重金属离子半径、轨道能级空位电子和吸附剂材料本身官能团吸附位点作用的不同有关。
2.4. 溶液pH对重金属吸附的影响
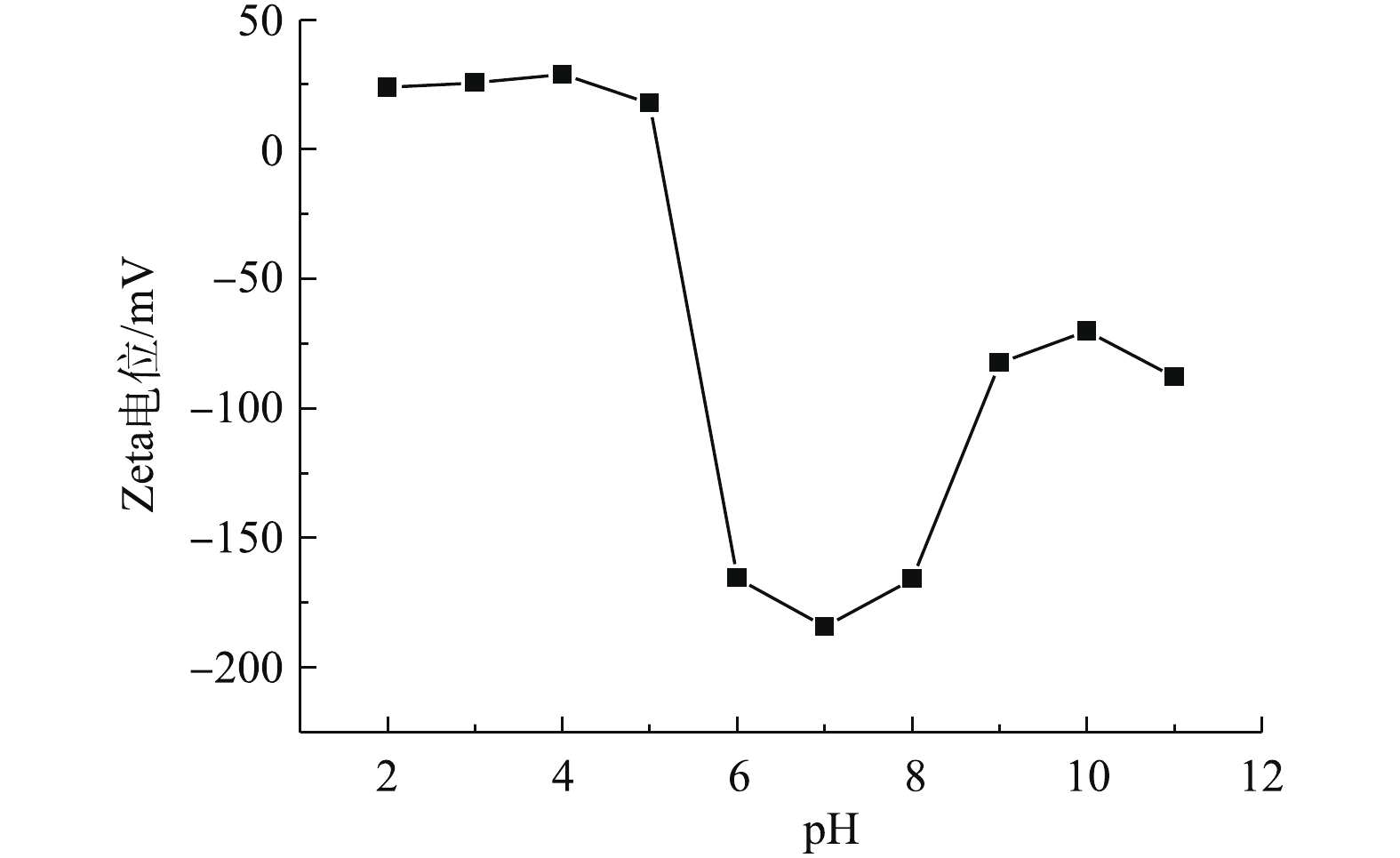
废水中重金属污染物的吸附量很大程度上受到溶液pH的影响,pH不仅会影响重金属离子在溶液中的解离程度和离子价态,同时还会影响吸附剂表面的电荷分布[39]。因此,研究pH对重金属离子的吸附量变化情况,可为实际废水处理工艺过程提供操作参数[40]。图6为材料的Zeta电位随着溶液pH的变化结果。如图6所示,当pH低于4.5时,材料的表面带有正电荷,当pH高于4.5时,Zeta电位发生明显变化,由17.8 mV降为?165.5 mV。pH=4.5为材料的电势转变点,随着材料表面电荷的变化,材料表面的性质也发生明显的变化。此外,也考察了pH对污染物去除性能的影响,结果如图7所示。由图7可知,当溶液pH较低时,材料2-D CNx的吸附量较低,随着溶液pH的增加,材料的吸附量开始出现增加。材料吸附重金属Pb2+的结果见图7(a),当溶液pH大于4.0时,可以发现材料的吸附量开始明显增加。根据离子解离平衡常数计算水中Pb2+的形态分布(图7(d)),可以发现,溶液pH低于5.0时,溶液主要以Pb2+的形式存在;但是溶液pH为2.0~5.0,材料的表面电势为正,这是由于材料的吸附量较低所致;当溶液pH增加到7.0时,材料对Pb2+的吸附量达到最大。由图7(a)可知,当溶液pH低于4.0时,材料对重金属Cd2+的去除量较低。但随着溶液pH的改变,材料表面带有的负电荷增加,材料的吸附量有明显的升高。当溶液的pH趋近于7.0时,材料的吸附量趋近于平衡。同样由2-D CNx去除水中的重金属Cu2+的结果可以发现,当溶液的pH低于4.0时,材料的吸附量较低,随着溶液pH升高,当溶液pH为6.0时,材料对重金属Cu2+的去除量达到最大。由Cu2+离子在水中分布形态(图7(c))可知,当pH低于6.0时,Cu2+在水中主要是以离子的形式存在。在溶液pH低于6.0时,材料可以去除溶液中大量存在的Cu2+。当溶液的pH继续增加时,材料的吸附量有所下降。
2.5. 吸附动态实验
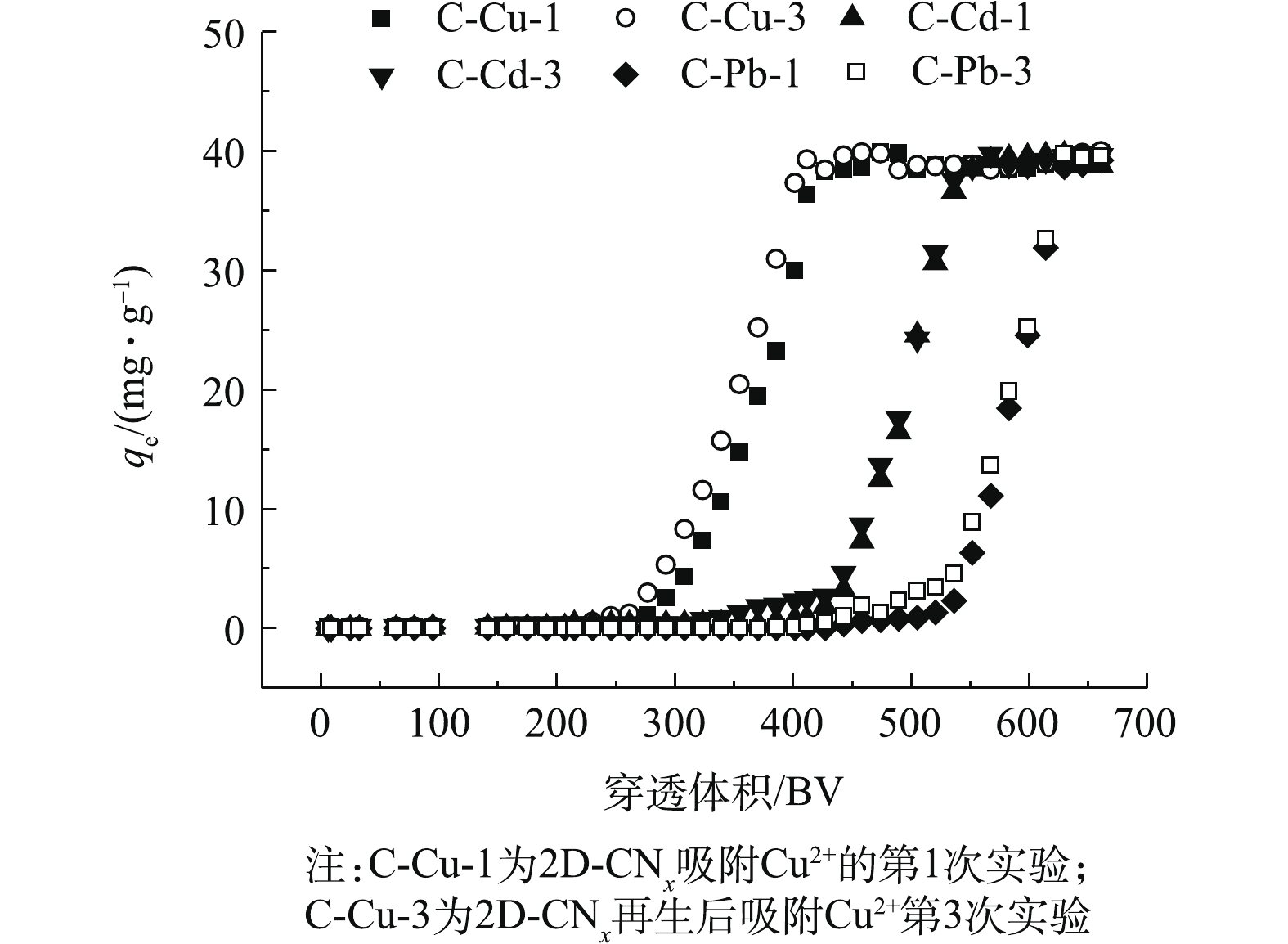
吸附柱动态实验中的Cd2+、Cu2+和Pb2+的浓度为40 mg·L?1,实验结果如图8所示。由图8中可知,重金属离子在吸附柱实验中重复循环3次之后,每种离子前后的吸附柱穿透体积(BV)几乎没有变化。在使用吸附柱去除重金属离子的过程中,吸附剂能够保持很好的循环稳定性。对于Cu2+离子,吸附剂处理量接近262 BV (1 508 mL),污染物Cd2+离子处理量接近411 BV (2 379 mL),重金属污染物Pb2+离子处理量接近505 BV (2 918 mL)。本研究中的吸附材料具有很好的重复利用性,这说明其具有较高的吸附和脱附效率。此外,重金属离子在吸附柱实验中重复循环3次之后,材料结构没有发生明显的变化,这说明材料具有良好的机械稳定性。2.6. 吸附机理
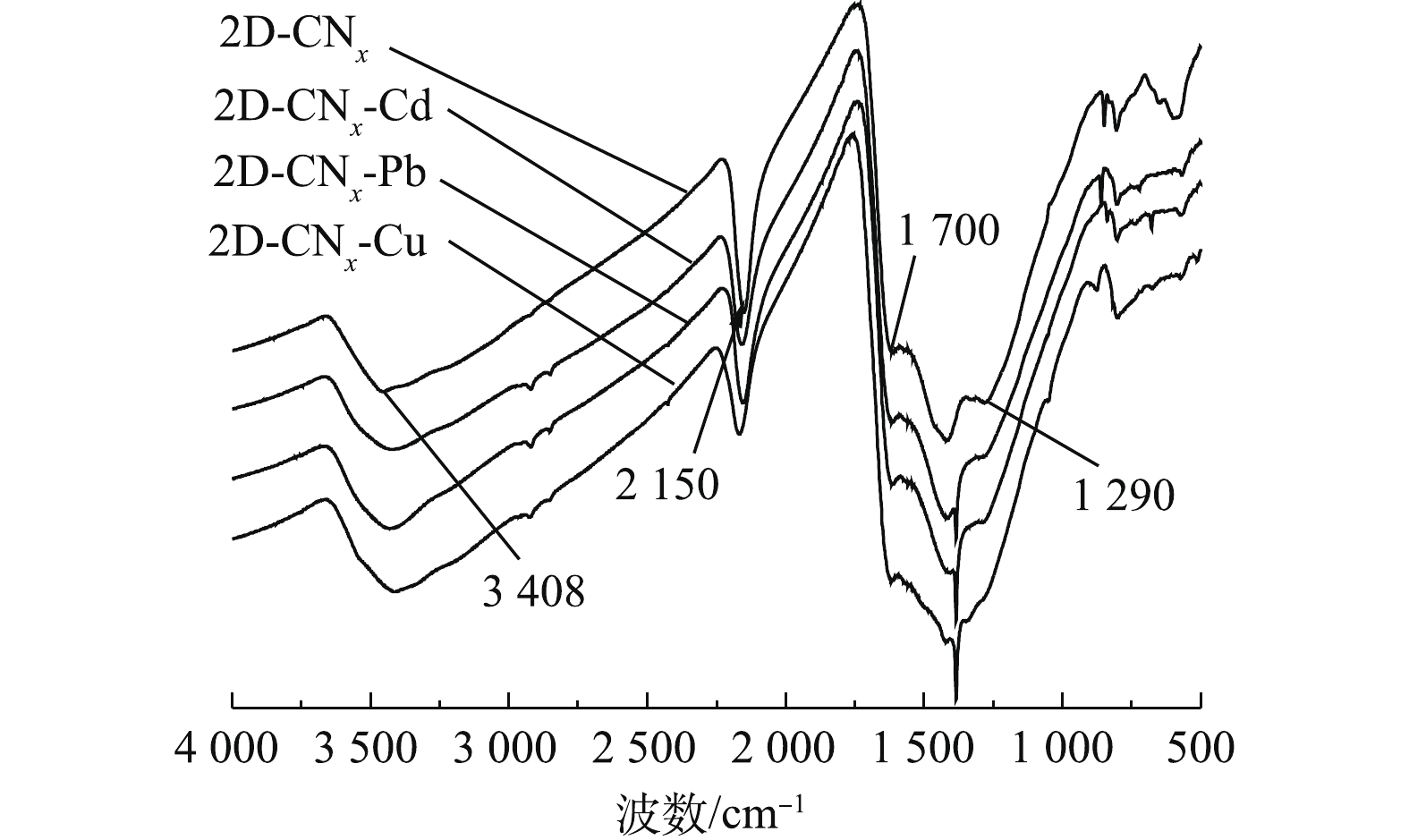
研究吸附剂对污染物的吸附机理有利于理解吸附剂与污染物的环境行为[17]。通常可以通过离子交换、配位和沉淀的方式去除重金属离子[7, 41]。通过对上述的吸附行为讨论可知,2-D CNx对重金属离子的吸附过程主要包括材料与离子之间的配位和离子交换的形式。为进一步确认其相互作用的机理,分别采用FT-IR和XPS分析手段,对材料吸附重金属离子前后的表面结构变化进行表征。材料与污染物作用前后的傅里叶红外光谱如图9所示。由图9可知,3 000~3 650 cm?1处峰是—NH2和H2O的伸缩振动峰[26],在吸附重金属离子之后有明显的变化,对应的619 cm?1处峰的指纹图谱位置在吸附了重金属离子之后出现新的伸缩振动峰,已有研究[21]表明,材料2-D CNx和重金属离子之间形成了离子键。位于1 290 cm?1处的—CN振动峰在吸附了重金属离子之后消失,可能是由于材料表面—NH2基团和重金属离子之间发生了络合作用[26]。
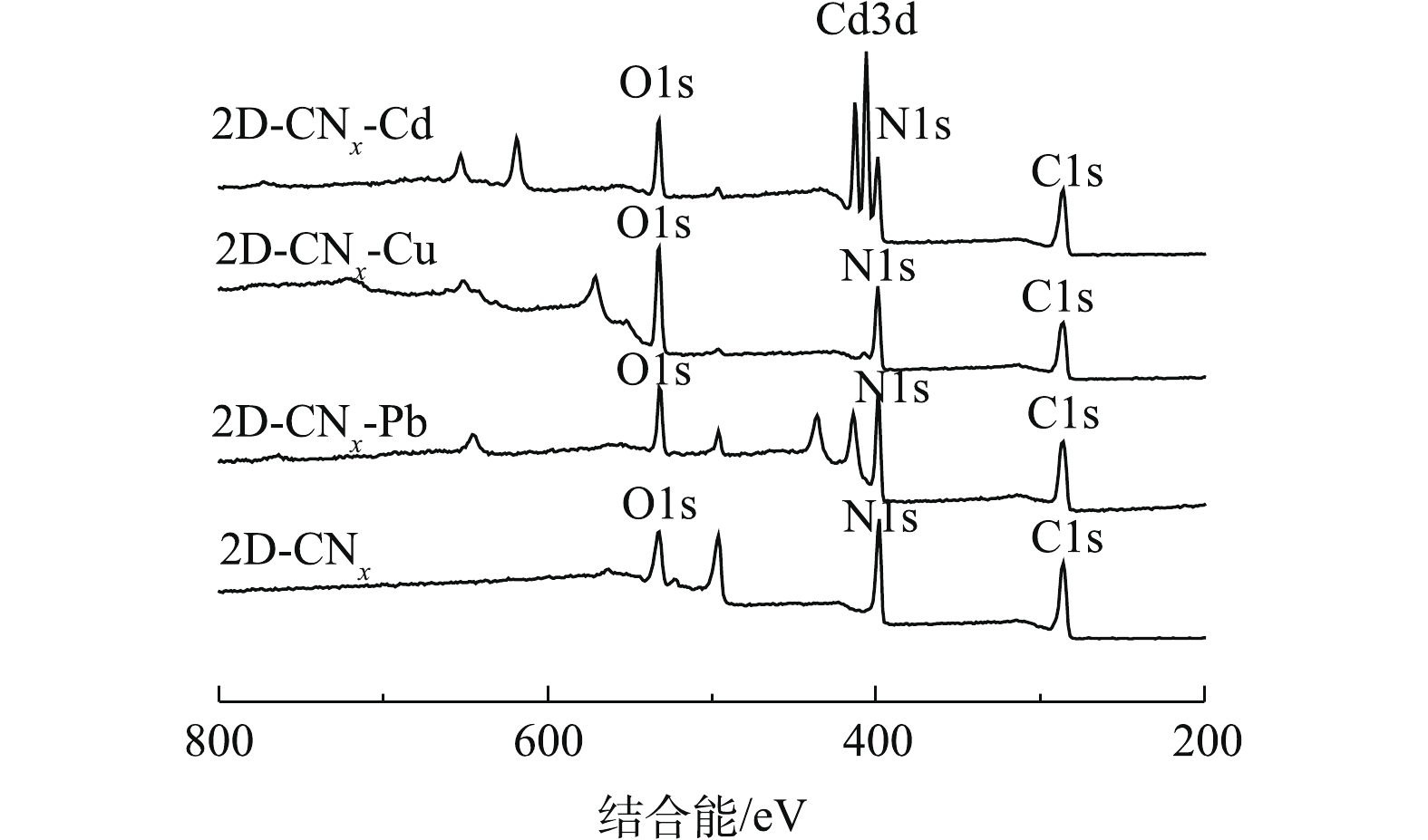
为了进一步探究吸附机理,利用XPS光谱对吸附前后材料表面官能团的变化进行了表征,结果如图10所示。由图10可知,在399、531 eV对应的N1s、O1s在吸附重金属离子后[42],其峰位均发生了明显的变化。
图11为2-D CNx中N1s和O1s的高分辨XPS图谱。由图11(a)可知,吸附重金属污染物之前的2-D CNx在398.2、398.9和400.9 eV分别对应着材料上的—NH2[42]、sp2杂化的吡咯形式的C—N—C[43]结构和石墨共轭结构的氮[44]。从吸附重金属离子以后的材料能级的变化可知,材料上sp2杂化的—NH2的能级有明显的向高能级移动的趋势,2-D CNx在吸附了重金属离子之后,材料上的一部分带有孤对电子的氮原子和重金属离子发生配位[45],氮原子的电子密度降低,向高能级移动[46]。由于类石墨中的氮占比较低,在吸附溶液重金属离子之后,此处的峰位消失。图11(b)为2-D CNx中O1s高分辨谱图中位于533.9 eV和532.1 eV的峰位分别对应着C—OH和—OH基团[47]。对比材料吸附重金属后的O1s变化可以发现,在吸附了Cd2+和Cu2+离子之后,O1s谱图中没有出现新的峰位[45],Pb2+离子在530.8 eV出现的新峰对应的是Pb—O—C基团[48]。这说明Cd2+和Cu2+主要与材料上—COOH发生了离子交换。综上所述,材料在吸附重金属离子的过程中,羧基和重金属离子的离子交换及氨基和重金属离子的配位络合起主要作用。
2)在重金属离子初始浓度为40 mg·L?1时,材料达到吸附平衡时,Cd2+、Cu2+和Pb2+的吸附量分别达到了79.4、56.0、63.5 mg·g?1,材料对重金属吸附量的大小顺序为Cd2+>Pb2+>Cu2+。材料的吸附等温线符合Langmuir模型。
3)通过测定pH对2-D CNx的Zeta电势及其对重金属离子吸附量的影响,发现2-D CNx的等电点在pH=4.0附近,材料2-D CNx对重金属离子在比较广泛的pH范围(3.0~9.0)内都具有比较好的吸附效果。
4)在吸附柱穿透实验中,可以发现吸附剂具有很好的重复利用性,可以说明吸附剂材料具有稳定的机械性能和较高的吸附和脱附效率。
5) 2-D CNx对重金属离子吸附机理主要归因为吸附材料表面的羧基基团和重金属离子之间的离子交换以及氨基基团和重金属离子的配位作用。
参考文献

 下载:
下载: 


 点击查看大图
点击查看大图