 ,1
,1Potassium channel Shaker play a protective role against cardiac aging in Drosophila
Xuewen Liu1, Hongmei Wu1, Ying Bai1, Qun Zeng1, Zemin Cao1, Xiushan Wu2, Min Tang ,1
,1通讯作者: 唐旻,博士,副教授,硕士生导师,研究方向:果蝇心衰的分子机制研究。E-mail:mtang2014@163.com
编委: 韩俊海
收稿日期:2020-08-11修回日期:2020-12-14网络出版日期:2021-01-20
| 基金资助: |
Received:2020-08-11Revised:2020-12-14Online:2021-01-20
| Fund supported: |
作者简介 About authors
刘学文,在读硕士研究生,专业方向:果蝇心衰的表观遗传调控。E-mail:

摘要
钾离子通道在心肌细胞动作电位复极过程中起着重要作用。钾离子通道蛋白种类繁多,已知钾离子通道蛋白KCNQ和HERG/eag参与心脏动作电位的形成,调节心脏收缩节律。钾离子通道蛋白Shaker是果蝇(Drosophila)体内发现的第一个电压门控钾离子通道,维持神经元和肌肉细胞的电兴奋性,但是目前其在成人心脏功能中的作用仍不清楚。本研究以果蝇为模型,高频电刺激模拟心脏应激状态,观察钾离子通道蛋白shaker基因突变体的心衰发生率。同时,利用心脏特异性启动子hand4.2 Gal4特异性敲低钾离子通道蛋白Shaker的表达;果蝇成体心脏生理学功能分析系统分析了1、3、5周龄特异性敲低钾离子通道蛋白Shaker的心脏表型。结果表明,shaker基因突变将严重影响果蝇心脏抗应激能力,表现在高频电刺激后的心力衰竭发生率显著性升高;心脏特异性敲低shaker基因导致5周龄果蝇心律失常发生率显著性增加;心脏特异性敲低HDAC3将显著降低果蝇寿命。综上所述,本研究推测钾离子通道蛋白Shaker在衰老过程中维护果蝇正常的心脏功能。
关键词:
Abstract
Potassium channels, which are the most diverse group of the ion channel family, play an important role in the repolarization of cardiomyocytes. Recent studies showed that potassium channels, such as KCNQ and HERG/eag, play an important role in regulating adult heart function through shaping the action potential and maintaining the rhythm of cardiac contraction. The potassium channel protein Shaker is the first voltage-gated potassium channel found in Drosophila to maintain the electrical excitability of neurons and muscle cells, but its role in adult cardiac function is still unclear. In this study, Drosophila was used as a model to study the role of Shaker channel in the maintenance of cardiac function under stress and aging. The incidence of heart failure was observed in shaker mutant after external electrical pacing, which simulates cardiac stress. Additionally, The cardiac-specific driver hand4.2 Gal4 was used to specifically knock down the expression of the potassium channel shaker in Drosophila. The cardiac parameter was analyzed at 1, 3, 5 weeks of age on cardiac specific knockdown of shaker using Drosophila adult cardiac physiological assay. The results showed that the mutation of shaker gene seriously affect the cardiac function under stress, demonstrated by significant increase in heart failure rate under electrical stimulation. In addition, cardiac specific knockdown of shaker increased the incidence of arrhythmias in Drosophila at the age of 5 weeks. Cardiac-specific knockdown of shaker reduces life span. Therefore, the results of this study suggest a vital role of the potassium channel shaker in maintaining normal cardiac function during aging.
Keywords:
PDF (606KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
刘学文, 吴红梅, 白瑛, 曾群, 曹泽民, 吴秀山, 唐旻. 钾离子通道蛋白Shaker对果蝇心脏衰老的保护作用. 遗传[J], 2021, 43(1): 94-99 doi:10.16288/j.yczz.20-253
Xuewen Liu.
心律失常是一种常见的心脏疾病,其患病率和死亡率随着年龄的增长而显著增加[1]。年龄相关性心律失常的发病机制一直是研究的热点。心脏结构和离子通道的重构是老年性心律失常发生的主要病理基础,但其发生的分子机制仍不清楚。
钾离子通道在心脏动作电位复极中起着重要作用[2,3,4,5]。钾离子通道家族作为心脏离子通道中种类最多的家族,由结构相似但功能不同的亚型组成,每一种钾离子通道都具有特定的动力学和电压依赖性[3]。在果蝇(Drosophila)和脊椎动物中,HERG/eag和KCNQ钾离子通道基因突变会导致心脏复极能力减弱和心脏动作电位延长,从而增加心律失常的发生率;且其基因表达水平在衰老心脏中也显著减少,这可能是年龄依赖性心脏功能障碍的重要标志之一[6,7,8,9]。钾离子通道蛋白Shaker是果蝇体内发现的第一个电压门控钾离子通道,维持神经元和肌肉细胞的电兴奋性[10,11],但其在衰老心脏中的作用仍不清楚。因此,本研究利用果蝇作为模型,研究钾离子通道蛋白Shaker在年龄依赖性心率失常发生发展中的功能。
1 材料与方法
1.1 果蝇品系
野生型果蝇w1118、shaker突变体果蝇shaker14、KCNQ突变体果蝇KCNQ186和shaker基因缺失系均来自于Bloomington果蝇品系中心。shaker的RNAi品系(v23673)来自于维也纳果蝇RNAi中心(VDRC)。心脏特异性表达的hand4.2 Gal4果蝇品系由美国Sanford Burnham医学研究所Rolf Bodmer实验室提供,Gal4与RNAi品系中的UAS序列结合启动下游RNA干扰序列的表达,在心脏中特异性敲低shaker基因的表达。1.2 电刺激实验
利用高频电刺激实验模拟人类心脏的应激状态,构建果蝇心衰模型,具体方法请参考文献[12,13]。利用脉冲电激仪循环交流电(6 Hz、40 V)刺激30 s,电刺激停止后的2 min内评估心脏功能,存在如下3种情况:(1)正常:心脏恢复正常而有节律的收缩跳动;(2)心脏纤颤:心脏表现为剧烈的收缩、回旋或者缓慢的蠕动等不正常行为;(3)心脏停搏:心脏停止跳动。心衰率是指果蝇发生心脏纤颤或心脏停搏的百分比。每个基因型检测150只以上,卡方检验进行统计学分析。1.3 果蝇成体心脏功能分析
在模拟果蝇生理缓冲液(108 mmol/L NaCl、5 mmol/L KCl、2 mmol/L CaCl2、8 mmol/L MgCl2、15 mmol/L HEPES、1 mmol/L NaH2PO4、4 mmol/L NaHCO3、10 mmol/L Sucrose、5 mmol/L Trehalose,pH 7.1)中解剖果蝇,剪去果蝇的大脑和胸部的腹神经节,排除神经对果蝇心跳的影响。暴露于模拟生理缓冲液中的心脏仍可以处于有节律的跳动,方法详见文献[14]。1周龄、3周龄和5周龄果蝇相当于人类的青年、中年和老年[15]。用高速EM-CCD摄像机(Hamamatsu C9300,日本)以130帧/秒的速度拍摄1周龄、3周龄和5周龄的果蝇心跳30 s,利用果蝇成体心脏功能分析软件分析获得M-mode心跳图谱,并测得心跳周期、收缩期和舒张期、收缩直径和舒张直径、缩短分数,以及心律失常指数等参数。心率失常指数是指果蝇心脏每个心跳周期与整个心跳周期中值的差异大小,代表果蝇不规律心跳的程度[7,14,16]。1.4 生存曲线分析
果蝇在25℃环境下培养,每管不超过25只。每隔3 d换一次果蝇食物管,并记录每管果蝇中的死亡数。根据每个时间点上果蝇死亡的数量,绘制生存曲线,分析果蝇的平均寿命。每个杂交组收集大约250只F1子代果蝇,并进行3次重复实验,以确保实验的可重复性。利用Graphpad Prism 8.0软件对数据进行Mantel-Cox统计学分析,结果呈现的是1次重复实验结果。2 结果与分析
2.1 shaker突变体心脏抗应激能力下降
老龄心脏在静息时表现功能正常,但由于生理储备下降在应激状态下表现为心衰。为了模拟人类心脏在应激状态下的生理表现,本研究利用脉冲电激仪对果蝇心脏进行高频电刺激30 s[12,15]。野生型w1118果蝇心脏出现心脏纤颤或心脏停搏的比例相对较低,心衰率为24%;钾离子通道蛋白纯和突变体KCNQ186果蝇的心衰率为75% (图1),与Ocorr等[7]研究数据相近。shaker14突变破坏了钾离子通道S5-S6跨膜区域,钾离子不能通过[17]。电刺激实验显示,shaker缺失系的杂合体(shaker Df/+)和纯合突变体(shaker14 homo)的心衰率分别为50%和56% (图1),具有显著性差异,表明Shaker钾离子通道功能的丧失或降低使电刺激诱发心衰的易感性显著增加,标志着心脏抗应激能力的下降。图1
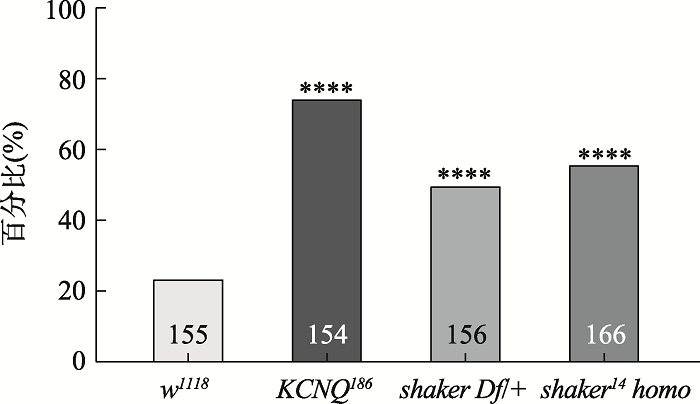 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1Shaker钾离子通道功能的丧失或降低增加电刺激诱发的心衰发生率
样本量分别标记在柱形图内,****:P<0.0001。
Fig. 1The loss or reduction of Shaker function increases the incidence of pacing-induced heart failure
2.2 钾离子通道蛋白Shaker维护老龄果蝇心脏的生理功能
为了进一步阐明钾离子通道蛋白Shaker在调节老龄心脏的生理学功能,本研究利用心脏特异性驱动子hand4.2 Gal4品系与shaker基因的RNAi品系杂交,获得F1代,在心脏中特异性敲低shaker (shaker KD)。利用高速EM-CCD摄像机拍摄果蝇心跳,获得M-mode心跳图,显示果蝇心脏壁随着心脏收缩和舒张的运动轨迹。与对照组相比,5周龄心脏特异性shaker KD果蝇的收缩期和舒张期延长,出现纤维性震颤(图2)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2心脏特异性敲低shaker基因的果蝇M-mode心跳图
M-mode图揭示了对照组(control, hand4.2/+)和心脏特异性敲低shaker组(shaker KD, hand4.2>shaker RNAi) 5周龄果蝇心脏壁在10 s内的运动轨迹。绿色代表舒张期,红色线条代表收缩期,蓝色线条代表心跳周期。
Fig. 2M-mode demonstrates the cardiac arrhythmia in cardiac specific shaker knockdown
基于M-mode心跳图谱分析测得心跳周期、收缩期和舒张期、收缩直径和舒张直径、缩短分数,以及心律失常指数等参数。结果发现,与对照组相比,1周龄和3周龄心脏特异性shaker KD果蝇心脏收缩期、舒张期以及心率不齐指数没有明显的异常;5周龄心脏特异性shaker KD果蝇心脏虽然代表收缩功能的缩短分数没有显著差异(图3G),但是心跳周期明显延长,包括收缩期和舒张期的延长,心律失常指数明显增加(图3:A~D),表明钾离子通道蛋白Shaker参与了老龄而非低龄果蝇心脏功能的维持。
正常果蝇的收缩期在0.15~0.25 s之间,舒张期在0.3~0.5 s之间。鉴于5周龄心脏特异性shaker KD果蝇出现了心脏纤颤和心脏短暂停搏,本研究记录了收缩期大于0.4 s和舒张期大于1 s的心跳为异常长收缩期(long systolic interval)和异常长舒张期(long diastolic interval)[18,19]。与对照组相比,心脏特异性shaker KD果蝇心脏从3周龄开始出现长时间的收缩或者舒张的发生率增加,并且随着年龄的增长,这种异常的表型会进一步加重(图3:E~F)。为了排除个别果蝇出现异常表型引起的实验误差,本研究对出现长收缩期的果蝇或者长舒张期的果蝇数量进行了统计,结果也显示出了相似的趋势(图3:H~I)。综上所述,钾离子通道蛋白Shaker在维持老龄果蝇心脏功能中具有非常重要作用。
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3心脏特异性敲低shaker基因导致年龄依赖性心衰
A~D、G:1周龄、3周龄和5周龄果蝇心脏特异性shaker KD (hand4.2>shaker RNAi)和对照组果蝇(hand4.2/+和shaker RNAi/+)的心脏生理功能参数,包括心跳周期、收缩期、舒张期、心律不齐指数和缩短分数。E, F:异常长收缩期(收缩期大于0.4 s)或者异常长舒张期(舒张期大于1 s)的百分比。H、I:含有长收缩期或者长舒张期的果蝇数量百分比所有数据均采用单因素方差分析。*:P<0.5;***:P<0.01。每个样本量为20~30个果蝇,error bar为SEM。
Fig. 3The cardiac specific shaker knockdown causes age-dependent deterioration of adult heart function
2.3 心脏特异性敲低shaker基因影响果蝇寿命
鉴于钾离子通道蛋白Shaker在维持衰老心脏功能方面的显著作用,本研究检测了心脏特异性shaker KD对果蝇寿命的影响。与对照组果蝇(51 d)相比,心脏特异性shaker KD果蝇的中位生存期(42 d)显著降低(图4),中位生存期缩短了17.6%,具有统计学显著意义,表明心脏特异性敲低钾离子通道蛋白Shaker缩短果蝇寿命,而心脏功能的衰退可能是决定寿命的重要因素。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4心脏特异性敲低shaker基因会影响果蝇生存
X轴为成体果蝇的存活时间(d),Y轴为存活果蝇的百分数。黑线和红线分别代表对照组(hand4.2/+)和shaker KD (hand4.2 > shaker RNAi)的生存曲线。样本量为250只果蝇,Mandel-Cox对数秩检验,****:P<0.0001。
Fig. 4Cardiac-specific shaker KD reduces life span
3 讨论
随着人类寿命的不断增加,对年龄相关性心脏功能障碍的研究越来越被关注。然而,对脊椎动物心脏衰老的研究存在着一些局限性。首先,在哺乳动物中与心衰发生相关的基因突变通常具有致死性;其次,哺乳动物寿命相对较长,不利于研究年龄相关性疾病的发病机制。果蝇是唯一具有心脏的无脊椎动物模型,其氧气供给不受心脏功能影响,心脏特异性敲低心衰发生相关基因的表达可能不致死;果蝇的生命周期短暂,有利于年龄相关性疾病的研究[18,20]。此外,果蝇具有与人类相似的心脏功能障碍,如扩张型心肌病、心律失常等;与人类心律失常相关的基因在果蝇中具有同样的功能。因此,果蝇是研究心衰的理想模型[18]。钾离子通道负责心脏动作电位的复极相,从而保证心脏节律性收缩;钾离子通道功能丧失,如KCNQ和HERG/eag,会导致果蝇或人类的年龄依赖性心律失常[7,8,9]。KCNQ突变体果蝇在1周龄时已经出现明显的心率失常,并且呈现年龄依赖性恶化。而心脏特异性shaker KD果蝇在1周龄和3周龄时表现正常,只有在5周龄时才表现出异常的生理功能,表明钾离子通道蛋白Shaker对低龄果蝇心脏功能不是必须的,但是参与了老龄果蝇心脏功能的维持。5周龄shaker KD果蝇心脏的收缩力没有受损,表明shaker KD不影响心脏结构的重塑。
心脏衰老的特征之一是心脏抗应激能力下降。本研究利用电刺激实验,发现shaker突变体对应激刺激诱发心衰的易感性较高,类似于人类心脏衰老。同时,心脏特异性shaker KD会显著提高老龄果蝇心律失常的发生率。我们推测心律失常发生率的增加和抗应激刺激能力的降低很可能是由于老龄果蝇心脏中shaker基因表达下调,心肌电位复极异常引起的。钾离子通道蛋白Shaker的研究可能为年龄依赖性心衰的治疗提供新的靶标。
致谢
感谢美国Sanford Burnham医学研究所Rolf Bodmer教授和湖南师范大学吴秀山教授提供果蝇成体心脏功能分析平台和心脏特异性驱动子hand4.2 Gal4;感谢瑞典斯德哥尔摩大学Mattias Mannervik教授对本论文的指导。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.jacc.2018.03.019URLPMID:29724357 [本文引用: 1]

Advances in medical care have led to an increase in the number of octogenarians and even older patients, forming an important and unique patient subgroup. It is clear that advancing age is an independent risk factor for the development of most arrhythmias, causing substantial morbidity and mortality. Patients >/=80 years of age have significant structural and electrical remodeling of cardiac tissue; accrue competing comorbidities; react differently to drug therapy; and may experience falls, frailty, and cognitive impairment, presenting significant therapeutic challenges. Unfortunately, very old patients are under-represented in clinical trials, leading to critical gaps in evidence to guide effective and safe treatment of arrhythmias. In this state-of-the-art review, we examine the pathophysiology of aging and arrhythmias and then present the available evidence on age-specific management of the most common arrhythmias, including drugs, catheter ablation, and cardiac implantable electronic devices.
DOI:10.1177/1074248417729880URLPMID:28946759 [本文引用: 1]

The development of novel drugs specifically directed at the ion channels underlying particular features of cardiac action potential (AP) initiation, recovery, and refractoriness would contribute to an optimized approach to antiarrhythmic therapy that minimizes potential cardiac and extracardiac toxicity. Of these, K(+) channels contribute numerous and diverse currents with specific actions on different phases in the time course of AP repolarization. These features and their site-specific distribution make particular K(+) channel types attractive therapeutic targets for the development of pharmacological agents attempting antiarrhythmic therapy in conditions such as atrial fibrillation. However, progress in the development of such temporally and spatially selective antiarrhythmic drugs against particular ion channels has been relatively limited, particularly in view of our incomplete understanding of the complex physiological roles and interactions of the various ionic currents. This review summarizes the physiological properties of the main cardiac potassium channels and the way in which they modulate cardiac electrical activity and then critiques a number of available potential antiarrhythmic drugs directed at them.
DOI:10.1152/physrev.00022.2013URL [本文引用: 2]

About 10 distinct potassium channels in the heart are involved in shaping the action potential. Some of the K+ channels are primarily responsible for early repolarization, whereas others drive late repolarization and still others are open throughout the cardiac cycle. Three main K+ channels drive the late repolarization of the ventricle with some redundancy, and in atria this repolarization reserve is supplemented by the fairly atrial-specific K(V)1.5, K(ir)3, K-Ca, and K-2P channels. The role of the latter two subtypes in atria is currently being clarified, and several findings indicate that they could constitute targets for new pharmacological treatment of atrial fibrillation. The interplay between the different K+ channel subtypes in both atria and ventricle is dynamic, and a significant up-and downregulation occurs in disease states such as atrial fibrillation or heart failure. The underlying posttranscriptional and posttranslational remodeling of the individual K+ channels changes their activity and significance relative to each other, and they must be viewed together to understand their role in keeping a stable heart rhythm, also under menacing conditions like attacks of reentry arrhythmia.
DOI:10.1038/nrcardio.2012.3URL [本文引用: 1]

The coordinated generation and propagation of action potentials within cardiomyocytes creates the intrinsic electrical stimuli that are responsible for maintaining the electromechanical pump function of the human heart. The synchronous opening and closing of cardiac Na+, Ca2+, and K+ channels corresponds with the activation and inactivation of inward depolarizing (Na+ and Ca2+) and outward repolarizing (K+) currents that underlie the various phases of the cardiac action potential (resting, depolarization, plateau, and repolarization). Inherited mutations in pore-forming alpha subunits and accessory beta subunits of cardiac K+ channels can perturb the atrial and ventricular action potential and cause various cardiac arrhythmia syndromes, including long QT syndrome, short QT syndrome, Brugada syndrome, and familial atrial fibrillation. In this Review, we summarize the current understanding of the molecular and cellular mechanisms that underlie K+-channel-mediated arrhythmia syndromes. We also describe translational advances that have led to the emerging role of genetic testing and genotype-specific therapy in the diagnosis and clinical management of individuals who harbor pathogenic mutations in genes that encode alpha or beta subunits of cardiac K+ channels.
DOI:10.1016/j.ccep.2016.01.007URLPMID:27261826 [本文引用: 1]

The cardiac action potential is generated by intricate flows of ions across myocyte cell membranes in a coordinated fashion to control myocardial contraction and the heart rhythm. Modulation of the flow of these ions in response to a variety of stimuli results in changes to the action potential. Abnormal or altered ion currents can result in cardiac arrhythmias. Abnormalities of autonomic regulation of potassium current play a role in the genesis of cardiac arrhythmias, and alterations in acetylcholine-activated potassium channels may play a key role in atrial fibrillation. Ischemia is another important modulator of cardiac cellular electrophysiology.
DOI:10.1016/j.hrthm.2005.07.025URLPMID:16253915 [本文引用: 1]

BACKGROUND: Long QT syndrome (LQTS) is a cardiovascular disorder characterized by prolonged QTc time, syncope, or sudden death caused by torsades de pointes and ventricular fibrillation. We investigated the clinical and electrophysiologic phenotype of individual mutations and the compound mutations in a family in which different genotypes could be found. OBJECTIVES: The purpose of this study was to determine the impact of genotype-based diagnostic assessment in LQTS. METHODS: We used cascade screening and functional analyses to investigate the phenotype in a family with LQTS. The contributions of the compound mutations in the KCNQ1 and KCNH2 genes (KCNQ1 R591H, KCNH2 R328C) were analyzed by heterologous expression in Xenopus laevis oocytes using two-electrode voltage clamp and by confocal imaging. RESULTS: KCNH2 R328C did not show any functional phenotype whereas KCNQ1 R591H resulted in severe reduction of current. Neither wild-type nor mutant channels affected each other functionally in coexpression experiments. Therefore, a direct interaction between KCNQ1 and KCNH2 was ruled out under these conditions. CONCLUSION: Assessment of novel mutational findings in LQTS should include accurate genetic and functional analysis. Notably, appropriate studies are needed if two or more mutations in different genes are present in one proband. Our findings prompt reconsideration of the impact of compound mutations in LQTS families and reinforce the need for thorough functional evaluation of novel ion channel mutations before assignment of pathogenic status.
DOI:10.1073/pnas.0609278104URLPMID:17360457 [本文引用: 4]

Population profiles of industrialized countries show dramatic increases in cardiovascular disease with age, but the molecular and genetic basis of disease progression has been difficult to study because of the lack of suitable model systems. Our studies of Drosophila show a markedly elevated incidence of cardiac dysfunction and arrhythmias in aging fruit fly hearts and a concomitant decrease in the expression of the Drosophila homolog of human KCNQ1-encoded K(+) channel alpha subunits. In humans, this channel is involved in myocardial repolarization, and alterations in the function of this channel are associated with an increased risk for Torsades des Pointes arrhythmias and sudden death. Hearts from young KCNQ1 mutant fruit flies exhibit prolonged contractions and fibrillations reminiscent of Torsades des Pointes arrhythmias, and they exhibit severely increased susceptibility to pacing-induced cardiac dysfunction at young ages, characteristics that are observed only at advanced ages in WT flies. The fibrillations observed in mutant flies correlate with delayed relaxation of the myocardium, as revealed by increases in the duration of phasic contractions, extracellular field potentials, and in the baseline diastolic tension. These results suggest that K(+) currents, mediated by a KCNQ channel, contribute to the repolarization reserve of fly hearts, ensuring normal excitation-contraction coupling and rhythmical contraction. That arrhythmias in both WT and KCNQ1 mutants become worse as flies age suggests that additional factors are also involved.
DOI:10.1038/nature04710URLPMID:16554806 [本文引用: 2]

hERG potassium channels are essential for normal electrical activity in the heart. Inherited mutations in the HERG gene cause long QT syndrome, a disorder that predisposes individuals to life-threatening arrhythmias. Arrhythmia can also be induced by a blockage of hERG channels by a surprisingly diverse group of drugs. This side effect is a common reason for drug failure in preclinical safety trials. Insights gained from the crystal structures of other potassium channels have helped our understanding of the block of hERG channels and the mechanisms of gating.
DOI:10.1371/journal.pgen.1006786URLPMID:28542428 [本文引用: 2]

Understanding the cellular-molecular substrates of heart disease is key to the development of cardiac specific therapies and to the prevention of off-target effects by non-cardiac targeted drugs. One of the primary targets for therapeutic intervention has been the human ether a go-go (hERG) K+ channel that, together with the KCNQ channel, controls the rate and efficiency of repolarization in human myocardial cells. Neither of these channels plays a major role in adult mouse heart function; however, we show here that the hERG homolog seizure (sei), along with KCNQ, both contribute significantly to adult heart function as they do in humans. In Drosophila, mutations in or cardiac knockdown of sei channels cause arrhythmias that become progressively more severe with age. Intracellular recordings of semi-intact heart preparations revealed that these perturbations also cause electrical remodeling that is reminiscent of the early afterdepolarizations seen in human myocardial cells defective in these channels. In contrast to KCNQ, however, mutations in sei also cause extensive structural remodeling of the myofibrillar organization, which suggests that hERG channel function has a novel link to sarcomeric and myofibrillar integrity. We conclude that deficiency of ion channels with similar electrical functions in cardiomyocytes can lead to different types or extents of electrical and/or structural remodeling impacting cardiac output.
DOI:10.1002/cne.10977URLPMID:14689489 [本文引用: 1]

While the larval neuromuscular junction (NMJ) of Drosophila has emerged as a model system to study synaptic function and development, little attention has been given to the study of the adult NMJ. Here we report an immunocytochemical and morphological characterization of an adult NMJ preparation of the prothorax. All muscles examined were innervated by small, uniform type II terminals (0.5-1.5 microm), a subset of which contained octopamine. Terminals classified as type I varied in their morphology across different muscles, ranging from strings or clusters of boutons (0.8-5.5 microm) to an elongate terminal (80-100 microm long) with few branches and contiguous swellings (3-15 microm) along its length. Analysis of the molecular composition of the NMJs during the first 5 days after eclosion revealed four major findings: 1) type I boutons increase in size during early adulthood; 2) Fasciclin II-immunoreactivity is not detectable at type I terminals, while DLG-immunoreactivity is observed at the synapse; 3) a Shaker-GFP fusion protein that localizes to all type I boutons in the larva is differentially localized at adult prothoracic NMJs; and 4) while all type I terminals contain glutamate, the glutamate receptor subunits, DGluRIIA and DGluRIIB, are expressed and clustered in only a subset of muscles. These findings suggest that maturation of the adult NMJ occurs during early adulthood and that muscle-specific properties may play a role in organizing synaptic components in the adult. Furthermore, these results demonstrate that there are major differences in the molecular organization of the adult and larval NMJs.
DOI:10.1046/j.1471-4159.2002.01092.xURLPMID:12354297 [本文引用: 1]

Subcellular localization of ion channels is crucial for the transmission of electrical signals in the nervous system. Here we show that Discs-Large (DLG), a member of the MAGUK (membrane-associated guanylate kinases) family in Drosophila, co-localizes with Shaker potassium channels (Sh Kch) in most synaptic areas of the adult brain and in the outer membrane of photoreceptors. However, DLG is absent from axonal tracts in which Sh channels are concentrated. Truncation of the C-terminal of Sh (including the PDZ binding site) disturbs its pattern of distribution in both CNS and retina, while truncation of the guanylate kinase/C-terminal domain of DLG induces ectopic localization of these channels to neuronal somata in the CNS, but does not alter the distribution of channels in photoreceptors. Immunocytochemical, membrane fractionation and detergent solubilization analysis indicate that the C-terminal of Sh Kch is required for proper trafficking to its final destination. Thus, several major conclusions emerge from this study. First, DLG plays a major role in the localization of Sh channels in the CNS and retina. Second, localization of DLG in photoreceptors but not in the CNS seems to depend on its interaction with Sh. Third, the guanylate kinase/C-terminal domain of DLG is involved in the trafficking of Shaker channels but not of DLG in the CNS. Fourth, different mechanisms for the localization of Sh Kch operate in different cell types.
DOI:10.2144/04371ST01URLPMID:15283201 [本文引用: 2]

The rapid life cycle and genetic tractability of Drosophila make it an ideal organism for large-scale genetic screens. Here we describe a novel assay for pupal heart rate and rhythmicity as well as techniques to measure adult cardiac stress response. These assays can be powerfully combined to concurrently screen for both mutations affecting cardiac function and mutations affecting the age-dependent decline in adult cardiac stress response. Mutations identified in such screens have the potential to contribute greatly to the understanding of both congenital heart disease and the regulation of age-dependent decline in cardiac function in the human population.
[本文引用: 1]
[本文引用: 1]
DOI:10.2144/000113078URLPMID:19317655 [本文引用: 2]

The genetic basis of heart development is remarkably conserved from Drosophila to mammals, and insights from flies have greatly informed our understanding of vertebrate heart development. Recent evidence suggests that many aspects of heart function are also conserved and the genes involved in heart development also play roles in adult heart function. We have developed a Drosophila heart preparation and movement analysis algorithm that allows quantification of functional parameters. Our methodology combines high-speed optical recording of beating hearts with a robust, semi-automated analysis to accurately detect and quantify, on a beat-to-beat basis, not only heart rate but also diastolic and systolic intervals, systolic and diastolic diameters, percent fractional shortening, contraction wave velocity, and cardiac arrhythmicity. Here, we present a detailed analysis of hearts from adult Drosophila, 2-3-day-old zebrafish larva, and 8-day-old mouse embryos, indicating that our methodology is potentially applicable to an array of biological models. We detect progressive age-related changes in fly hearts as well as subtle but distinct cardiac deficits in Tbx5 heterozygote mutant zebrafish. Our methodology for quantifying cardiac function in these genetically tractable model systems should provide valuable insights into the genetics of heart function.
DOI:10.1038/ng1476URLPMID:15565107 [本文引用: 2]

Insulin-IGF receptor (InR) signaling has a conserved role in regulating lifespan, but little is known about the genetic control of declining organ function. Here, we describe progressive changes of heart function in aging fruit flies: from one to seven weeks of a fly's age, the resting heart rate decreases and the rate of stress-induced heart failure increases. These age-related changes are minimized or absent in long-lived flies when systemic levels of insulin-like peptides are reduced and by mutations of the only receptor, InR, or its substrate, chico. Moreover, interfering with InR signaling exclusively in the heart, by overexpressing the phosphatase dPTEN or the forkhead transcription factor dFOXO, prevents the decline in cardiac performance with age. Thus, insulin-IGF signaling influences age-dependent organ physiology and senescence directly and autonomously, in addition to its systemic effect on lifespan. The aging fly heart is a model for studying the genetics of age-sensitive organ-specific pathology.
[本文引用: 1]
URLPMID:1702382 [本文引用: 1]

Mutations in the Shaker (Sh) locus of Drosophila melanogaster have differing effects on action potential duration and repolarization in neurons as well as on A-type K+ channels (IA) in muscle. The molecular basis of three exemplary Sh alleles (ShKS133, ShE62 and Sh5) has been identified. They are point mutations in the Sh transcription unit expressing aberrant voltage-gated A-type K+ channels. Replicas of each mutation have been introduced by in vitro mutagenesis into Sh cDNA. The expression of in vitro transcribed mutant Sh cRNA in Xenopus laevis oocytes reproduced the specific phenotypic traits of each Sh allele. The lack of IA in ShKS133 is due to a missense mutation within a sequence motif occurring in all hitherto characterized voltage-gated K+ channel forming proteins. The reduction of IA in ShE62 is due to a mutation in an AG acceptor site. The intervening sequence between exons 19 and 20 is not spliced in ShE62 RNA. As a consequence, ShE62 flies do not contain the full complement of Sh K+ forming proteins. Finally, the Sh5 mutation leads to an altered voltage dependence of K+ channel activation and inactivation as well as to an accelerated rate of recovery from inactivation. This is due to a missense mutation altering the amino acid sequence of the proposed transmembrane segment S5 of the Sh K+ channels. Segment S5 is located adjacently to the presumed voltage sensor of voltage-gated ion channels. The results explain the altered properties of excitable cells in Sh mutants and provide a general model for the possible role of A-type K+ channels in modulating action potential profiles.
DOI:10.1016/j.exger.2010.11.035URL [本文引用: 3]

With age, cardiac performance declines progressively and the risk of heart disease, a primary cause of mortality, rises dramatically. As the elderly population continues to increase, it is critical to gain a better understanding of the genetic influences and modulatory factors that impact cardiac aging. In an attempt to determine the relevance and utility of the Drosophila heart in unraveling the genetic mechanisms underlying cardiac aging, a variety of heart performance assays have recently been developed to quantify Drosophila heart performance that permit the use of the fruit fly to investigate the heart's decline with age. As for the human heart, Drosophila heart function also deteriorates with age. Notably, with progressive age the incidence of cardiac arrhythmias, myofibrillar disorganization and susceptibility to heart dysfunction and failure all increase significantly. We review here the evidence for an involvement of the insulin-TOR pathway, the K(ATP) channel subunit dSur, the KCNQ potassium channel, as well as Dystrophin and Myosin in fly cardiac aging, and discuss the utility of the Drosophila heart model for cardiac aging studies. (C) 2010 Elsevier Inc.
DOI:10.1002/cphy.c140063URLPMID:26140710 [本文引用: 1]

Aging is a major risk factor for the development of cardiovascular disease, with the majority of affected patients being elderly. Progressive changes to myocardial structure and function occur with aging, often in concert with underlying pathologies. However, whether chronological aging results in a remodeled
DOI:10.1016/S0034-5288(18)34880-XURL [本文引用: 1]
