 ,武汉大学生命科学学院,细胞稳态湖北省重点实验室,武汉 430072
,武汉大学生命科学学院,细胞稳态湖北省重点实验室,武汉 430072An overview of bile acid synthesis and its physiological and pathological functions
Xiao Liu, Yan Wang ,Hubei Province Key Laboratory of Cell Homeostasis, College of Life Sciences, Wuhan University, Wuhan 430072, China
,Hubei Province Key Laboratory of Cell Homeostasis, College of Life Sciences, Wuhan University, Wuhan 430072, China通讯作者:
编委: 陈雁
收稿日期:2019-01-9修回日期:2019-03-18网络出版日期:2019-05-20
| 基金资助: |
Received:2019-01-9Revised:2019-03-18Online:2019-05-20
| Fund supported: |
作者简介 About authors
刘笑,硕士研究生,专业方向:脂类代谢E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (408KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
刘笑, 王琰. 胆汁酸的合成调控及其在生理与病理中的功能机制[J]. 遗传, 2019, 41(5): 365-374 doi:10.16288/j.yczz.19-011
Xiao Liu, Yan Wang.
胆固醇是机体内膜性结构的重要组成物质,其代谢紊乱会引发动脉粥样硬化和冠心病等一系列代谢性疾病[1]。体内胆固醇的来源可以分为外源食物摄取和内源机体合成。机体无法直接将胆固醇分解,但是可以利用胆固醇为原料,经过一系列的酶促催化反应将其转化为胆汁酸。肝脏合成的胆汁酸以及部分游离胆固醇以胆汁的形式从肝脏分泌进入胆管,并最终分泌至小肠。进入小肠中的胆汁酸95%以上会被小肠重新吸收,然后通过肝脏门静脉循环进入肝脏,另外5%左右会以粪便的形式排出体外[2]。机体通过调节胆汁酸的合成、分泌及重吸收等过程精确调节体内胆汁酸及胆固醇的稳态平衡。而体内的胆汁酸也是一种信号分子,能够与其受体核受体法呢醇X受体(farnesoid X receptor, FXR)和细胞膜表面受体G蛋白偶联受体(cell membrane surface receptor-G protein coupled receptor, TGR5)等相互作用,启动下游信号通路。本文将从胆汁酸的生物合成、肝肠循环及胆汁酸合成限速酶CYP7A1的表达调控等方面,总结近年来胆汁酸的合成调控及功能机制研究进展,以期为胆汁酸代谢调控分子机制的研究提供参考。
1 胆汁酸的生物合成
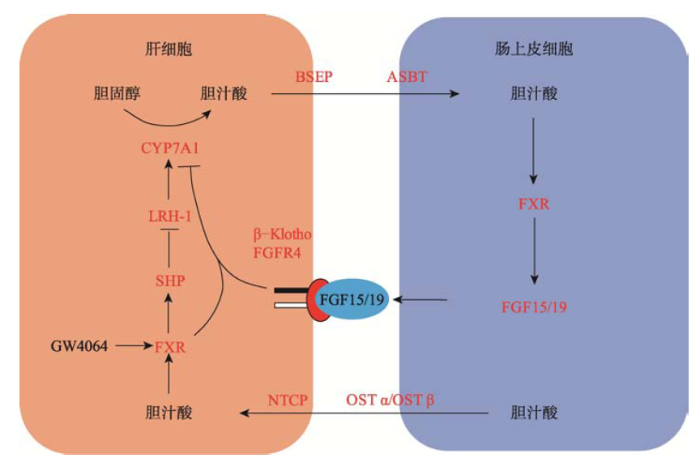
体内胆固醇水平的稳态主要由胆固醇的外源摄取、胆固醇的体内合成及胆固醇外排协调控制的,其中胆固醇经过一系列的酶促反应生成胆汁酸是胆固醇代谢的主要去路[3]。人体内胆汁酸的合成通路包括经典通路和非经典通路[4]。经典通路是在肝脏中,以定位于肝细胞内质网上的胆固醇7-α羟化酶(cholesterol 7α-hydroxylase, CYP7A1)为主要限速酶经过一系列的催化反应发生的,生成胆酸(cholic acid, CA)和鹅脱氧胆酸(chenodeoxycholic acid, CDCA)两种疏水性初级胆汁酸。非经典通路发生在多种组织及巨噬细胞中,以定位于线粒体的甾醇27A羟化酶(sterol 27A-hydroxylase, CYP27A1)和定位于内质网的氧甾醇和类固醇7α-羟化酶(oxysterol and steroid 7α-hydroxylase, CYP7B1)启动发生的[5,6]。Axelson等[7]的研究认为,非经典通路主要发生在一些病理状态下,当肝脏中CYP7A1的活性下降时,非经典通路通过产生鹅脱氧胆酸调节体内的代谢平衡。肝脏中生成的疏水性初级胆汁酸可以被甘氨酸或牛磺酸共价修饰形成胆酸盐。胆酸盐较初级胆汁酸的水溶性增加,降低了胆汁酸的毒性[8],使其可以被分泌到小肠。小肠中的肠道菌群可进一步代谢胆酸盐,使其脱去羟基,移去甘氨酸和牛磺酸形成次级胆汁酸,即脱氧胆酸(deoxycholic acid, DCA)和石胆酸(lithocholic acid, LCA)。CA、DCA及CDCA可以被小肠的刷状缘细胞重吸收经过门静脉循环被运回到肝脏[9]。2 胆汁酸的肝肠循环
肝脏中生成的胆酸盐通过肝细胞表面的胆汁酸转运蛋白-胆盐输出泵(bile salt export pump, BSEP)被运送到胆小管,并储存在胆囊中。当进食后,胆囊收缩,将胆汁酸分泌到肠道[10],少部分胆汁酸可通过被动吸收的方式被十二指肠吸收。其中95%的胆汁酸在回肠中被主动吸收[9],这一过程依赖于小肠刷状缘细胞表面的Na+依赖的胆汁酸转运体(apical sodium dependent bile acid transporter, ASBT)。进入小肠细胞内的胆汁酸可以从肠上皮细胞极性膜一侧转移到基底膜,通过基底膜上的有机溶质转运体(organic solute transporter α and β heterdimer, OSTα/OSTβ)异源二聚体排出细胞,进而被转运到肝脏门静脉。到达肝脏的胆汁酸被肝细胞细胞膜表面的Na+依赖的牛磺酸盐协同转运肽(Na+-dependent taurocholate cotransport peptide, NTCP)吸收进入肝细胞。这一过程被称为胆汁酸的肝肠循环。人体内的胆汁酸总量大约有3 g,每天可以进行4~12次的肝肠循环。人体粪便中每天排出的胆汁酸大约有0.5 g,这部分胆汁酸通过肝脏中胆汁酸的从头合成途径生成,从而维持胆汁酸总量的动态平衡[4,7]。胆汁酸的肝肠循环促进了脂类及维生素等营养物质的乳化和吸收[11],并且使肝脏内胆汁酸的合成和小肠内胆汁酸的重吸收协同作用,共同维持机体胆汁酸及胆固醇的代谢平衡。
3 以CYP7A1为靶标的胆汁酸合成的代谢调控
CYP7A1作为胆汁酸合成的关键限速酶[3],其表达调控对于维持机体胆汁酸的稳态发挥了重要作用。研究发现,FXR作为胆汁酸的受体,在机体胆汁酸负反馈调节过程中发挥了重要作用[12,13]。FXR主要表达于肝脏和小肠[14],响应胆汁酸的刺激,在小肠和肝脏中分别通过不同的调控途径负反馈抑制CYP7A1的表达。机体中CYP7A1的表达水平除了受FXR调节之外,还受到FXR非依赖途径的调节,这一途径受到多种细胞因子、激素和酶的调节。这些因子共同作用,确保机体能够响应不同环境刺激,维持机体的正常运行。3.1 FXR依赖的CYP7A1表达调控
3.1.1 肝脏中FXR参与的CYP7A1表达调控FXR作为胆汁酸的感应器负反馈抑制CYP7A1的表达[12,15]。FXR高表达于肝脏和小肠,在肝脏中CA及CDCA可以激活FXR核受体活性[5]。但是CYP7A1启动子区没有FXR的结合域,FXR通过与其他基因相互作用间接抑制CYP7A1的表达。活化的FXR首先与视黄酸X受体α (retinoid X receptor α, RXRα)结合形成异源二聚体,此异源二聚体可与目的基因启动子区的法呢醇X受体响应元件(farnesoid X receptor response element, FXREs)结合,进而上调或抑制基因的表达[16]。Goodwin等[15]经过大量筛选找到了FXR特异性的激活剂GW4064,发现GW4064对FXR的激活作用是CDCA的1000倍。GW4064刺激人和大鼠肝细胞时,小异二聚体伴侣(small heterodimer partner-1, SHP-1)的mRNA含量明显增加[17]。SHP-1是一个非典型的核孤儿受体家族成员,它缺乏DNA结合域,含有一个N端受体二聚化结合域,SHP-1通过其N端二聚化受体结合域招募其他受体,并通过抑制这些受体的转录来调节其他下游基因的表达。SHP-1在肝脏中低表达,在胆汁酸刺激的情况下表达量迅速升高。SHP-1 KO小鼠中,CYP7A1的表达水平明显上调,胆汁酸池明显增大。SHP-1过表达小鼠中胆汁酸池明显减小,并伴随甘油三酯的堆积[18,19]。SHP-1可以与核孤儿受体肝脏相关同系物1 (liver related homologue-1, LRH-1)结合并抑制此受体的活性。LRH-1是核受体家族的胞内转录因子,可以与CYP7A1的启动子区结合,上调CYP7A1的转录[17,20]。另一方面,LRH-1可以通过促进肠上皮细胞分泌成纤维细胞生长因子15 (fibroblast growth factor 15, FGF15)进而抑制CYP7A1的合成[16,18]。
综上所述,在肝脏中,CA及CDCA可以结合并活化FXR,活化的FXR首先与RXRα结合形成异源二聚体,此异源二聚体可以与SHP-1的启动子区结合并上调SHP-1的表达,SHP-1通过与LRH-1相互作用从而抑制CYP7A1的表达(图1)[17,19]。
图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1胆汁酸的肝肠循环及代谢调控
Fig. 1The enterohepatic circulation and metabolic regulation of bile acids
3.1.2 小肠中FXR参与的CYP7A1表达调控
小肠中的FXR通过调节内分泌成纤维细胞生长因子15/19 (fibroblast growth factor 15/19, FGF15/19)的表达来抑制肝脏中CYP7A1的表达[20,21,22]。FGF19是成纤维细胞生长因子(fibroblast growth factor, FGFs)亚家族的成员之一,包括FGF19、FGF21和FGF23,是胆汁酸合成、葡糖糖吸收、脂代谢、维生素D和磷酸盐稳态的重要调控因子。小鼠中没有FGF19,研究发现小鼠FGF15蛋白的氨基酸序列与人FGF19蛋白的氨基酸序列有51%的同源性,并发挥了相似作用[23]。FGF19高表达于十二指肠和回肠,低表达于肝脏[24]。当小肠中的胆汁酸浓度增加时FXR被激活,活化的FXR进而上调FGF19的表达,FGF19可以通过旁分泌和内分泌途径发挥作用(图1)。FGF19分泌到血液后随肝门静脉循环被运回到肝脏,与肝细胞表面的成纤维细胞生长因子受体4 (fibroblast growth factor receptors 4, FGFR4)结合并磷酸化激活FGFR4受体酪氨酸激酶活性,通过有丝分裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)级联反应抑制CYP7A1的表达[25,26]。FGF19与FGFR4的作用需要β-Klotho的辅助。β-Klotho是一个大小为130 kDa的1型跨膜蛋白,主要表达于肝脏、脂肪以及胰腺[27]。β-Klotho缺失小鼠与FGFR4基因敲除鼠的表型非常一致,都伴随着CYP7A1转录水平的增加和胆汁酸池的增大[21]。β-Klotho可与FGFs作用并促进FGFs与受体的结合[28]。研究发现FGFR4与β-Klotho的相互作用发生在内质网。FGFR4含有3个糖基化修饰位点(N112、N258和N290),在内质网中形成核心糖基化FGFR4,核心糖基化FGFR4转运到高尔基体后经过进一步的修饰形成终端糖基化FGFR4。在β-Klotho存在时,FGFR4主要以终端糖基化形式存在。在内质网中β-Klotho与核心糖基化FGFR4相互结合,促进了核心糖基化FGFR4的蛋白酶体降解,从而使细胞内的终端糖基化FGFR4得到富集。研究还发现FGF19只与终端糖基化FGFR4结合并磷酸化激活FGFR4受体酪氨酸激酶活性,进而抑制CYP7A1的表达[29,30]。小肠中的FXR对肝脏中胆汁酸的合成起了重要的负反馈调节作用[31]。
3.2 FXR非依赖的CYP7A1表达调控
3.2.1 组蛋白去乙酰化酶参与的胆汁酸代谢调控胆固醇水平过高会引发动脉粥样硬化以及心脑血管等疾病。胆固醇转变为胆汁酸是胆固醇代谢的主要去路,在这个过程中发挥关键催化作用的酶是胆固醇7-α羟化酶CYP7A1[3]。研究发现,在HepG2细胞中,用CDCA刺激1 h后CYP7A1的表达有显著下调,但此时FXR、SHP和FGF19的表达没有变化,刺激16 h后SHP和FGFG19的mRNA含量明显增加[32]。但是用FXR的特异性激活剂GW4064肝脏中的胆固醇在CYP7A1等一系列酶的催化作用下可代谢为胆汁酸。一方面,肝脏中的胆汁酸通过激活FXR/SHP信号通路,抑制LRH-1的表达进而抑制的CYP7A1的表达。另一方面,肝脏中合成的胆汁酸在BSEP和ASBT的作用下被吸收进入小肠。在小肠中,胆汁酸激活FXR通路,刺激肠上皮细胞分泌FGF15/19,分泌的FGF15/19通过肝肠循环途径进入肝脏与肝细胞表面的FGFR4及β-Klotho相互作用抑制CYP7A1的表达。小肠中的胆汁酸通过OSTα/OSTβ和NTCP的作用被吸收进入肝细胞。GW4064是FXR的特异性激活剂。
刺激1 h[33],并未看到CYP7A1的下调。这说明起始CYP7A1的下调不依赖于FXR途径[34]。研究还发现在CDCA刺激的初始阶段,组蛋白去乙酰化酶7 (histone deacetylase 7, HDCA7)参与了CYP7A1的抑制调控。HDCA7正常情况下定位在细胞膜上,但是在BA刺激情况下会移位到细胞核内,并被招募到CYP7A1的启动子区,与组蛋白去乙酰化酶3(histone deacetylase 3, HDAC3)、视黄酸和甲状腺受体沉默介质(silencing mediator for retinoid and thyroid receptors, SMRT-α)以及核受体辅阻遏物(nuclear receptor corepressor, N-COR)相互作用促进RNA聚合酶2的解离,从而抑制CYP7A1的表达[32,35]。
3.2.2 胰高血糖素参与的胆汁酸代谢调控
在肝脏中胰高血糖素通过蛋白激酶A (protein kinase A, PKA)通路激活磷酸烯醇丙酮酸羧化激酶(phosphoenolpyruvate carboxykinase, PEPCK)并磷酸化肝细胞核因子(hepatocyte nuclear factor 4α, HNF4α)第304位的丝氨酸残基,从而抑制CYP7A1的表达。HNF4α是一个核转录因子,可以与CYP7A1的DR-1序列结合,从而激活CYP7A1的转录[36]。但是当HNF4α第304位丝氨酸残基被磷酸化后就会降低其与CYP7A1的结合能力和反式激活能力。因CYP7A1特异性的在肝脏中表达,PKA依赖的CYP7A1的抑制是肝脏特异性的,但是PKA依赖的PEPCK的活化在肝脏和肾脏中都存在。胰高血糖素在饥饿时分泌增加,所以CYP7A1的表达受饥饿进食影响[37]。胰高血糖素刺激下CYP7A1的转录被抑制,使体内胆汁酸的合成被抑制,进一步在胆汁酸乳化作用下脂的吸收水平下降[38],从而确保糖异生最大程度的被活化以维持体内的糖代谢和能量代谢。
3.2.3 细胞因子参与的胆汁酸代谢调控
肝细胞生长因子(hepatocyte growth factor, HGF)通过与酪氨酸激酶受体c-Met结合使其磷酸化被活化,进而调控了下游包括Ras、MAPK、PIP3及PKC等信号通路,在促进细胞生长、增殖、凋亡、创伤修复和组织再生等过程中发挥了重要作用[39]。肝脏部分切除后,在肝脏再生过程中胆汁酸的合成及CYP7A1的表达被抑制,同时血液中HGF的含量明显上调,HGF在肝再生过程中对胆汁酸的代谢调控发挥了重要作用[40,41,42]。研究发现,在人的原代肝细胞中,HGF可以显著抑制CYP7A1的表达。其作用机理是HGF通过与c-Met结合激活c-Met的磷酸酪氨酸激酶活性,通过磷酸化Erk1/2、JNK及PKC来抑制CYP7A1的表达。同时,HGF可以上调SHP-1的表达,进而抑制CYP7A1的转录(图2)[43]。HGF对CYP7A1的抑制,在肝损伤再生过程中发挥了重要作用,使肝细胞内胆汁酸浓度维持在一个较低水平,防止高胆汁酸浓度对细胞的毒害作用。
图2
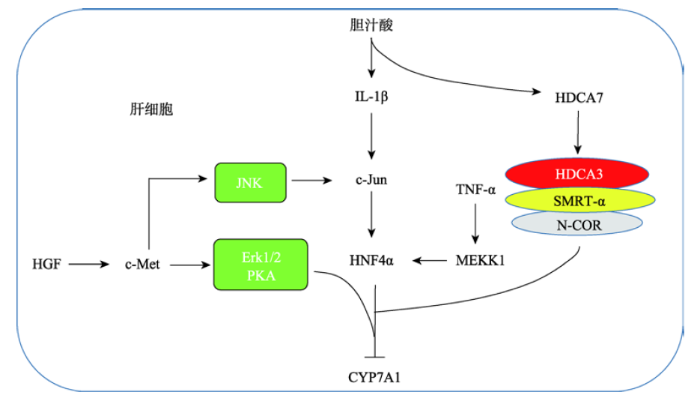 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2FXR非依赖的CYP7A1表达调控
Fig. 2FXR-independent regulation of CYP7A1 metabolism
肿瘤坏死因子α (tumor necrosis factor α, TNF-α)通过激活MAPKs家族的促分裂原活化蛋白激酶1 (mitogen-activated protein kinase kinase kinase 1, MEKK-1)进而磷酸化结合在CYP7A1胆汁酸响应元件序列区的HNF4α,从而降低HNF4α与CYP7A1的结合,抑制转录因子HNF4α的反式激活能力,从而下调CTP7A1的表达(图2)[34]。
胆汁酸可以激活肝脏巨噬细胞分泌炎症因子IL-1β,同时IL-1β又可以抑制胆汁酸的合成[43]。研究发现,IL-1β可以激活c-Jun的转录,一方面c-Jun可以抑制HNF4α招募过氧化物酶体增生激活受体γ的共活化因子1α (peroxisome proliferator-activated receptor γ co-activator 1α, PGC1-α);另一方面c-Jun可以被c-Jun-NH2末端激酶JNK磷酸化活化并抑制HNF4α与CYP7A1的结合,从而抑制CYP7A1的表达,以保护肝细胞免受炎症介导的毒害作用(图2)[44]。
4 其他胆汁酸受体参与的代谢调控
4.1 TGR5参与的代谢调控
胆汁酸作为细胞膜表面蛋白-G蛋白偶联受体TGR5的配体在能量代谢过程中发挥了重要作用[5,45]。TGR5广泛表达于小肠及胆囊的内皮细胞、肝窦内皮细胞和星状巨噬细胞,但是在肝实质细胞中不表达[46,47]。研究发现,在人和小鼠脂肪细胞中,DCA和LCA可以激活TGR5,活化的TGR5通过腺苷酸环化酶(cyclic adenosine monophosphate, cAMP)通路促进了甘油三酯的分解、脂肪酸的β氧化及线粒体的分裂和形成,从而促进了皮下白色脂肪组织的米色化和能量代谢[48]。在小鼠的棕色脂肪组织中,胆汁在肝脏中,FXR非依赖的CYP7A1表达调控主要有以下几条途径:(1)胆汁酸通过激活炎症因子IL-1β,进一步激活c-Jun的转录,通过磷酸化HNF4α来抑制CYP7A1的表达;(2)胆汁酸也可激活HDCA7,在HDAC3以及共轭抑制子SMRT-α和N-COR的相互作用下抑制CYP7A1的表达;(3)肿瘤坏死因子TNF-α通过激活MEKK1进而磷酸化HNF4α来抑制CYP7A1的表达;(4)肝细胞生长因子HGF通过激活c-Met,进而激活Erk1/2、PKA或JNK通路从而抑制CYP7A1的表达。酸通过与TGR5结合,可激活腺苷酸环化酶,进而激活2型碘化甲状腺氨酸脱碘酶(type2 iodothyronine deiodinases, D2),D2可以使抑制型的甲状腺激素T4转变为活跃型的甲状腺激素T3[49],从而促进能量代谢,抑制肥胖的发生并提高胰岛素敏感性[50]。在小鼠肠内分泌细胞系中,胆汁酸通过激活TGR5进而促进了胰高血糖素样肽-1 (glucagon-like peptide-1, GLP-1)的分泌。GLP-1可刺激胰岛素的合成,促进胰岛素从胰岛β细胞分泌,在调节血糖平衡,抑制糖尿病发生过程中发挥了重要作用[46,51]。最近的研究发现,当用小肠FXR的特异性激动剂Fexaramine (FEX)刺激小鼠时,可诱导肠道微生物分泌LCA,分泌的LCA可激活TGR5/GLP-1通路,进而提高了胰岛素的敏感性以及白色脂肪组织的棕色化,促进了能量代谢[47]。这为治疗肥胖、糖尿病和非酒精性脂肪肝的研究提供了靶标。
胆汁酸激活的TGR5代谢通路在天然免疫、细胞的增殖和迁移以及癌症发生过程中也发挥重要的作用。最近的研究发现,病毒感染后诱导胆汁酸合成与转运通路的激活,胞内的胆汁酸通过其受体TGR5激活RIG-1和MAVS通路,在抗病毒天然免疫过程中发挥重要作用[52]。也有文献报道,TGR5被胆汁酸(包括LCA、DCA、CDCA和CA)活化后,通过Janus激酶2/信号转导和转录活化因子3 (janus kinase 2/signal transducer and activator of transcription 3, JAK2/STAT3)通路可促进非小细胞肺癌的增值和迁移[53,54],促进癌症的发生,这为非小细胞肺癌的治疗提供了靶点[55]。
4.2 孕烷X受体参与的代谢调控
研究发现,在慢性胆汁淤积性肝病患者体内,LCA的浓度偏高[56]。胆汁淤积,即胆汁流动停止或减少,会导致营养代谢失衡,脂质吸收不良,并导致对肝脏具有毒性的胆汁酸的淤积,从而使肝脏遭受不可逆的损伤[57]。肝脏主要通过两条途径排出毒性胆汁酸:一是羟基化;二是氨基酸修饰。孕烷X受体(pregnane X receptor, PXR)可以与孕烷孕烯醇酮16α-碳腈以及LCA结合并被激活,活化的PXR通过激活细胞色素P450-3A (cytochrome P450-3A, CYP3A)的表达促进LCA的6羟基化[58,59],增加了疏水性LCA的水溶性,从而降低其毒性。研究还发现,丹参酮IIA (tanshinone IIA, Tan IIA)是PXR的有效激活剂,活化的PXR通过诱导CYP3A的表达降低LCA的毒性[60]。Tan IIA是从丹参根中提取的天然活性物质,具有肝保护作用[61]。PXR可以与HNF4α和PGC1-α相互作用调节CYP7A1的表达。半合成药物利福平,可以激活PXR核受体活性,活化的PXR可以与HNF4α相互作用,抑制PGC1-α与HNF4α的相互作用,进而抑制CYP7A1的转录[62]。
4.3 维生素D受体参与的代谢调控
维生素D受体(vitamin D receptor, VDR)在小肠中高表达,在人的肝细胞中表达量较低,在小鼠的肝脏中不表达。VDR作为LCA的受体,对于小肠中毒性胆汁酸LCA的代谢具有重要意义[63]。研究发现LCA对VDR的敏感性是PXR的10倍。在LCA或维生素D作用下VDR可被活化,活化的VDR通过CYP3A途径对LCA进行羟基化,从而降低毒性胆汁酸的浓度[64]。小肠中VDR的缺失可降低小肠CYP3A的表达,抑制LCA的代谢,但同时可间接上调胆汁酸转运蛋白的表达,促进胆汁酸的肝肠循环,使大量的毒性胆汁酸被转运到肝脏,造成肝脏胆汁淤积并产生肝毒性。小肠中的VDR对于维持小肠屏障具有重要意义,在小肠中过表达CYP3A可促进小肠LCA的羟基化,保护小肠屏障[65]。
5 结语与展望
胆汁酸作为信号分子广泛参与了体内的糖脂代谢和能量代谢。近年来,对CYP7A1依赖的胆汁酸的合成调控已有深入的研究。形成了以CYP7A1为核心,核受体、细胞膜表面受体、细胞因子和酶等共同参与的代谢调控网络。多基因共同调控以确保机体在不同生理及外界刺激条件下体内代谢稳态的维持。然而,在该领域内仍有一些问题尚未解决。胆汁酸受体FXR能够响应胆汁酸和GW4064的刺激,抑制胆汁酸合成限速酶CYP7A1的表达,GW4064激活的FXR通路主要通过SHP-1来抑制CYP7A1的表达,但是胆汁酸激活的FXR通路存在SHP-1依赖和SHP-1非依赖两条途径[18,19]。SHP-1非依赖的胆汁酸负反馈调节通路的分子机制尚不清楚。TGR5是已知的胆汁酸膜蛋白受体,该受体主要表达在胆囊等器官,在肝脏和小肠等胆汁酸代谢相关重要器官几乎不表达。越来越多的证据表明,胆汁酸是体内一类非常重要的信号分子,在肝脏和小肠等胆汁酸代谢重要组织中是否存在其他的胆汁酸的膜蛋白受体仍完全未知。这些问题的解决是全面认识胆汁酸及胆固醇代谢的重要途径。
(责任编委: 陈雁)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLMagsci [本文引用: 1]

<p>心血管病已成为威胁我国人群健康的首要疾病,而胆固醇代谢紊乱是心血管病发生发展的重要危险因素之一。近年来高通量技术的推广和群体基因组学的发展极大地促进了复杂性状(或疾病)易感基因或突变的发现,为深入解析胆固醇代谢紊乱的遗传学病因提供了机会。文章整合传统遗传分析和近期GWAS筛查的结果,对胆固醇代谢紊乱的分子遗传研究进展进行了综述,结合通路富集分析揭示胆固醇代谢紊乱的功能背景,以期更好地理解胆固醇代谢紊乱致病的分子机制,为其防治提供线索。</p>
URLMagsci [本文引用: 1]

<p>心血管病已成为威胁我国人群健康的首要疾病,而胆固醇代谢紊乱是心血管病发生发展的重要危险因素之一。近年来高通量技术的推广和群体基因组学的发展极大地促进了复杂性状(或疾病)易感基因或突变的发现,为深入解析胆固醇代谢紊乱的遗传学病因提供了机会。文章整合传统遗传分析和近期GWAS筛查的结果,对胆固醇代谢紊乱的分子遗传研究进展进行了综述,结合通路富集分析揭示胆固醇代谢紊乱的功能背景,以期更好地理解胆固醇代谢紊乱致病的分子机制,为其防治提供线索。</p>
URLPMID:15554243 [本文引用: 1]

Lithocholic acid, a monohydroxy, secondary bile acid, is formed by bacterial 7-dehydroxylation of the primary bile acid chenodeoxycholic acid (CDCA) and of the secondary bile acid ursodeoxycholic acid (UDCA). Lithocholic acid and its precursor CDCA are toxic when fed to the rabbit, rhesus monkey, and baboon, but not when CDCA, as well as UDCA, is used for therapeutic purposes in man. Older studies showed that the species specific toxicity of lithocholic acid could be explained by efficient sulfation of lithocholic acid in man and in chimpanzee, but not in the rabbit, rhesus monkey, or baboon. Rodents detoxify lithocholic acid by hydroxylation, but this does not occur in species in which it is toxic. Recent studies suggest that lithocholic acid induces its own detoxification by activating nuclear receptors to promote transcription of genes encoding sulfotransferase. In addition, work with CaCo2 cells suggest that lithocholic acid may undergo sulfation in the enterocyte and be effluxed back into the intestinal lumen. The evolution of trihydroxy bile acids in vertebrates may have occurred to decrease the formation of lithocholic acid. Lithocholic acid is a rare example of a toxic endobiotic; a variety of mechanisms have evolved to solve the problem of efficient detoxification.
URLPMID:2090709 [本文引用: 3]

Abstract Lymphatic drainage leads to a significant stimulation of both the cholesterol 7 alpha-hydroxylase and HMG-CoA reductase activity in rats (Bj rkhem et al. 1978. Biochem. Biophys. Res. Commun. 85: (532-540). This finding was confirmed here and it was also shown that ligation of the lymph duct leads to a similar but less pronounced effect. Ligation of the lymph duct or lymph fistulation of bile duct-ligated or cholestyramine-treated rats did not further increase 7 alpha-hydroxylase or the HMG-CoA reductase activity. However, treatment of lymph fistula rats with cholestyramine led to a significant further stimulation of both 7 alpha-hydroxylase and HMG-CoA reductase activity. Intravenous infusion of lymph into bile fistula rats led to a significant inhibition of both cholesterol 7 alpha-hydroxylase activity and HMG-CoA reductase activity. A corresponding infusion of cholesterol-enriched Intralipid led to inhibition of HMG-CoA reductase without effect on cholesterol 7 alpha-hydroxylase activity. The results show that cholesterol 7 alpha-hydroxylase is feedback-regulated by bile acids in a situation where the flux of cholesterol to the liver is interrupted also. The possibility is discussed that there is a factor in the lymph that down-regulates cholesterol 7 alpha-hydroxylase. If such a factor exists, it requires an intact enterohepatic circulation for its effect. The stimulatory effect of cholestyramine on HMG-CoA reductase also in lymph fistula rats shows that the previously demonstrated suppressive effect of bile acids on HMG-CoA reductase is not only due to the effect of bile acids on intestinal absorption of cholesterol.(ABSTRACT TRUNCATED AT 250 WORDS).
URL [本文引用: 2]

Bile acids formed from cholesterol in the liver are defined as “primary” bile acids. In man, these are chenodeoxycholic acid and cholic acid. Following conjugation with glycine or taurine the bile acids are secreted into the bile and stored in the gallbladder. After participating in the intestinal digestive process the bile acids are reabsorbed from the ileum, and return to the liver via the portal system. This enterohepatic circulation preserves the bile acids for repeated utilization.
URLPMID:23897684 [本文引用: 3]

Bile acids are important physiological agents for intestinal nutrient absorption and biliary secretion of lipids, toxic metabolites, and xenobiotics. Bile acids also are signaling molecules and metabolic regulators that activate nuclear receptors and G protein-coupled receptor (GPCR) signaling to regulate hepatic lipid, glucose, and energy homeostasis and maintain metabolic homeostasis. Conversion of cholesterol to bile acids is critical for maintaining cholesterol homeostasis and preventing accumulation of cholesterol, triglycerides, and toxic metabolites, and injury in the liver and other organs. Enterohepatic circulation of bile acids from the liver to intestine and back to the liver plays a central role in nutrient absorption and distribution, and metabolic regulation and homeostasis. This physiological process is regulated by a complex membrane transport system in the liver and intestine regulated by nuclear receptors. Toxic bile acids may cause inflammation, apoptosis, and cell death. On the other hand, bile acid-activated nuclear and GPCR signaling protects against inflammation in liver, intestine, and macrophages. Disorders in bile acid metabolism cause cholestatic liver diseases, dyslipidemia, fatty liver diseases, cardiovascular diseases, and diabetes. Bile acids, bile acid derivatives, and bile acid sequestrants are therapeutic agents for treating chronic liver diseases, obesity, and diabetes in humans. (C) 2013 American Physiological Society.
URLPMID:5698910 [本文引用: 1]

Bile acids are derived from cholesterol to facilitate intestinal nutrient absorption and biliary secretion of cholesterol. Recent studies have identified bile acids as signaling molecules that activate nuclear farnesoid X receptor (FXR) and membrane G protein-coupled bile acid receptor-1 (Gpbar-1, also known as TGR5) to maintain metabolic homeostasis and protect liver and other tissues and cells from bile acid toxicity. Bile acid homeostasis is regulated by a complex mechanism of feedback and feedforward regulation that is not completely understood. This review will cover recent advances in bile acid signaling and emerging concepts about the classic and alternative bile acid synthesis pathway, bile acid composition and bile acid pool size, and intestinal bile acid signaling and gut microbiome in regulation of bile acid homeostasis.
URL [本文引用: 2]

http://linkinghub.elsevier.com/retrieve/pii/002247319090182R
URLPMID:5098396 [本文引用: 1]

The properties of sodium fusidate micelles were determined by a spectral shift technique, surface tension measurements, and ultracentrifugal analysis. The critical micellar concentrations, mean molecular areas, and apparent aggregation numbers were estimated as a function of the concentration of counterion (0.001-1.0 m Na(+)) at 20 degrees C. The critical micellar concentrations were studied over a temperature range of 10 degrees C to 40 degrees C at one counterion concentration (0.001 m Na(+)), and from these data the standard thermo-dynamic functions of micellization were calculated. The ability of sodium fusidate solutions to solubilize the insoluble swelling amphiphiles, lecithin and monoolein, was investigated, and the results were compared with the solubilizing properties of sodium taurocholate. The critical micellar concentrations of sodium fusidate approximated those of sodium taurocholate. The values fell in the range of 1.44-4.56 mm, varying with the technique used, counterion concentration, and temperature. The percentage of counterions bound to fusidate micelles in water, calculated from the log critical micellar concentration-log Na(+) curve, was estimated to be negligible, which compares with sodium taurocholate micelles. The critical micellar concentration of sodium fusidate exhibited a minimum at 20 degrees C, a phenomenon observed with other ionic detergents and with bile salts. Micelle formation in sodium fusidate solutions was shown to be primarily entropy-driven at 10 degrees and 20 degrees C, whereas at 30 degrees and 40 degrees C the enthalpy factor predominated. From the surface tension measurements the molecular areas of sodium fusidate and sodium taurocholate were calculated. The mean molecular area of fusidate was 101 A(2), whereas sodium taurocholate possessed a molecular area of 88 A(2). It was demonstrated that the sodium fusidate molecule, like a bile salt molecule, lies with its longitudinal axis horizontal at an air-water interface. The apparent aggregation number of sodium fusidate micelles increased from 5 to 16 as the concentration of counterion increased from 0.01 to 0.60 m Na(+). These values are slightly larger than the corresponding aggregation numbers of sodium taurocholate micelles.
URLPMID:28390813 [本文引用: 2]

Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG), are the most effective and durable treatments for morbid obesity and potentially a viable treatment for type 2 diabetes (T2D). The resolution rate of T2D following these procedures is between 40 and 80% and far surpasses that achieved by medical management alone. The molecular basis for this improvement is not entirely understood, but has been attributed in part to the altered enterohepatic circulation of bile acids. In this review we highlight how bile acids potentially contribute to improved lipid and glucose homeostasis, insulin sensitivity and energy expenditure after these procedures. The impact of altered bile acid levels in enterohepatic circulation is also associated with changes in gut microflora, which may further contribute to some of these beneficial effects. We highlight the beneficial effects of experimental surgical procedures in rodents that alter bile secretory flow without gastric restriction or altering nutrient flow. This information suggests a role for bile acids beyond dietary fat emulsification in altering whole body glucose and lipid metabolism strongly, and also suggests emerging roles for the activation of the bile acid receptors farnesoid x receptor (FXR) and G-protein coupled bile acid receptor (TGR5) in these improvements. The limitations of rodent studies and the current state of our understanding is reviewed and the potential effects of bile acids mediating the short- and long-term metabolic improvements after bariatric surgery is critically examined.
URL [本文引用: 1]

胆汁酸在肝脏南胆同醇合成,是机体类同醇物质的主要清除途径,胆汁酸代谢在维持机体的胆同醇稳态中起重要的作用。本文就胆汁酸合成的机制、生理功能、肝肠循环、胆汁酸障碍相关疾病及胆汁酸合成的调节等方面的一些进展作一简要综述。
URL [本文引用: 1]

胆汁酸在肝脏南胆同醇合成,是机体类同醇物质的主要清除途径,胆汁酸代谢在维持机体的胆同醇稳态中起重要的作用。本文就胆汁酸合成的机制、生理功能、肝肠循环、胆汁酸障碍相关疾病及胆汁酸合成的调节等方面的一些进展作一简要综述。
URLPMID:4629214 [本文引用: 1]

The classical functions of bile acids include acting as detergents to facilitate the digestion and absorption of nutrients in the gut. In addition, bile acids also act as signaling molecules to regulate glucose homeostasis, lipid metabolism and energy expenditure. The signaling potential of bile acids in compartments such as the systemic circulation is regulated in part by an efficient enterohepatic circulation that functions to conserve and channel the pool of bile acids within the intestinal and hepatobiliary compartments. Changes in hepatobiliary and intestinal bile acid transport can alter the composition, size, and distribution of the bile acid pool. These alterations in turn can have significant effects on bile acid signaling and their downstream metabolic targets. This review discusses recent advances in our understanding of the inter-relationship between the enterohepatic cycling of bile acids and the metabolic consequences of signalingviabile acid-activated receptors, such as farnesoid X nuclear receptor (FXR) and the G-protein-coupled bile acid receptor (TGR5). This review discusses recent advances in our understanding of the inter-relationship between the enterohepatic cycling of bile acids and the metabolic consequences of signalingviabile acid-activated receptors such as farnesoid X nuclear receptor (FXR) and the G-protein-coupled bile acid receptor (TGR5).
URLPMID:8895049 [本文引用: 2]

Abstract A stable HepG2 cell line harboring a human cholesterol 7 alpha-hydroxylase (CYP7A) minigene/luciferase reporter gene construct was selected for studying transcriptional regulation of CYP7A gene promoter. Insulin and phorbol 12-myristate-13-acetate (PMA) strongly repressed the promoter activity as measured with luciferase activity expressed in the cells. The promoter activity of the 5' progressive deletion/luciferase reporter gene constructs was studied in a transient transfection assay in HepG2 cells. PMA represses the promoter activity and the response elements were localized in the -184/-151 and -134/-81 regions. Insulin also represses the promoter activity and response element was mapped in the -298/-81 region. Surprisingly, glucocorticoid receptor (GR) strongly inhibited promoter activity in the presence of dexamethasone, and response elements were localized in the -298/-151 and the -150/+24 regions. Thyroid hormone receptor also repressed promoter activity and response elements were localized in the -150/+24 and upstream regions. Cotransfection of CYP7A chimeric constructs with an expression vector carrying liver-enriched transcription factor HNF3 alpha stimulated the reporter gene activity, but cotransfection with GR plasmid interfered with the HNF3 alpha-stimulated activity possibly through competition for binding to overlapping GR/HNF3 binding sites. Thus, human cholesterol 7 alpha-hydroxylase gene promoter is strongly repressed by insulin, PMA, and steroid/thyroid hormones and results in the low level of cholesterol 7 alpha-hydroxylase expression in the human liver.
URLPMID:4629217 [本文引用: 1]

The bile-acid-activated nuclear receptor, farnesoid X receptor (FXR), is involved in the pathophysiology of a wide range of diseases of gastrointestinal tract, including inflammatory bowel disease, colorectal cancer and type 2 diabetes. In this review, authors discuss current knowledge of the roles of FXR in physiology of the digestive system and the related diseases. [Display omitted]
URLPMID:29195686 [本文引用: 1]

Pre/probiotics and fecal transplantation are tools available to manipulate microbiota and bile acid composition in the host. This has been shown to have a profound effect on host physiology. Improving these techniques could be a reasonable approach to treat metabolic disease.
URLPMID:11030332 [本文引用: 2]

Bile acids repress the transcription of cytochrome P450 7A1 (CYP7A1), which catalyzes the rate-limiting step in bile acid biosynthesis. Although bile acids activate the farnesoid X receptor (FXR), the mechanism underlying bile acid–mediated repression of CYP7A1 remained unclear. We have used a potent, nonsteroidal FXR ligand to show that FXR induces expression of small heterodimer partner 1 (SHP-1), an atypical member of the nuclear receptor family that lacks a DNA-binding domain. SHP-1 represses expression of CYP7A1 by inhibiting the activity of liver receptor homolog 1 (LRH-1), an orphan nuclear receptor that is known to regulate CYP7A1 expression positively. This bile acid–activated regulatory cascade provides a molecular basis for the coordinate suppression of CYP7A1 and other genes involved in bile acid biosynthesis.
[本文引用: 2]
URLPMID:15973435 [本文引用: 3]

SHP (small heterodimer partner) is an important component of the feedback regulatory cascade, which controls the conversion of cholesterol to bile acids. In order to identify the bona fide molecular targets of SHP, we performed global gene expression profiling combined with chromatin immunoprecipitation assays in transgenic mice constitutively expressing SHP in the liver. We demonstrate that SHP affects genes involved in diverse biological pathways, and in particular, several key genes involved in consecutive steps of cholesterol degradation, bile acid conjugation, transport and lipogenic pathways. Sustained expression of SHP leads to the depletion of hepatic bile acid pool and a concomitant accumulation of triglycerides in the liver. The mechanism responsible for this phenotype includes SHP-mediated direct repression of downstream target genes and the bile acid sensor FXR, and an indirect activation of PPAR and SREBP-1c genes. We present evidence for the role of altered chromatin configurations in defining distinct gene-specific mechanisms by which SHP mediates differential transcriptional repression. The multiplicity of genes under its control suggests that SHP is a pleiotropic regulator of diverse metabolic pathways.
URLPMID:8650544 [本文引用: 3]

SHP is an orphan member of the nuclear hormone receptor superfamily that contains the dimerization and ligand-binding domain found in other family members but lacks the conserved DNA binding domain. In the yeast two-hybrid system, SHP interacted with several conventional and orphan members of the receptor superfamily, including retinoid receptors, the thyroid hormone receptor, and the orphan receptor MB67. SHP also interacted directly with these receptors in vitro. In mammalian cells, SHP specifically inhibited transactivation by the superfamily members with which it interacted. These results suggest that SHP functions as a negative regulator of receptor-dependent signaling pathways.
URLPMID:10359768 [本文引用: 3]

Cholesterol 7伪 -hydroxylase is the first and rate-limiting enzyme in a pathway through which cholesterol is metabolized to bile acids. The gene encoding cholesterol 7伪 -hydroxylase, CYP7A, is expressed exclusively in the liver. Overexpression of CYP7A in hamsters results in a reduction of serum cholesterol levels, suggesting that the enzyme plays a central role in cholesterol homeostasis. Here, we report the identification of a hepatic-specific transcription factor that binds to the promoter of the human CYP7A gene. We designate this factor CPF, for CYP7A promoter binding factor. Mutation of the CPF binding site within the CYP7A promoter abolished hepatic-specific expression of the gene in transient transfection assays. A cDNA encoding CPF was cloned and identified as a human homolog of the Drosophila orphan nuclear receptor fushi tarazu F1 (Ftz-F1). Cotransfection of a CPF expression plasmid and a CYP7A reporter gene resulted in specific induction of CYP7A-directed transcription. These observations suggest that CPF is a key regulator of human CYP7A gene expression in the liver.
URL [本文引用: 2]
URLPMID:16075061 [本文引用: 2]

We have generated a line of mutant mouse that lacks betaKlotho, a protein that structurally resembles Klotho. The synthesis and excretion of bile acids were found to be dramatically elevated in these mutants, and the expression of 2 key bile acid synthase genes, cholesterol 7alpha-hydroxylase (Cyp7a1) and sterol 12alpha-hydroxylase (Cyp8b1), was strongly upregulated. Nuclear receptor pathways and the enterohepatic circulation, which regulates bile acid synthesis, seemed to be largely intact; however, bile acid-dependent induction of the small heterodimer partner (SHP) NR0B2, a common negative regulator of Cyp7a1 and Cyp8b1, was significantly attenuated. The expression of Cyp7a1 and Cyp8b1 is known to be repressed by dietary bile acids via both SHP-dependent and -independent regulations. Interestingly, the suppression of Cyp7a1 expression by dietary bile acids was impaired, whereas that of Cyp8b1 expression was not substantially altered in betaklotho mice. Therefore, betaKlotho may stand as a novel contributor to Cyp7a1-selective regulation. Additionally, betaKlotho-knockout mice exhibit resistance to gallstone formation, which suggests the potential future clinical relevance of the betaKlotho system.
URLPMID:25584736 [本文引用: 1]

This review focuses on the latest understanding of the molecular mechanisms underlying the complex interactions between intestine and liver bile acid signaling, gut microbiota, and their impact on whole-body lipid, glucose and energy metabolism.Hepatic bile acid synthesis is tightly regulated by the bile acid negative feedback mechanisms. Modulating the enterohepatic bile acid signaling greatly impacts the whole-body metabolic homeostasis. Recently, a positive feedback mechanism through intestine farnesoid X receptor (FXR) antagonism has been proposed to link gut microbiota to the regulation of bile acid composition and pool size. Two studies identified intestine Diet1 and hepatic SHP-2 as novel regulators of CYP7A1 and bile acid synthesis through the gut-liver FXR-fibroblast growth factor 15/19-FGF receptor four signaling axis. New evidence suggests that enhancing bile acid signaling in the distal ileum and colon contributes to the metabolic benefits of bile acid sequestrants and bariatric surgery.Small-molecule ligands that target TGR5 and FXR have shown promise in treating various metabolic and inflammation-related human diseases. New insights into the mechanisms underlying the bariatric surgery and bile acid sequestrant treatment suggest that targeting the enterohepatic circulation to modulate gut-liver bile acid signaling, incretin production and microbiota represents a new strategy to treat obesity and type 2 diabetes.
URLPMID:18692401 [本文引用: 1]

Endocrine fibroblast growth factors (FGFs) control a variety of physiological processes including suppression of bile acid synthesis in hepatocytes, promotion of lipolysis in adipocytes, and inhibition of phosphate reabsorption and vitamin D biosynthesis in renal tubular cells. Endocrine FGFs require the Klotho gene family of transmembrane proteins as co-receptors to bind cognate FGF receptors. Importantly, expression of endocrine FGFs is regulated by nuclear receptors whose lipophilic ligands are generated under the control of these hormones in their target organs. Thus, novel endocrine axes have emerged that regulate diverse metabolic processes through feedback loops composed of the FGF, Klotho, and nuclear receptor gene families. This review covers roles of Klotho family proteins in the regulation of activity and expression of endocrine FGFs.
URLPMID:4073476 [本文引用: 1]

Bile salts play crucial roles in allowing the gastrointestinal system to digest, transport and metabolize nutrients. They function as nutrient signaling hormones by activating specific nuclear receptors (FXR, PXR, Vitamin D) and G-protein coupled receptors [TGR5, sphingosine-1 phosphate receptor 2 (S1PR2), muscarinic receptors]. Bile acids and insulin appear to collaborate in regulating the metabolism of nutrients in the liver. They both activate the AKT and ERK1/2 signaling pathways. Bile acid induction of the FXR-伪 target gene, small heterodimer partner (SHP), is highly dependent on the activation PKC味, a branch of the insulin signaling pathway. SHP is an important regulator of glucose and lipid metabolism in the liver. One might hypothesize that chronic low grade inflammation which is associated with insulin resistance, may inhibit bile acid signaling and disrupt lipid metabolism. The disruption of these signaling pathways may increase the risk of fatty liver and non-alcoholic fatty liver disease (NAFLD). Finally, conjugated bile acids appear to promote cholangiocarcinoma growth via the activation of S1PR2.
URLPMID:15750181 [本文引用: 1]

The fibroblast growth factor (FGF) receptor complex is a regulator of adult organ homeostasis in addition to its central role in embryonic development and wound healing. FGF receptor 4 (FGFR4) is the sole FGFR receptor kinase that is significantly expressed in mature hepatocytes. Previously, we showed that mice lacking mouse FGFR4 (mR4 –/– ) exhibited elevated fecal bile acids, bile acid pool size, and expression of liver cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme for canonical neutral bile acid synthesis. To prove that hepatocyte FGFR4 was a negative regulator of cholesterol metabolism and bile acid synthesis independent of background, we generated transgenic mice overexpressing a constitutively active human FGFR4 (CahR4) in hepatocytes and crossed them with the FGFR4-deficient mice to generate CahR4/mR4 –/– mice. In mice expressing active FGFR4 in liver, fecal bile acid excretion was 64%, bile acid pool size was 47%, and Cyp7a1 expression was 10–30% of wild-type mice. The repressed level of Cyp7a1 expression was resistant to induction by a high cholesterol diet relative to wild-type mice. Expression of CahR4 in mR4 –/– mouse livers depressed bile acid synthesis below wild-type levels from the elevated levels observed in mR4 –/– . Levels of phosphorylated c-Jun N-terminal kinase (JNK), which is part of a pathway implicated in bile acid-mediated repression of synthesis, was 30% of wild-type levels in mR4 –/– livers, whereas CahR4 livers exhibited an average 2-fold increase. However, cholate still strongly induced phospho-JNK in mR4 –/– livers. These results confirm that hepatocyte FGFR4 regulates bile acid synthesis by repression of Cyp7a1 expression. Hepatocyte FGFR4 may contribute to the repression of bile acid synthesis through JNK signaling but is not required for activation of JNK signaling by bile acids.
URLPMID:26505219 [本文引用: 1]

The bile acid (BA)-sensing nuclear receptor, farnesoid X receptor (FXR), regulates postprandial metabolic responses, including inhibition of BA synthesis, by inducing the intestinal hormone, fibroblast growth factor (FGF)15 (FGF19 in human). In this study, we tested a novel hypothesis that FXR not only induces intestinal FGF15 but also primes the liver for effectively responding to the signal by transcriptional induction of the obligate coreceptor for FGF15, β-Klotho (βKL). Activation of FXR by a synthetic agonist, GW4064, in mice increased occupancy of FXR and its DNA-binding partner, retinoid X receptor-α, at FGF15-signaling component genes, particularly βKL, and induced expression of these genes. Interestingly, mRNA levels of Fgfr4, the FGF15 receptor, were not increased by GW4064, but protein levels increased as a result of βKL-dependent increased protein stability. Both FGF receptor 4 and βKL protein levels were substantially decreased in FXR-knockout (KO) mice, and FGF19 signaling, monitored by phosphorylated ERK, was blunted in FXR-KO mice, FXR-KO mouse hepatocytes, and FXR-down-regulated human hepatocytes. Overexpression of βKL in FXR-lacking hepatocytes partially restored FGF19 signaling and inhibition by FGF19 of Cyp7a1, which encodes the rate-limiting BA biosynthetic enzyme. In mice, transient inductions of intestinal Fgf15 and hepatic βKL were temporally correlated after GW4064 treatment, and pretreatment of hepatocytes with GW4064 before FGF19 treatment enhanced FGF19 signaling, which was abolished by transcriptional inhibition or βKL down-regulation. This study identifies FXR as a gut-liver metabolic coordinator for FGF15/19 action that orchestrates transient induction of hepatic βKL and intestinal Fgf15/19 in a temporally correlated manner.
URLPMID:11044614 [本文引用: 1]

We report here the identification of mouse βklotho ( βkl), which encodes a type I membrane protein with high resemblance to Klotho (KL). Both βKL and KL consist of two internal repeats with homology to family 1 glycosidases, while these essential glutamates for the enzymatic activities were not conserved. The identical pattern of substitution and variation in the substituted amino acids between these two proteins indicate that they likely to form a unique family within the glycosidase family 1 superfamily. During mouse embryonic development, strong βkl expression was detected in the yolk sac, gut, brown and white adipose tissues, liver and pancreas, and in the adult, predominantly in the liver and pancreas. Despite the high structural similarity between βKL and KL, their expression profiles were considerably different and βkl expression was not induced in kl-deficient mouse mutants.
URLPMID:10809780 [本文引用: 1]

Abstract Heparan sulfate-regulated transmembrane tyrosine kinase receptor FGFR4 is the major FGFR isotype in mature hepatocytes. Fibroblast growth factor has been implicated in the definition of liver from foregut endoderm where FGFR4 is expressed and stimulation of hepatocyte DNA synthesis in vitro. Here we show that livers of mice lacking FGFR4 exhibited normal morphology and regenerated normally in response to partial hepatectomy. However, the FGFR4 (-/-) mice exhibited depleted gallbladders, an elevated bile acid pool and elevated excretion of bile acids. Cholesterol- and bile acid-controlled liver cholesterol 7alpha-hydroxylase, the limiting enzyme for bile acid synthesis, was elevated, unresponsive to dietary cholesterol, but repressed normally by dietary cholate. Expression pattern and cholate-dependent, cholesterol-induced hepatomegaly in the FGFR4 (-/-) mice suggested that activation of receptor interacting protein 140, a co-repressor of feed-forward activator liver X receptor alpha, may mediate the negative regulation of cholesterol- and bile acid-controlled liver cholesterol 7alpha-hydroxylase transcription by FGFR4 and cholate. The results demonstrate that transmembrane sensors interface with metabolite-controlled transcription networks and suggest that pericellular matrix-controlled liver FGFR4 in particular may ensure adequate cholesterol for cell structures and signal transduction.
URLPMID:20683963 [本文引用: 1]

De novo bile acid synthesis in the liver needs to be tightly regulated in order to maintain optimal bile flow and prevent cholestasis. In the liver, fibroblast growth factor 19 (FGF19) regulates bile acid synthesis by down-regulating messenger RNA levels of cytochrome P450 7A1 (CYP7A1). FGF19 acts through fibroblast growth factor receptor 4 (FGFR4), and β-Klotho has recently been recognized as a modulator of FGFR4 activity. However, its precise mechanism of action has not been thoroughly described. We show here that β-Klotho is an endoplasmic reticulum–resident protein that affects the cellular abundance of different FGFR4 glycoforms. β-Klotho binds and directs the core glycoform of FGFR4 to the proteasome, and it allows only a terminal glycoform to reach the plasma membrane. Only the terminal FGFR4 glycoform is phosphorylated upon FGF19 treatment of HepG2 cells, and this shows that only fully glycosylated FGFR4 is active in CYP7A1 down-regulation. Conclusion: β-Klotho enhances FGF19 signaling by binding the inactive, core-glycosylated FGFR4 and preventing it from reaching the surface. These results indicate that β-Klotho is an indirect regulator of FGFR4, whereas glycosylation is the master switch for FGF19 activity and regulation of bile acid synthesis. (HEPATOLOGY 2010)
URLPMID:19706524 [本文引用: 1]

FGF19 is a hormone that regulates bile acid and glucose homeostasis. Progress has been made in identifying cofactors for receptor activation. However, several functions of FGF19 have not yet been fully defined, including the actions of FGF19 on target tissues, its FGF receptor specificity, and the contributions of other cofactors, such as heparin. Here, we explore the requirements for FGF19-FGFR/co-receptor interactions and signaling in detail. We show that 0Klotho was essential for FGF19 interaction with FGFRs 1c, 2c, and 3c, but FGF19 was able to interact directly with FGFR4 in the absence of 尾Klotho in a heparin-dependent manner. Further, FGF19 activated FGFR4 signaling in the presence or absence of pKlotho, but activation of FGFRs 1c, 2c, or 3c was completely 0Klotho dependent We then generated an FGF19 molecule, FGF19dCTD, which has a deletion of the C-terminal region responsible for 尾Klotho interaction. We determined that 0Klotho-dependent FGFRIc 2c, and 3c interactions and activation were abolished, and 尾Klothoindependent FGFR4 activation was preserved; therefore, FGF19dCTD is an FGFR4-specific activator. This unique FGF19 molecule specifically activated FGFR4-dependent signaling in liver and suppressed CYP7A1 expression in vivo, but was unable to activate signaling in adipose where FGFR4 expression is very low. Interestingly, unlike FGF19, treatment of oblob mice with FGF19dCTD failed to improve glucose levels and insulin sensitivity. These results suggest that FGF19-regulated liver bile acid metabolism could be independent of its glucose-lowering effect and direct FGFR activation in adipose tissue may play an important role in the regulation of glucose homeostasis.
[本文引用: 1]
URLPMID:17654698 [本文引用: 2]

The transcription of the gene ( CYP7A1 ) encoding cholesterol 7-hydroxylase, a key enzyme in cholesterol homeostasis, is repressed by bile acids via multiple mechanisms involving members of the nuclear receptor superfamily. Here, we describe a regulatory mechanism that can be exploited for modulating bile acid synthesis. By dissecting the mechanisms of CYP7A1 transcription, we found that bile acids stimulate the sequential recruitment of the histone deacetylases (HDACs) 7, 3, and 1, and of the corepressor SMRT (silencing mediator of retinoid and thyroid receptors-) and the nuclear corepressor. Bile acids, but not the farnesoid X receptor-selective agonist GW4064, increase the nuclear concentration of HDAC7, which promotes the assembly of a repressive complex that ultimately represses CYP7A1 transcription. Interestingly, despite its high basal expression level, small heterodimer partner (SHP) is associated with the CYP7A1 promoter only at a later stage of bile acid repression. Gene silencing with small interfering RNA confirms that HDAC7 is the key factor required for the repression of CYP7A1 transcription, whereas knockdown of SHP does not prevent the down-regulation of CYP7A1 . Administration of the HDAC inhibitors valproic acid or trichostatin A to genetically hypercholesterolemic mice increases Cyp7a1 messenger RNA and bile acid synthesis and consequently markedly reduces total plasma and low-density lipoprotein cholesterol. Conclusion: By using a combination of molecular, cellular, and animal models, our study highlights the importance of HDACs in the feedback regulation of CYP7A1 transcription and identifies these enzymes as potential targets to modulate bile acid synthesis and for the treatment of hypercholesterolemia. (H EPATOLOGY 2007.)
URLPMID:11030331 [本文引用: 1]

The catabolism of cholesterol into bile acids is regulated by oxysterols and bile acids, which induce or repress transcription of the pathway's rate-limiting enzyme cholesterol 7伪-hydroxylase (CYP7A1). The nuclear receptor LXR伪 binds oxysterols and mediates feed-forward induction. Here, we show that repression is coordinately regulated by a triumvirate of nuclear receptors, including the bile acid receptor, FXR; the promoter-specific activator, LRH-1; and the promoter-specific repressor, SHP. Feedback repression of CYP7A1 is accomplished by the binding of bile acids to FXR, which leads to transcription of SHP. Elevated SHP protein then inactivates LRH-1 by forming a heterodimeric complex that leads to promoter-specific repression of both CYP7A1 and SHP. These results reveal an elaborate autoregulatory cascade mediated by nuclear receptors for the maintenance of hepatic cholesterol catabolism.
URL [本文引用: 2]
URLPMID:10809664 [本文引用: 1]

Abstract The corepressor SMRT mediates repression by thyroid hormone receptor (TR) as well as other nuclear hormone receptors and transcription factors. Here we report the isolation of a novel SMRT-containing complex from HeLa cells. This complex contains transducin beta-like protein 1 (TBL1), whose gene is mutated in human sensorineural deafness. It also contains HDAC3, a histone deacetylase not previously thought to interact with SMRT. TBL1 displays structural and functional similarities to Tup1 and Groucho corepressors, sharing their ability to interact with histone H3. In vivo, TBL1 is bridged to HDAC3 through SMRT and can potentiate repression by TR. Intriguingly, loss-of-function TRbeta mutations cause deafness in mice and humans. These results define a new TR corepressor complex with a physical link to histone structure and a potential biological link to deafness.
URLPMID:10627496 [本文引用: 1]

Abstract The gene for cholesterol 7alpha-hydroxylase (CYP7A1) contains a sequence at nt -149 to -118 that was found to play a large role in determining the overall transcriptional activity and regulation of the promoter. Hepatocyte nuclear factor 4 (HNF4) and chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) synergistically activate transcription of the CYP7A1 promoter. Transactivation of CYP7A1 by HNF4 in the human hepatoma cell line, HepG2, was enhanced by cotransfection with COUP-TFII or the basal transcription element binding protein (BTEB). HNF4 prepared from rat liver nuclear extracts bound to oligomers homologous to the nt -146 to -134 sequences in electrophoretic mobility shift assays (EMSA), which corresponded to a conserved region containing a direct repeat of hormone response elements spaced by one nucleotide (DR1). The sequences surrounding this DR1 were found to be essential for the HNF4 transactivation. In vitro-translated COUP-TFII was found to bind the adjacent sequences from nt -139 to -128 (DR0), but COUP-TFII interacted with this region at a much lower affinity than to the COUP-TFII-site at nt -72 to -57 (DR4). Mutations at nt -139 to -128 or nt -72 to -57 reduced the COUP-TFII and HNF4 synergy; however, these COUP-TFII-binding sequences were not absolutely required for the cooperative effect of HNF4 and COUP-TFII on transactivation. These results indicated that the observed transactivation was the result of protein/protein interactions facilitated by the juxtaposition of the binding elements.
URLPMID:12865425 [本文引用: 1]

Bile acid metabolism plays an essential role in cholesterol homeostasis and is critical for the initiation of atherosclerotic disease. However, despite the recent advances, the molecular mechanisms whereby bile acids regulate gene transcription and cholesterol homeostasis in mammals still need further investigations. Here, we show that bile acids suppress transcription of the gene (CYP7A1) encoding cholesterol 7alpha-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis, also through an unusual mechanism not involving the bile acid nuclear receptor, farnesoid X receptor. By performing cell-based reporter assays, protein/protein interaction, and chromatin immunoprecipitation assays, we demonstrate that bile acids impair the recruitment of peroxisome proliferator-activated receptor-gamma coactivator-1alpha and cAMP response element-binding protein-binding protein by hepatocyte nuclear factor-4alpha, a master regulator of CYP7A1. We also show for the first time that bile acids inhibit transcription of the gene (PEPCK) encoding phosphoenolpyruvate carboxykinase, the rate-limiting enzyme in gluconeogenesis, through the same farnesoid X receptor-independent mechanism. Chromatin immunoprecipitation assay revealed that bile acid-induced dissociation of coactivators from hepatocyte nuclear factor-4alpha decreased the recruitment of RNA polymerase II to the core promoter and downstream in the 3'-untranslated regions of these two genes, reflecting the reduction of gene transcription. Finally, we found that Cyp7a1 expression was stimulated in fasted mice in parallel to Pepck, whereas the same genes were repressed by bile acids. Collectively, these results reveal a novel regulatory mechanism that controls gene transcription in response to extracellular stimuli and argue that the transcription regulation by bile acids of genes central to cholesterol and glucose metabolism should be viewed dynamically in the context of the fasted-to-fed cycle.
URL [本文引用: 1]
URLPMID:10951502 [本文引用: 1]

Abstract Summary: While the targeted disruption of a gene is a powerful tool for investigating the physiological functions of that gene, disruption of a gene essential for embryogenesis leads to embryonic death. Rescue of the defect(s) causing embryonic death should promote survival, thus permitting further evaluation of the roles that the gene plays later in the developmental process. Disruption of the gene for mouse hepatocyte growth factor/scatter factor (HGF/SF) leads to middle-stage embryonic lethality because of a defect in placental development. Here we report that a single injection of HGF/SF at embryonic day 9.5 (E9.5) into the amniotic cavity of HGF / SF 61/61 embryos rescued the placental defect and resulted in the survival of the embryos until term. Histological analysis suggested that HGF/SF is also required at the late stage of development for tissue organogenesis. Thus, injection of a secreted factor can be a useful method to rescue the defects causing embryonic lethality. genesis 27:99–103, 2000. 08 2000 Wiley-Liss, Inc.
URL [本文引用: 1]
URLPMID:16614213 [本文引用: 1]

Liver mass depends on one or more unidentified humoral signals that drive regeneration when liver functional capacity is diminished. Bile acids are important liver products, and their levels are tightly regulated. Here, we identify a role for nuclear receptor-dependent bile acid signaling in normal liver regeneration. Elevated bile acid levels accelerate regeneration, and decreased levels inhibit liver regrowth, as does the absence of the primary nuclear bile acid receptor FXR. We propose that FXR activation by increased bile acid flux is a signal of decreased functional capacity of the liver. FXR, and possibly other nuclear receptors, may promote homeostasis not only by regulating expression of appropriate metabolic target genes but also by driving homeotrophic liver growth.
URLPMID:18398918 [本文引用: 1]

Previous studies from our laboratory have demonstrated that hepatocytes can transdifferentiate into biliary epithelium (BE) both in vivo and in vitro ; however, the mechanisms are unclear. The current study was designed to investigate the mechanisms of hepatocyte transdifferentiation in vitro . Rat hepatocytes were cultured in roller bottles to obtain hepatocyte organoid cultures, which were stimulated with various growth factors (GFs) including hepatocyte growth factor (HGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), stem cell factor (SCF), macrophage-stimulating protein (MSP), fibroblast growth factor-a (FGF-a), fibroblast growth factor-b (FGF-b), and fibroblast growth factor-8b (FGF-8b). Only the cultures treated with HGF, EGF, and their combination exhibited formation of hepatocyte-derived biliary epithelium (BE) despite the presence and activation of all the pertinent cognate membrane receptors of the rest of the GFs. Microarray analysis of the organoid cultures identified specific up-regulation of approximately 500 target genes induced by HGF and EGF, including members of the extracellular matrix (ECM) protein family, Wnt/-catenin pathway, transforming growth factor beta (TGF-)/bone morphogenetic protein (BMP) pathway, and CXC (cysteine-any amino acid-cysteine) chemokines. To investigate the downstream signaling involved in hepatocyte to biliary epithelial cell (BEC) transdifferentiation, we investigated expression and activities of mitogen-activated protein (MAP) kinases [extracellular signal-regulated kinase (ERK)1/2, p38, and c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK)] as well as serine/threonine kinase AKT. The analysis indicated that AKT phosphorylation was particularly increased in cultures treated with HGF, EGF, and their combination. Whereas phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 completely inhibited biliary epithelium formation, AKT inhibitor could only moderately reduce formation of BE in the organoid cultures treated with HGF+EGF. Most of the HGF+EGF target genes were altered by LY294002. Conclusion: Taken together, these data indicate that hepatocyte to BE transdifferentiation is regulated by HGF and EGF receptors and that PI3 kinase-mediated signaling independent of AKT is a crucial component of the transdifferentiation process. (H EPATOLOGY 2008.)
URL [本文引用: 2]
URLPMID:10823815 [本文引用: 1]

Abstract In the studies reported herein, we show that two complementary experimental models: inbred strains of mice (i.e. C57BL/6 and C3H/HeJ), and a differentiated line of rat hepatoma cells (i.e. L35 cells), require the activation of cytokines by monocyte/macrophages to display bile acid negative feedback repression of cholesterol 7alpha-hydroxylase (CYP7A1). Feeding a bile acid-containing atherogenic diet for 3 weeks to C57BL/6 mice led to a 70% reduction in the expression of hepatic CYP7A1 mRNA, whereas no reduction was observed in C3H/HeJ mice. The strain-specific response to repression of CYP7A1 paralleled the activation of hepatic cytokine expression. Studies using cultured THP-1 monocyte/macrophages showed that the hydrophobic bile acid chenodeoxycholate, a well established potent repressor of CYP7A1, induced the expression of mRNAs encoding interleukin 1 (IL-1) and tumor necrosis factor alpha (TNFalpha). In contrast, the hydrophilic bile acid ursodeoxycholate, which does not repress CYP7A1, did not induce cytokine mRNA expression by THP-1 cells. Chenodeoxycholate activation of cytokines by THP-1 cells was blocked by the peroxisome proliferator-activated receptor gamma agonist rosiglitazone. The expression of cytokines (e.g. IL-1 and TNFalpha) by THP-1 cells paralleled with the ability of these cells to produce conditioned medium that when added to rat L35 hepatoma cells, repressed CYP7A1. Moreover, rosiglitazone, which blocks cytokine activation by macrophages, also blocked the repression of CYP7A1 normally exhibited by C57BL/6 mice fed the bile acid-containing atherogenic diet. The combined data indicate that the activation of cytokines may mediate CYP7A1 repression caused by feeding mice an atherogenic diet containing bile acids.
URLPMID:26424786 [本文引用: 1]

Obesity and diabetes mellitus are the leading causes of renal disease. In this study, we determined the regulation and role of the G protein-coupled bile acid receptor TGR5, previously shown to be regulated by high glucose and/or fatty acids, in obesity-related glomerulopathy (ORG) and diabetic nephropathy (DN). Treatment of diabetic db/db mice with the selective TGR5 agonist INT-777 decreased proteinuria, podocyte injury, mesangial expansion, fibrosis, and CD68 macrophage infiltration in the kidney. INT-777 also induced renal expression of master regulators of mitochondrial biogenesis, inhibitors of oxidative stress, and inducers of fatty acid 尾-oxidation, including sirtuin 1 (SIRT1), sirtuin 3 (SIRT3), and Nrf-1. Increased activity of SIRT3 was evidenced by normalization of the increased acetylation of mitochondrial superoxide dismutase 2 (SOD2) and isocitrate dehydrogenase 2 (IDH2) observed in untreated db/db mice. Accordingly, INT-777 decreased mitochondrial H2O2 generation and increased the activity of SOD2, which associated with decreased urinary levels of H2O2 and thiobarbituric acid reactive substances. Furthermore, INT-777 decreased renal lipid accumulation. INT-777 also prevented kidney disease in mice with diet-induced obesity. In human podocytes cultured with high glucose, INT-777 induced mitochondrial biogenesis, decreased oxidative stress, and increased fatty acid 尾-oxidation. Compared with normal kidney biopsy specimens, kidney specimens from patients with established ORG or DN expressed significantly less TGR5 mRNA, and levels inversely correlated with disease progression. Our results indicate that TGR5 activation induces mitochondrial biogenesis and prevents renal oxidative stress and lipid accumulation, establishing a role for TGR5 in inhibiting kidney disease in obesity and diabetes.
URLPMID:15721318 [本文引用: 2]

Bile acids play essential roles in the absorption of dietary lipids and in the regulation of bile acid biosynthesis. Recently, a G protein-coupled receptor, TGR5, was identified as a cell-surface bile acid receptor. In this study, we show that bile acids promote glucagon-like peptide-1 (GLP-1) secretion through TGR5 in a murine enteroendocrine cell line STC-1. In STC-1 cells, bile acids promoted GLP-1 secretion in a dose-dependent manner. As STC-1 cells express TGR5 mRNA, we examined whether bile acids induce GLP-1 secretion through TGR5. RNA interference experiments showed that reduced expression of TGR5 resulted in reduced secretion of GLP-1. Furthermore, transient transfection of STC-1 cells with an expression plasmid containing TGR5 significantly enhanced GLP-1 secretion, indicating that bile acids promote GLP-1 secretion through TGR5 in STC-1 cells. Bile acids induced rapid and dose-dependent elevation of intracellular cAMP levels in STC-1 cells. An adenylate cyclase inhibitor, MDL12330A, significantly suppressed bile acid-promoted GLP-1 secretion, suggesting that bile acids induce GLP-1 secretion via intracellular cAMP production in STC-1 cells.
URLPMID:29486523 [本文引用: 2]

Abstract Bile acids activate farnesoid X receptor (FXR) and G protein-coupled bile acid receptor-1 (Gpbar-1, aka TGR5) to regulate bile acid metabolism and glucose and insulin sensitivity. FXR and TGR5 are co-expressed in the enteroendocrine L cells but their roles in integrated regulation of metabolism are not completely understood. We reported recently that activation of FXR induces TGR5 to stimulate glucagon-like peptide-1 (GLP-1) secretion to improve insulin sensitivity and hepatic metabolism. In this study, we used the intestine-restricted FXR agonist fexaramine (FEX) to study the effect of activation of intestinal FXR on the gut microbiome, bile acid metabolism, and FXR and TGR5 signaling. The current study revealed that FEX markedly increased taurolithocholic acid (TLCA), increased fibroblast growth factor 15 (FGF15) and FGF21 and GLP-1 secretion, improved insulin and glucose tolerance, and promoted white adipose tissue browning in mice. Analysis of 16S ribosomal RNA sequences of the gut microbiome identified the FEX-induced and LCA-producing bacteria Acetatifactor and Bacteroides. Antibiotic treatment completely reversed the FEX-induced metabolic phenotypes and inhibited TLCA synthesis, adipose tissue browning, and liver bile acid synthesis gene expression, but further increased intestinal FXR target gene expression. FEX treatment effectively improved lipid profiles, increased GLP-1 secretion, improved glucose and insulin tolerance, and promoted adipose tissue browning, while antibiotic treatment reversed the beneficial metabolic effects of FEX in obese and diabetic mice. This study uncovered a novel mechanism in which activation of intestinal FXR shaped the gut microbiota to activate TGR5/GLP-1 signaling to improve hepatic glucose and insulin sensitivity and increase adipose tissue browning. The gut microbiota plays a critical role in bile acid metabolism and signaling to regulate metabolic homeostasis in health and disease. This article is protected by copyright. All rights reserved.
URLPMID:5770450 [本文引用: 1]

Remodelling of energy storing white fat into energy expending beige fat could be a promising strategy to reduce adiposity. Here, we show that the bile acid-responsive membrane receptor TGR5 mediates beiging of the subcutaneous white adipose tissue (scWAT) under multiple environmental cues including cold exposure and prolonged high-fat diet feeding. Moreover, administration of TGR5-selective bile acid mimetics to thermoneutral housed mice leads to the appearance of beige adipocyte markers and increases mitochondrial content in the scWAT ofTgr5+/+mice but not in theirTgr561/61littermates. This phenotype is recapitulated in vitro in differentiated adipocytes, in which TGR5 activation increases free fatty acid availability through lipolysis, hence fuelling β-oxidation and thermogenic activity. TGR5 signalling also induces mitochondrial fission through the ERK/DRP1 pathway, further improving mitochondrial respiration. Taken together, these data identify TGR5 as a druggable target to promote beiging with potential applications in the management of metabolic disorders. White adipose tissue can undergo a process of beiging and acquire functional characteristics similar to brown adipose tissue, including the ability to dissipate energy via uncoupled respiration. Here, Velazquez-Villegas et al. show that activation of the bile acid membrane receptor, TGR5, leads to white adipocyte beiging by promoting mitochondrial fission.
URL [本文引用: 1]
URLPMID:16400329 [本文引用: 1]

Abstract While bile acids (BAs) have long been known to be essential in dietary lipid absorption and cholesterol catabolism, in recent years an important role for BAs as signalling molecules has emerged. BAs activate mitogen-activated protein kinase pathways, are ligands for the G-protein-coupled receptor (GPCR) TGR5 and activate nuclear hormone receptors such as farnesoid X receptor alpha (FXR-alpha; NR1H4). FXR-alpha regulates the enterohepatic recycling and biosynthesis of BAs by controlling the expression of genes such as the short heterodimer partner (SHP; NR0B2) that inhibits the activity of other nuclear receptors. The FXR-alpha-mediated SHP induction also underlies the downregulation of the hepatic fatty acid and triglyceride biosynthesis and very-low-density lipoprotein production mediated by sterol-regulatory-element-binding protein 1c. This indicates that BAs might be able to function beyond the control of BA homeostasis as general metabolic integrators. Here we show that the administration of BAs to mice increases energy expenditure in brown adipose tissue, preventing obesity and resistance to insulin. This novel metabolic effect of BAs is critically dependent on induction of the cyclic-AMP-dependent thyroid hormone activating enzyme type 2 iodothyronine deiodinase (D2) because it is lost in D2-/- mice. Treatment of brown adipocytes and human skeletal myocytes with BA increases D2 activity and oxygen consumption. These effects are independent of FXR-alpha, and instead are mediated by increased cAMP production that stems from the binding of BAs with the G-protein-coupled receptor TGR5. In both rodents and humans, the most thermogenically important tissues are specifically targeted by this mechanism because they coexpress D2 and TGR5. The BA-TGR5-cAMP-D2 signalling pathway is therefore a crucial mechanism for fine-tuning energy homeostasis that can be targeted to improve metabolic control.
URLPMID:5511840 [本文引用: 1]

Worldwide, metabolic diseases such as obesity and type 2 diabetes have reached epidemic proportions. A major regulator of metabolic processes that gained interest in recent years is the bile acid receptor TGR5. This G-protein coupled membrane receptor can be found predominantly in the intestine, where it is mainly responsible for the secretion of the incretins glucagon-like peptide 1 (GLP-1) and peptide YY (PYY). The aim of this study was i) to identify plant extracts with TGR5-activating potential, ii) to narrow down their activity to the responsible constituents, and iii) to assess whether the intestinal microbiota produces transformed metabolites with a different activity profile. Chenodeoxycholic acid (CDCA) served as positive control for both, the applied cell-based luciferase reporter gene assay for TGR5 activity and the biotransformation assay using mouse fecal slurry. The suitability of the workflow was demonstrated by the biotransformation of CDCA to lithocholic acid (LCA) resulting in a distinct increase in TGR5 activity. Based on a traditional Tibetan formula, nineteen plant extracts were selected and investigated for TGR5 activation. Extracts from the commonly used spices Syzygium aromaticum (SaroE, clove), Pimenta dioica (PdioE, allspice), and Kaempferia galanga (KgalE, aromatic ginger) significantly increased TGR5 activity. After biotransformation, only KgalE showed significant differences in its metabolite profile, which however did not alter its TGR5 activity compared to non-transformed KgalE. UHPLC-HRMS analysis revealed triterpene acids (TTAs) as the main constituents of the extracts SaroE and PdioE. Identification and quantification of TTAs in these two extracts as well as comparison of their TGR5 activity with reconstituted TTA mixtures allowed the attribution of the TGR5 activity to TTAs. EC50s were determined for the main TTAs, i.e. oleanolic acid (2.2 ± 1.6 08M), ursolic acid (1.1 ± 0.2 08M), as well as for the hitherto unknown TGR5 activators corosolic acid (0.5 ± 1.0 08M) and maslinic acid (3.7 ± 0.7 08M). In conclusion, extracts of clove, allspice, and aromatic ginger activate TGR5, which might play a pivotal role in their therapeutic use for the treatment of metabolic diseases. Moreover, the TGR5 activation of SaroE and PdioE could be pinpointed solely to TTAs.
[本文引用: 1]
URLPMID:5338933 [本文引用: 1]

Lung cancer has the highest morbidity and mortality in the world, and non-small cell lung carcinomas (NSCLC) account for 80% of cases of lung cancer. The mechanism of NSCLC is still largely unknown, and finding novel targets is of great importance for the treatment of NSCLC. The current study was designed to evaluate the role of miR-148a in NSCLC cell proliferation and invasion and to investigate the possible molecular mechanisms. We found that miR-148a expression was decreased in NSCLC tissues and cell lines. Upregulation of miR-148a significantly decreased A549 cell proliferation, and downregulation of miR-148a significantly increased A549 cell proliferation. Upregulation of miR-148a markedly increased apoptotic cell death and inhibited cell invasion potential. Upregulation of miR-148a significantly decreased signal transducer and activator of transcription 3 (STAT3) expression and 3-untranslated region luciferase activity. Downregulation of miR-148a significantly increased STAT3 expression. Overexpression of STAT3 significantly inhibited the effect of miR-148a on cell viability and invasion potential. In conclusion, we found that miR-148a inhibited NSCLC cell proliferation and invasion potential through the inhibition of STAT3. Our findings highlight miR-148a/STAT3 axis as a novel therapeutic target for the inhibition of NSCLC growth.
URLPMID:28377453 [本文引用: 1]

Oncogene-specific changes in cellular signaling have been widely observed in lung cancer. Here, we investigated how these alterations could affect signaling heterogeneity and suggest novel therapeutic strategies. We compared signaling changes across six human bronchial epithelial cell (HBEC) strains that were systematically transformed with various combinations of , , and ncogenic alterations commonly found in non mall cell lung cancer (NSCLC). We interrogated at single-cell resolution how these alterations could affect classic readouts (-CATENIN, SMAD2/3, phospho-STAT3, P65, FOXO1, and phospho-ERK1/2) of key pathways commonly affected in NSCLC. All three oncogenic alterations were required concurrently to observe significant signaling changes, and significant heterogeneity arose in this condition. Unexpectedly, we found two mutually exclusive altered subpopulations: one with STAT3 upregulation and another with SMAD2/3 downregulation. Treatment with a STAT3 inhibitor eliminated the upregulated STAT3 subpopulation, but left a large surviving subpopulation with downregulated SMAD2/3. A bioinformatics search identified , a gene downstream of SMAD2/3, as a novel pharmacologically accessible target of our transformed HBECs. Combination treatment with STAT3 and BCL6 inhibitors across a panel of NSCLC cell lines and in xenografted tumors significantly reduced tumor cell growth. We conclude that BCL6 is a new therapeutic target in NSCLC and combination therapy that targets multiple vulnerabilities (STAT3 and BCL6) downstream of common oncogenes, and tumor suppressors may provide a potent way to defeat intratumor heterogeneity.
URLPMID:29074425 [本文引用: 1]

react-text: 104 OBJECTIVE: To inquire into the influence of silencing HMGB1 expression by small interfering RNA (siRNA) on cell growth, proliferation, invasion and metastasis of colorectal cancer LoVo cells both in vitro and in vivo. /react-text react-text: 105 /react-text
URLPMID:8862472 [本文引用: 1]

Clin Chim Acta. 1996 Jul 30;251(2):173-86. Clinical Trial
URLPMID:27871908 [本文引用: 1]

In conclusion, we found that acetylated DCA and CA are potent ligands of PXR. Whether the acetylated bile acid derivatives are novel endogenous ligands of PXR with detoxification or physiological functions should be further studied in ongoing experiments.
URLPMID:24399466 [本文引用: 1]

The bile salt export pump (BSEP/Bsep; gene symbol ABCB11 / Abcb11 ) translocates bile salts across the hepatocyte canalicular membrane into bile in humans and mice. In humans, mutations in the ABCB11 gene cause a severe childhood liver disease known as progressive familial intrahepatic cholestasis type 2. Targeted inactivation of mouse Bsep produces milder persistent cholestasis due to detoxification of bile acids through hydroxylation and alternative transport pathways. The purpose of the present study was to determine whether functional expression of hepatic cytochrome P450 (CYP) and microsomal epoxide hydrolase (mEH) is altered by Bsep inactivation in mice and whether bile acids regulate CYP and mEH expression in Bsep 61/61 mice. CYP expression was determined by measuring protein levels of Cyp2b, Cyp2c and Cyp3a enzymes and CYP-mediated activities including lithocholic acid hydroxylation, testosterone hydroxylation and alkoxyresorufin O -dealkylation in hepatic microsomes prepared from female and male Bsep 61/61 mice fed a normal or cholic acid (CA)-enriched diet. The results indicated that hepatic lithocholic acid hydroxylation was catalyzed by Cyp3a/Cyp3a11 enzymes in Bsep 61/61 mice and that 3-ketocholanoic acid and murideoxycholic acid were major metabolites. CA feeding of Bsep 61/61 mice increased hepatic Cyp3a11 protein levels and Cyp3a11-mediated testosterone 2β-, 6β-, and 15β-hydroxylation activities, increased Cyp2b10 protein levels and Cyp2b10-mediated benzyloxyresorufin O -debenzylation activity, and elevated Cyp2c29 and mEH protein levels. We propose that bile acids upregulate expression of hepatic Cyp3a11, Cyp2b10, Cyp2c29 and mEH in Bsep 61/61 mice and that Cyp3a11 and multidrug resistance-1 P-glycoproteins (Mdr1a/1b) are vital components of two distinct pathways utilized by mouse hepatocytes to expel bile acids.
URLPMID:11248085 [本文引用: 1]

The pregnane X receptor (PXR) is the molecular target for catatoxic steroids such as pregnenolone 16伪-carbonitrile (PCN), which induce cytochrome P450 3A (CYP3A) expression and protect the body from harmful chemicals. In this study, we demonstrate that PXR is activated by the toxic bile acid lithocholic acid (LCA) and its 3-keto metabolite. Furthermore, we show that PXR regulates the expression of genes involved in the biosynthesis, transport, and metabolism of bile acids including cholesterol 7伪-hydroxylase (Cyp7a1) and the Na+-independent organic anion transporter 2 (Oatp2). Finally, we demonstrate that activation of PXR protects against severe liver damage induced by LCA. Based on these data, we propose that PXR serves as a physiological sensor of LCA, and coordinately regulates gene expression to reduce the concentrations of this toxic bile acid. These findings suggest that PXR agonists may prove useful in the treatment of human cholestatic liver disease.
URLPMID:25660334 [本文引用: 1]

Tanshinone IIA (Tan IIA) is one of the main natural active ingredients purified from Salvia miltiorrhiza radix, which has long been used in clinical practice in China to treat diseases including liver fibrosis, Alzheimer's disease, and cardiovascular diseases. Tan IIA has hepatoprotective properties, and is an efficacious PXR agonist. Our study was designed to observe the function and mechanism of the hepatoprotective properties of Tan IIA. HepG2 cells were used to investigate the vitrol effects of Tan IIA on PXR and CYP3A4. Gut-formed LCA is hepatotoxic, and has been implicated in the pathogenesis of cholestatic diseases. To further. investigate the hepatoprotective mechanisms of Tan IIA against LCA-induced cholestasis in vivo, we choose the normal mice and siRNA-treated mice. The in vitro study demonstrated that the effect of Tan IIA on CYP3A4 was mediated by transactivation of PXR in a dose- and time-dependent manner. The in vivo experiments using PXR siRNA revealed that Tan IIA could protect against LCA-induced hepatotoxicity and cholestasis in a dose-dependent manner. These effects were partially caused by the upregulation of PXR, as well as Cyp3all, Cyp3a13, and Mdr1, which are the enzymes responsible for LCA metabolism. This is the first report showing that the hepatoprotective effects of Tan HA are partly mediated by PXR. (C) 2015 Published by Elsevier Ireland Ltd.
URLPMID:10630370 [本文引用: 1]

Tanshinone II-A is a derivative of phenanthrene-quinone isolated from Salvia miltiorrhiza BUNGE, a traditional herbal medicine that is known to induce antiinflammatory, anti-oxidative and cytotoxic activity. We have examined cellular effects of Tanshione II-A on HL60 human promyelocytic leukemic cells and K562 human erythroleukemic cells. Tanshione II-A induced a dose- and time-dependent DNA fragmentation into the multiples of 180 bp and specific proteolytic cleavage of poly(ADP-ribose) polymerase in both cell lines. PI-staining and flow cytometry analysis of K562 cells following Tanshione II-A treatment showed an increase of the cells possessing hypodiploid DNA indicative of apoptotic state of cells. Caspase-3 activity was significantly increased during Tanshinone II-A treatment of both HL60 and K562 cells, whereas caspase-1 activity was not changed. These results suggest that Tanshione II-A induced HL60 and K562 cellular apoptosis that may be associated with the selective members of caspase family. Copyright 2000 Academic Press.
URL [本文引用: 1]

\0
URL [本文引用: 1]

The vitamin D receptor (VDR) is a nuclear receptor that mediates the biological action of the active form of vitamin D, 1伪,25-dihydroxyvitamin D3[1,25(OH)2D3], and regulates calcium and bone metabolism. Lithocholic acid (LCA), which is a secondary bile acid produced by intestinal bacteria, acts as an additional physiological VDR ligand. Despite recent progress, however, the physiological function of the LCA DR axis remains unclear. In this study, in order to elucidate the differences in VDR action induced by 1,25(OH)2D3and LCA, we compared their effect on the VDR target gene induction in the intestine of mice. While the oral administration of 1,25(OH)2D3induced theCyp24a1expression effectively in the duodenum and jejunum, the LCA increased target gene expression in the ileum as effectively as 1,25(OH)2D3. 1,25(OH)2D3, but not LCA, increased the expression of the calcium transporter geneTrpv6in the upper intestine, and increased the plasma calcium levels. Although LCA could induce an ilealCyp24a1expression as well as 1,25(OH)2D3, the oral LCA administration was not effective in the VDR target gene induction in the kidney. No effect of LCA on the ilealCyp24a1expression was observed in the VDR-null mice. Thus, the results indicate that LCA is a selective VDR ligand acting in the lower intestine, particularly the ileum. LCA may be a signaling molecule, which links intestinal bacteria and host VDR function.
URLPMID:24343899 [本文引用: 1]

Abstract Vitamin D receptor (VDR) mediates vitamin D signaling involved in bone metabolism, cellular growth and differentiation, cardiovascular function, and bile acid regulation. Mice with an intestine-specific disruption of VDR (Vdr(ΔIEpC)) have abnormal body size, colon structure, and imbalance of bile acid metabolism. Lithocholic acid (LCA), a secondary bile acid that activates VDR, is among the most toxic of the bile acids that when overaccumulated in the liver causes hepatotoxicity. Because cytochrome P450 3A4 (CYP3A4) is a target gene of VDR-involved bile acid metabolism, the role of CYP3A4 in VDR biology and bile acid metabolism was investigated. The CYP3A4 gene was inserted into Vdr(ΔIEpC) mice to produce the Vdr(ΔIEpC)/3A4 line. LCA was administered to control, transgenic-CYP3A4, Vdr(ΔIEpC), and Vdr(ΔIEpC)/3A4 mice, and hepatic toxicity and bile acid levels in the liver, intestine, bile, and urine were measured. VDR deficiency in the intestine of the Vdr(ΔIEpC) mice exacerbates LCA-induced hepatotoxicity manifested by increased necrosis and inflammation, due in part to over-accumulation of hepatic bile acids including taurocholic acid and taurodeoxycholic acid. Intestinal expression of CYP3A4 in the Vdr(ΔIEpC)/3A4 mouse line reduces LCA-induced hepatotoxicity through elevation of LCA metabolism and detoxification, and suppression of bile acid transporter expression in the small intestine. This study reveals that intestinal CYP3A4 protects against LCA hepatotoxicity.
URL [本文引用: 1]
