 ,, 何建波西南大学生命科学学院,淡水鱼类资源与生殖发育教育部重点实验室,重庆 400715
,, 何建波西南大学生命科学学院,淡水鱼类资源与生殖发育教育部重点实验室,重庆 400715Progress of GATA6 in liver development
Ling Zhang ,, Jianbo HeKey Laboratory of Freshwater Fish Reproduction and Development, Ministry of Education, School of Life Sciences, Southwest University, Chongqing 400715, China
,, Jianbo HeKey Laboratory of Freshwater Fish Reproduction and Development, Ministry of Education, School of Life Sciences, Southwest University, Chongqing 400715, China第一联系人:
收稿日期:2017-05-8修回日期:2017-12-13网络出版日期:--
| 基金资助: |
Received:2017-05-8Revised:2017-12-13Online:--
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (724KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张玲, 何建波. GATA6在肝脏发育中的作用及调控机制. 遗传[J], 2018, 40(1): 22-32 doi:10.16288/j.yczz.17-163
Ling Zhang, Jianbo He.
肝脏发育是高度复杂的动态过程,主要包括内胚层肝脏特化(specification)、肝芽(liver bud)生长、肝母细胞(hepatoblast)增殖分化以及肝脏形态发生4个阶段,整个过程需要转录因子偶联细胞信号进行严密精确的时空调控。转录因子能识别特定的DNA序列,常与其他因子相互作用从而激活或抑制基因转录。研究表明,肝脏的正常发育和功能维持离不开GATA家族、叉头框A蛋白(forkhead box A,FoxA)家族和肝细胞核因子(hepatocyte nuclear factor, HNF)家族等肝内富集转录因子间形成的复杂网络的调控。其中,GATA6在内胚层肝脏谱系决定、肝脏特化、肝芽生长以及肝母细胞增殖分化等阶段发挥重要的调控作用。为了深入阐明GATA6在肝脏发育中转录调控的分子机制,本文梳理了GATA6在肝脏发育各阶段扮演的角色,并探讨了其“先锋因子(pioneer factor)”作用、肝向重编程作用以及其与肝脏诱导信号间相互作用的转录调控机制。
1 GATA6结构特点和功能
GATA家族成员广泛存在于动物、植物和真菌中,在早期内胚层图式建成、细胞增殖分化以及细胞运动等方面具有重要作用[1]。通过对24种脊椎动物GATA基因进行进化分析,结果发现在脊椎动物早期发生了两次主要的基因复制事件:第一次复制产生了GATA2、GATA3和GATA1;第二次复制产生了GATA4、GATA5和GATA6[2]。生物进化导致这一家族成员不断扩充,并产生了组织特异性表达模式。基于序列相似性,GATA家族成员可分为两类:GATA1/2/3 主要表达于造血系统、神经系统和免疫系统,对造血细胞与淋巴细胞的分化具有重要作用;GATA4/5/6主要在脊椎动物肝脏、胰腺、心脏、肺、肠道、生殖腺等器官中表达,通过与其他组织富集转录因子相互作用行使发育调控功能[3,4,5,6]。GATA家族成员之间同源性高,在不同物种间的进化高度保守,成员间的功能既有拮抗又有重叠,共同的结合基序为5°-(T/A)GATA(A/G)-3°,该序列最早是在鸡(Gallus gallus)的珠蛋白基因启动子上被发现[7]。GATA6是GATA转录因子家族成员之一,具有两个用于DNA结合的保守锌指结构—Cys-X2- Cys-X17-Cys-X2-Cys。靠近C端的锌指结构被称为C-finger,主要负责特异识别WGATAR (W=A或T,R=A或G) DNA碱基序列,从而使其能结合到特定位点;靠近N端的锌指结构被称为N-finger,主要通过与GATA互作蛋白的结合来调节C-finger的DNA结合能力[8]。在人类基因组中,编码GATA6的基因组DNA包含33088个碱基,位于18号染色体长臂(18q11.2)。由于从不同起始密码子起始转录,GATA6又可分为L型(595aa, 64 kDa)和S型(449aa, 52 kDa),这两种亚型能相互作用,L型转录活性比S型高[9]。
近年来,通过对小鼠(Mus musculus)和斑马鱼(Danio rerio)等模式动物的深入研究,科研人员逐渐揭示了GATA6在脊椎动物胚胎发育中的重要作用[10]。GATA6是组织特异性的重要转录调节因子,在胚胎发育过程中调节细胞增殖、分化和迁移,主要参与原肠运动、中内胚层特化、间充质向上皮细胞转化以及肝脏、胰腺、心脏等器官的发生过程[11,12]。GATA6影响多种组织器官的发育。在小鼠中利用四倍体补偿技术敲除gata6后发现对肝脏早期发育影响较大。Zhao等[13]通过分子和生物化学方法证明GATA6在肝脏发生过程中具有自主调控功能。此外,大量研究还揭示了GATA6作为“先锋因子”在肝脏内胚层中起始肝脏特异性基因转录,是肝脏发育早期阶段所必需的少数因子之一[14,15]。
GATA6在细胞定型、谱系转换和转分化方面的作用也不容忽视。有研究表明,GATA6是谱系选择信号通路中的关键调节因子,可启动细胞命运转换开关[16]。通常情况下,GATA6通过与其他转录因子或信号分子的相互作用来发挥调控功能。与其他GATA因子相似,GATA6也可能通过与半限制转录因子(semi-restrict transcription factor)相互作用来调控细胞类型特化和决定[2]。此外,已有研究证明GATA家族成员均具有重编程功能,可诱导细胞产生多能性,表明GATA家族也许是细胞命运转换的重要介质[17]。最新研究表明,损伤会诱导表皮的GATA6阳性细胞去分化并重新进入具有持续自我更新能力的干细胞状态[18]。
越来越多的研究表明,GATA家族成员的异常表达与癌症的发生发展及预后密切相关。Kwei等[19]通过基因组表达谱分析鉴定出gata6是一个潜在的癌基因,在胰胆管癌中高表达。然而,肿瘤恶化过程中GATA6究竟是促癌因子还是抑癌因子,目前还尚有争议。一些研究发现GATA6的作用取决于肿瘤来源的组织背景,科研人员将GATA6这种“在不同组织来源的恶性肿瘤中表达变化及作用明显不同”的模式解释为“组织来源特异性”[20]。此外,GATA6能调节巨噬细胞的表型,肝脏中的Kupffer细胞也是特化的巨噬细胞,因此推测GATA6可能在肝脏再生过程中也发挥一定作用[21]。
2 GATA6在肝脏发育中的作用
肝脏是人体中最大的消化腺,也是最重要的代谢器官之一,在体内参与消化、解毒、合成、分泌以及免疫防御等过程,肝脏稳态和功能的正常维持对机体极为重要。然而,我国人口众多,大约3亿人患有肝炎、非酒精脂肪肝、酒精肝等肝功能损伤性疾病,肝癌发病率和致死率一直居高不下。每年大约有40万人死于肝癌,占世界肝癌死亡人数的50%以上[22]。因此,深入解析肝脏发育机制以及开发新型的肝病治疗技术迫在眉睫。正向和反向遗传学分析已经证明,哺乳动物和斑马鱼肝脏发育的调控机制高度保守[10,23]。肝脏发育涉及不同基因时空上的相互作用,需要来源于其邻近器官或组织中多种信号分子的参与[24]。越来越多的研究表明,与发育相关的信号通路能够激活关键转录因子的表达,这些转录因子通过反馈调节方式维持参与器官发育的基因调控网络,如GATA 家族、FoxA家族、HNF家族以及C/EBPα(CCAAT- enhancer-binding proteins)、Prox1(prospero homeobox protein 1)和Hhex(hematopoietically-expressed homeobox protein)等肝内富集转录因子间的复杂调控网络不仅对肝脏早期发育至关重要,而且对肝脏细胞的表型及功能发挥也起到关键作用[25]。
大量研究表明,GATA4/6表达于前肠内胚层,对小鼠和斑马鱼的肝脏发育具有重要作用[13]。GATA6既可作为“先锋因子”调控肝脏早期发育,又能够与FoxA、BMP(bone morphogenetic protein)、FGF(fibroblast growth factor)、HNF等信号分子相互作用调控肝脏发育。例如,GATA6可作为hnf4的上游调控子来调控肝脏命运[26];科研人员在小鼠胚胎干细胞(embryonic stem cell, ESC)的体外分化模型中也观察到GATA6等转录因子在肝脏发育过程发挥了关键的调控作用[27,28]。
2.1 GATA6在内胚层肝脏谱系决定中的作用
诸多研究表明,GATA6对内胚层肝脏谱系决定至关重要。在胚胎发育早期,gata6是内细胞团(inner cell mass, ICM)谱系特化基因调节网络的关键基因,参与原始内胚层(primary endoderm, PrE)细胞命运特化和发育程序的启动。Bessonnard等[29]发现 GATA6、NANOG和FGF/ERK信号通路通过彼此间相互作用来决定ICM的细胞命运。GATA因子特别是GATA4/6,参与了原肠运动以及内胚层前体细胞的肝向分化过程[30,31]。此外,有报道称GATA4/6可通过抑制Hedgehog信号通路来调节前肠内胚层图式建成[32]。GATA和FoxA2因子参与限定性内胚层(definitive endoderm, DE)向肝脏—胰腺前体细胞的分化;GATA、Hhex和SOX17(SRY-box containing gene 17)等因子参与肝胰前体细胞向肝母细胞和胰腺前体细胞的分化[25]。研究表明,GATA4/5/6处于DE发育程序的核心,以自主调节和交互调节方式调控DE发育[33]。此外,GATA6也参与脏壁内胚层分化过程[34];GATA6和FoxA2通过调节Wnt6的表达并激活经典Wnt信号通路,进而诱导胚外内胚层(extraembryonic endoderm, XEN)形成[35]。在小鼠胚胎中,通过靶向抑制gata6表达可致使XEN发育缺陷,进而导致内胚层分化缺陷和早期胚胎性致死[36]。这些研究进一步说明GATA6在内胚层形成中具有重要的调节作用。
DE先形成初级肠管,然后沿前后轴(anterior-posterior axis, AP轴)形成前肠、中肠和后肠,该过程由邻近中胚层分泌的各种信号分子,如BMP、RA (retinoic acid)、FGF和Wnt等参与调控[37]。不同转录因子和信号诱导分子沿AP轴的表达差异和相互作用赋予肠管发育成不同器官的“感受性(competence)”,使得肠管能对各种诱导信号产生应答,最终决定肠管的发育命运。
内源性FoxA2和GATA4/6能够诱导腹侧前肠内胚层前体细胞分化为肝脏和胰腺[24]。研究表明,GATA4与GATA6同时表达于前肠内胚层并且在功能上互补,对肝脏早期发育具有重要作用[38]。小鼠中的GATA、FoxA和HNF转录因子家族成员与一些信号通路的协同作用能够促进肝脏谱系分化重要基因表达并且抑制胰腺形成[39]。值得注意的是,这些FoxA和GATA因子在随后的发育阶段也会表达并发挥不同作用。由此可见,GATA因子能直接调控肝脏发育,对肝脏特异性基因的表达非常重要[26]。此外,BMP、FGF和Wnt家族也促进多潜能的腹侧前肠内胚层向肝脏方向发育[40]。例如,在内胚层特化期间BMP信号对肝脏发生具有重要作用[41],通过染色质免疫共沉淀证明GATA6能直接结合于bmp2的启动子区域,以一种自分泌的正向调控方式促进内胚层的分化和成熟[42]。
内胚层细胞向肝脏特化过程需要GATA4/5/6、FoxA2、Hhex等转录因子的参与,这些转录因子以及它们之间的协同作用在胚胎早期器官形成中扮演重要角色[43]。阐明DE肝向分化过程中核心转录因子的作用以及其与下游靶基因的调控机制对于深入理解肝脏发育至关重要。然而,目前关于肝脏内胚层图式建成的直接数据较少,还需进一步探索。
2.2 GATA6在肝脏特化中的作用
小鼠E8.5天时,腹侧前肠内胚层肝脏命运已特化。前肠腹侧区域上皮细胞增厚形成肝盲囊(liver diverticulum),这是肝脏发育的第一个形态发生信号[25,44]。移植实验证明,来自心脏中胚层的FGF信号和来自横隔间充质(septum transversum mesenchyme, STM)及侧板中胚层的BMP信号在小鼠肝脏特化诱导过程中有重要作用[45]。GATA6通过与BMP、FGF和HNF等信号分子的相互作用来影响肝脏特化。研究发现,来自小鼠移植体中的BMP信号是通过维持GATA4/6的表达促进肝脏特化。Rossi等[45]运用分子标志物和功能评价等方法证明BMP信号通过调节GATA4的水平以及FGF信号通路来控制肝脏早期基因表达。在斑马鱼和小鼠中,BMP信号在肝脏特化诱导阶段促进肝向分化,抑制胰腺发育[46]。GATA6能直接结合bmp2的启动子,而BMP2信号可下调胰腺特异性基因pdx1的表达[47],然而肝脏特化过程中GATA6是否与bmp2相互作用还需进一步研究。间充质细胞表达的BMP4对肝脏诱导也非常重要,研究表明GATA6能够结合在bmp4启动子的GATA结合位点。在斑马鱼中研究发现,抑制BMP信号表现出同gata6基因纯合敲除类似的表型,这说明GATA6和BMP信号的相互作用可能影响心脏发育[48,49],然而,它们是否依赖相同的调控机制影响肝脏发育还有待进一步研究。Wnt信号对肝脏特化也很重要[50],Whissell等[51]研究表明,GATA6 通过抑制bmp表达促进结肠腺瘤干细胞自我更新,而且在结肠癌中GATA6是Wnt和BMP信号通路的一个关键调节因子。
在斑马鱼受精18~26 h后的胚胎中抑制FGF受体会同时下调gata6、gata4、hhex和prox1等基因的表达[46]。但Zhao等[13]研究发现,小鼠肝脏特化不需要GATA6参与,并推测在肝脏特化期间GATA4能够补偿GATA6的功能,而GATA6单独调控肝脏下一阶段的发育。此外,HNF1/3/4在哺乳动物肝脏特化中发挥重要作用[52]。研究发现GATA6的纯合突变体会导致GATA4表达严重下调以及HNF4表达缺失[34,53]。Delaforest等[54]利用shRNA敲降HNF4α后能够阻碍肝脏特化,并且抑制GATA4/6和FoxA1/2的表达。此外,Hhex也可通过抑制脱中胚蛋白(eomesodermin)表达来促进肝脏特化[55]。以上结果说明,gata6可能作为上游调控基因来调节肝脏命运特化。
近年来,随着对肝脏发育机理的深入研究,Zaret等[56]提出了“先锋因子”假说。在肝脏发育特别是肝脏特化阶段存在一系列的“先锋因子”,例如FoxA1(HNF3A)、FoxA2(HNF3B)、GATA4和GATA6,这些因子能够改变染色质的伸缩程度,使染色质处于“开放状态”,因而易与其他转录因子结合从而启动肝脏特异性基因转录。FoxA和GATA因子在发育过程能够与增强子结合,对肝脏特化诱导至关重要。体外研究表明,体外分离的内胚层细胞仍保持诱导成为肝脏的“感受性”。小鼠中的白蛋白增强子体内足迹法结果显示,在未被诱导产生肝脏但具有“感受性”的内胚层中(E9.5~E12.5),内胚层转录因子重要的结合位点被FoxA1、FoxA2、GATA4和GATA6占据,因此允许接收肝脏诱导信号。在小鼠E14.5天时,“感受性”丧失,而这些位点也不再被占据[15,24]。由此可见,GATA6同GATA4和FoxA可作为“先锋因子”发挥染色质重塑功能。
内胚层命运特化之前,“先锋因子”先赋予特异性基因表达的“感受性”,即占据内胚层肝脏特异性基因增强子的结合位点,发育诱导信号可通过“先锋因子”发挥作用,此过程相当于是对染色质状态的预图式建成(pre-pattern)[57]。然而诱导信号与先锋因子相互作用影响靶基因染色质状态的分子机制还需进一步研究。不同的发育阶段或基因调控网络存在不同的“先锋因子”。此外,“先锋因子”在癌症中也会异常表达。GATA因子在癌症中同样能够发挥“先锋因子”作用,并可作为药物靶标;GATA因子和FoxA因子涉及很多激素依赖性癌症,例如雄激素介导的肝癌发生[15]。
2.3 GATA6维持肝芽生长
在小鼠中,肝脏特化后上皮细胞表达肝脏基因(alp、hnf4α)并开始增厚,由原来的立方上皮细胞逐渐形成肝脏假复层柱状上皮,进而形成肝盲囊。随后,包围内胚层富含层粘连蛋白的基底膜被打破,肝母细胞增殖加快(E9.5),不断向STM迁移和分层,肝芽向外生长,成为小鼠胚胎造血来源。其中肝脏间充质和STM分泌的生长因子,包括FGF、BMP、HGF(hepatocyte growth factor)、Wnt、TGF-β(tumor growth factor β)和RA等,能够促进肝母细胞的增殖、迁移和存活[24,40,58]。GATA家族成员在肝芽生长时也同样发挥重要作用[26]。在斑马鱼中,敲除任意一个gata4/5/6基因会导致肝芽生长出现严重缺陷,而肝芽特化正常;同时敲除任意这两种基因都会阻碍肝脏特化,说明GATA4/5/6在肝脏内胚层特化时功能冗余,而在随后的肝芽生长中则各自发挥特异作用[25,59]。GATA4/6调节肝芽向STM迁移和生长,此时GATA4/6之间功能冗余[60]。Zhao等[13]利用四倍体补偿技术在小鼠中研究发现,gata6基因缺失导致细胞分化和肝芽生长受阻,但肝脏特化正常,说明GATA6在肝芽生长过程中有关键作用。此外,GATA6在肝芽生长中可能通过调节HNF转录因子发挥重要作用。例如,XEN发育所需的同源域蛋白HNF1β可能位于GATA6的下游,调节肝芽增厚过程并维持FoxA1/2/3的表达[25]。
肝芽生长需要多种因子的参与。Hhex转录因子参与立方上皮向假复层柱状上皮转换过程[61];Prox1因子能瓦解细胞间联系,促使肝芽生长[62];Klf6(krüppel-like factor 6)促进肝芽生长[63],而Tbx3(T-box 3)促进肝母细胞复制以及迁移[64]。肝脏形态发生过程中,尽管大量关键基因已陆续被发现,然而它们在不同信号通路中的表达和功能不同,暗示在肝芽生长中还存在其他较为重要的基因和信号通路。
2.4 GATA6在肝脏形态发生中的作用
在小鼠胚胎E13天时,具有双向分化潜能的肝母细胞开始增殖迁移,在不同转录因子和信号通路调控网络的作用下分别分化为肝细胞和胆管细胞。肝实质中,未与肝门静脉联系的肝母细胞逐渐分化为成熟的肝细胞,并在细胞顶面形成胆小管,获得上皮形态特征;E17天时,邻近肝门静脉的肝母细胞形成管状扩张(ductal plate remodeling,也称胆管盘重塑),被门静脉间充质包围进而形成肝内胆管[58]。而谱系追踪研究显示,由肝胆管、胆囊管、胆总管和胆囊组成的肝外胆管起源于前肠内胚层的Pdx1阳性细胞,和腹侧胰腺(而不是肝脏)共起源[65]。在肝脏形态发生阶段,由小鼠造血前体细胞分泌的抑瘤蛋白M(oncostatin M, OSM)能够在体外诱导肝母细胞增殖,并促进其向具有代谢功能的成熟肝脏细胞分化,而TGFβ和Notch信号指导双潜能胚胎肝脏前体细胞向胆管细胞命运分化[25,58]。此外,Hex(homeobox protein)对于前肠器官形态发生和细胞分化维持也具有重要作用。在hex纯合敲除的小鼠中,尽管肝细胞转录因子表达以及肝芽形成正常,但肝脏前体细胞不能迁移到STM,因此肝脏不能生长并且形态发生受到抑制[38]。HNF1α和HNF4α 也能够调节肝脏特异性基因表达,对肝脏结构以及特异性功能的维持有重要作用[53]。有研究表明,BMP2可能是肝细胞增殖的负调节子。体外研究发现,BMP2可抑制肝癌细胞生长,然而该结论还须利用肝细胞再生模型进行体内实验证实[66]。此外,内皮细胞不仅在肝脏特化中发挥重要功能,而且其在肝脏形态发生中也至关重要,斑马鱼血管系统对胆管分化和肝细胞极化有重要作用[58]。总之,肝脏细胞成熟和异质化这一过程主要由Wnt和Notch信号通路介导,涉及FGF、BMP、HNF等一系列信号分子间的相互作用[40,44]。
图1
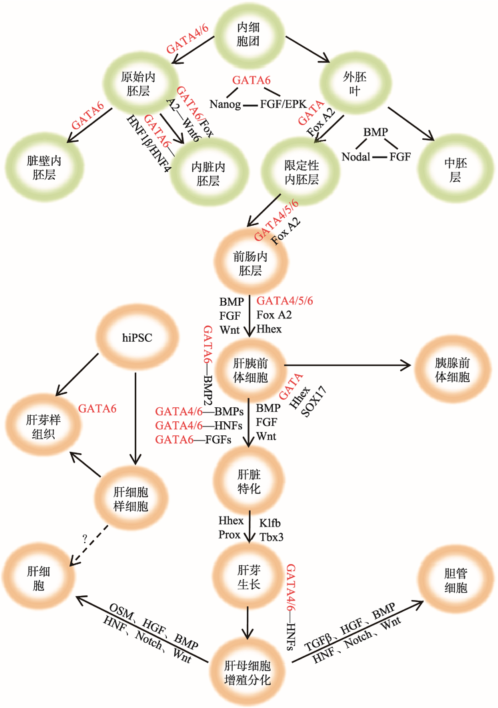 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1GATA6在早期胚胎及肝脏发育中的调控作用示意图
图中所示本文提到的参与胚胎早期发育和肝脏发育各阶段的主要转录因子、信号分子和信号通路之间的关系。“—”表示两者间存在相互作用关系;“→”表示发育过程中的分化方向或发育阶段的递进关系。带箭头的虚线表示目前还没有研究证明有此转换过程。
Fig. 1The role of GATA6 in early embryogenesis and liver development
研究表明,在已分化的肝细胞中敲除gata4或同时敲除gata4/6后,基因表达谱和肝脏功能几乎没有改变,说明此发育阶段存在特殊的转录调控网络以维持肝脏功能,GATA4/6可能不是转录调控复合物的核心元件[24]。已知GATA6表达于胚胎肝细胞(fetal hepatocyte, fHep)、成体肝细胞以及胆管细胞以维持细胞分化状态,然而关于成熟肝细胞中GATA6的转录调控机制研究还很匮乏[60],目前只有少数研究聚焦于肝脏生长和形态发生过程所需要的关键基因。
3 GATA6的肝向重编程作用
研究发现,GATA家族成员均可代替OCT4 (octamer-binding transcription factor-4)将小鼠胃细胞重编程为多能性细胞[17],其中GATA3/4/6取代OCT4的能力最强。GATA因子在细胞重编程过程中主要通过C-finger的DNA结合位点发挥作用,这暗示GATA因子可能通过促进染色质成环远距离控制DNA的相互作用,以这种染色质结构重塑的方式重建多能性基因的表达[17]。此外,各种谱系特化力量的势均力敌对于恢复多能性至关重要,然而传统观念认为向目标细胞转变就需要激活目标细胞特定的转录因子,事实可能并非如此[8,67]。有研究表明,Sall4在重编程过程中可作为GATA家族成员的直接靶标,调节肝脏前体细胞和造血细胞的分化[17]。
目前已证实GATA家族所有成员都具有重编程功能,可能是细胞命运转化的一个重要介导物,但这一作用长期以来一直没有被重视[17]。最近在一项聚焦于指导细胞命运向内胚层和中胚层谱系分化的转录因子的研究中,科研人员利用遗传工程使人类自体同源诱导多能干细胞(autologous hiPSCs)过表达GATA6从而快速诱导出3个胚层,并成功诱导出胚胎肝细胞、胆管细胞、内皮细胞、星形细胞和周细胞样细胞,这些细胞通过共发育和自组织(self-organization)形成了包括血管样结构和造血样过程的异质性肝芽状组织,尽管该组织无复杂的三维结构,但它包含多种细胞类型并且表现出与肝脏发育关键特征类似的表型[68]。
此前已有研究发现,在小鼠ES细胞的谱系分化中,GATA4主要负责接收细胞位置或聚集信息,而GATA6主要感知形态发生信号,如视黄酸(retinoic acid, RA)信号[28]。当然,细胞命运决定还可能由其他转录因子与GATA6的相对水平、GATA6的表达动力学以及细胞定位共同调节[68]。此外,“先锋因子”的活性对具有重编程细胞能力的转录因子至关重要,目前已有研究证明在细胞重编程过程中“先锋因子”同样发挥重要作用[15,69,70],但GATA家族成员是否和表观调节子相互作用还需进一步探索。
近年来随着诱导多功能干细胞(induced pluripotent stem cell, iPSC)研究的飞速发展,细胞转分化和细胞谱系决定的研究取得了巨大进展。早在几十年前,人们就利用过表达特定的转录因子实现了细胞谱系间的转换。目前调节肝脏发育的转录因子已经被应用于人类成纤维细胞的肝向重编程[71,72]。例如GATA4、HNF1和FoxA3三者联合能够将小鼠成纤维细胞重编程为有功能的肝细胞样细胞,并且在体内移植后能够发挥肝细胞正常功能[73],说明GATA家族、HNF家族以及FoxA家族作为关键转录因子在肝向命运决定调控网络中发挥重要作用。在体外诱导分化实验中,通过模仿体内肝脏发育模式,人为添加促进肝向分化的因子或化合物,可将小鼠ES细胞诱导为早期肝细胞[74]。利用hiPSC起源的肝细胞样细胞、内皮细胞以及从脐带分离的中内胚层细胞可以产生具备肝脏基本功能的肝芽组织[75]。一系列在体内外利用人多潜能干细胞产生肝脏细胞的方案已相继实施。
总之,GATA6在细胞重编程中具有重要作用。谱系特化子(如GATA因子等)能直接激活特定基因或调节表观遗传因子的表达,诱导细胞产生多能性,促进器官修复再生,因此具有巨大的研究价值和重要的临床意义。未来可将GATA6等关键转录因子与自组织hiPSC诱导结合以改进组织模型(如药物毒性筛选模型)和治疗应用[68,76]。深入研究指导多潜能前体细胞形成特定器官的特异性因子以及它们之间的相互作用,以期解开细胞命运转换之谜[77]。
4 结语与展望
GATA6 的基本功能是控制细胞分化、调节细胞周期,是脊椎动物器官发育过程中的关键转录因子。此外,GATA6可扮演“先锋因子”和“染色质重塑因子”,在细胞命运转换、谱系特化和重编程过程中发挥重要作用。最新研究表明,GATA6和其他器官组织(如肺和毛囊)的再生也密切相关[78]。Zhang等[79]发现GATA6通过Wnt信号通路可调节肺支气管上皮细胞分化和气管再生。GATA6在肝脏发育中的作用逐渐清晰,然而GATA6是否在肝脏再生过程中也同样发挥重要作用还有待进一步研究。总之,GATA6在肝脏发育中的功能及其调控机制的进一步揭示为诱导产生肝实质细胞提供了理论指导,对于靶向GATA6等关键转录因子或其调控的基因进行肝病临床治疗也具有重大意义。众所周知,治疗晚期肝病的肝脏移植技术目前还面临一系列挑战,即供体来源有限、免疫排斥严重以及体外诱导产生肝脏细胞的重编程效率不高等。因此,未来可着重发展转化医学研究,重点从以下两方面突破:第一,利用谱系追踪建立肝脏发育的细胞来源图谱,重点鉴定各种前体细胞的形态学和功能特征;第二,深入探索肝脏发育的分子机制,或利用转分化技术直接诱导产生功能性肝细胞。肝功能受损对人体健康危害极大,因此利用现有研究基础完善和开发新的肝病治疗方法迫在眉睫。虽然目前的生物人工肝装置可以缓解严重肝脏功能障碍,但实际临床应用还存在诸多限制因素。此外,目前的肝细胞移植治疗也未取得突破性进展。人体肝脏能够再生但再生能力有限,因此提高肝脏内源性再生能力将是另一治疗策略。
迄今为止,调节肝脏发育的许多基因和分子信号途径已被鉴定,但肝脏发育的关键基因及分子调控机制还需进一步研究。例如,转录因子如何协同作用发挥功能赋予内胚层不同空间域“感受性”以发育成肝脏这样的组织?组织细胞间如何相互作用以调节肝脏细胞的成熟?因此,鉴定肝脏发育和自稳态功能维持过程中控制肝脏命运转换的特异性信号元件,如配体、受体、转录因子,以及它们之间的调控网络,对于深入理解肝脏发育、再生、肝病治疗有重要意义。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:18367552 [本文引用: 1]

One poorly understood mechanism of developmental patterning involves the intermingled differentiation of different cell types that then sort out to generate pattern. Examples of this are known in nematodes and vertebrates, and in Dictyostelium it is the major mechanism. However, a general problem with this mechanism is the possibility that different inducers are required for each cell type that arises independently of positional information. Consistent with this idea, in Dictyostelium the signalling molecule DIF acts as a position-independent signal and was thought only to regulate the differentiation of a single cell type (pstO). The results presented here challenge this idea. In a novel genetic selection to isolate genes required for DIF signal transduction, we found a mutant (dimC(-)) that is a hypomorphic allele of a GATA family transcription factor (gtaC). gtaC expression is directly regulated by DIF, and GtaC rapidly translocates to the nucleus in response to DIF. gtaC(-) null cells showed some hallmark DIF signalling defects. Surprisingly, other aspects of the mutant were distinct from those of other DIF signalling mutants, suggesting that gtaC regulates a subset of DIF responses. For example, pstO cell differentiation appeared normal. However, we found that pstB cells were mislocalised and the pstB-derived basal disc was much reduced or missing. These defects are due to a failure to respond to DIF as they are phenocopied in other DIF signalling mutants. These findings therefore identify a novel small-molecule-activated GATA factor that is required to regulate the cell type-specific effects of DIF. They also reveal that a non-positional signal can regulate the differentiation of multiple cell types through differential interpretation in receiving cells.
URLPMID:24368683 [本文引用: 2]

GATA transcription factors perform conserved and essential roles during animal development, including germ-layer specification, hematopoiesis, and cardiogenesis. The evolutionary history and the changes in selection pressures following duplication of the six GATA family members in vertebrates have not been completely understood. Recently, we explored multiple databases to find GATAs in different vertebrate species. Using these sequences, we have performed molecular phylogenetic analyses using Maximum Likelihood and Bayesian methods, and statistical tests of tree topologies, to ascertain the phylogenetic relationship and selection pressures among GATA proteins. Seventy-one full-length cDNA sequences from 24 vertebrate species were extracted from multiple databases. By phylogenetic analyses, we investigated the origin, conservation, and evolution of the GATAs. Six GATA genes in vertebrates might be formed by gene duplication. The inferred evolutionary transitions that separate members which belong to different gene clusters correlated with changes in functional properties. Selection analysis and protein structure analysis were combined to explain Darwinian selection in GATA sequences and these changes brought putative biological significance. 26 positive selection sites were detected in this process. This study reveals the evolutionary history of vertebrate GATA paralogous and positively selected sites likely relevant for the distinct functional properties of the paralogs. It provides a new perspective for understanding the origin and evolution and biological functions of GATAs, which will help to uncover the GATAs' biological roles, evolution and their relationship with associated diseases; in addition, other complex multidomain families and also larger superfamilies can be investigated in a similar way.
URL [本文引用: 1]
URLPMID:9811575 [本文引用: 1]

Gene inactivation studies have shown that members of the GATA family of transcription factors are critical for endoderm differentiation in mice, flies and worms, yet how these proteins function in such a conserved developmental context has not been understood. We use in vivo footprinting of mouse embryonic endoderm cells to show that a DNA-binding site for GATA factors is occupied on a liver-specific, transcriptional enhancer of the serum albumin gene. GATA site occupancy occurs in gut endoderm cells at their pluripotent stage: the cells have the potential to initiate tissue development but they have not yet been committed to express albumin or other tissue-specific genes. The GATA-4 isoform accounts for about half of the nuclear GATA-factor-binding activity in the endoderm. GATA site occupancy persists during hepatic development and is necessary for the activity of albumin gene enhancer. Thus, GATA factors in the endoderm are among the first to bind essential regulatory sites in chromatin. Binding occurs prior to activation of gene expression, changes in cell morphology or functional commitment that would indicate differentiation. We suggest that GATA factors at target sites in chromatin may generally help potentiate gene expression and tissue specification in metazoan endoderm development.
URLPMID:2526999 [本文引用: 1]

Background GATA factors 4/5/6 have been implicated in the development of the heart and endodermal derivatives in vertebrates. Work in zebrafish has indicated that GATA5 is required for normal development earlier than GATA4/6. However, the GATA5 knockout mouse has no apparent embryonic phenotype, thereby questioning the importance of the gene for vertebrate development. Results In this study we show that in Xenopus embryos GATA5 is essential for early development of heart and liver precursors. In addition, we have found that in Xenopus embryos GATA4 is important for development of heart and liver primordia following their specification, and that in this role it might interact with GATA6. Conclusion Our results suggest that GATA5 acts earlier than GATA4 to regulate development of heart and liver precursors, and indicate that one early direct target of GATA5 is homeobox gene Hex.
URLPMID:16887115 [本文引用: 1]

Gata4, Gata5, and Gata6 represent a subfamily of zinc-finger transcriptional regulators that are important in the development and differentiation of numerous tissues, including many endodermally-derived organs. We demonstrate that Gata4 and Gata6 have overlapping expression patterns in the early pancreatic epithelium. Later, Gata4 becomes restricted to exocrine tissue and Gata6 becomes restricted to a subset of endocrine cells. In addition, we show Gata6, but not Gata4, physically interacts with Nkx2.2, an essential islet transcription factor. To begin determining the roles that Gata4 and Gata6 play during pancreatic development, we expressed Gata4-Engrailed and Gata6-Engrailed dominant repressor fusion proteins in the pancreatic epithelium and in the islet. At e17.5, transgenic Gata6-Engrailed embryos exhibit two distinct phenotypes: a complete absence of pancreas or a reduction in pancreatic tissue. In the embryos that do form pancreas, there is a significant reduction of all pancreatic cell types, with the few differentiated endocrine cells clustered within, or in close proximity to, enlarged ductal structures. Conversely, the majority of transgenic Gata4-Engrailed embryos do not have a pancreatic phenotype. This study suggests that Gata6 is an important regulator of pancreas specification.
URLPMID:8321208 [本文引用: 1]

Members of the GATA family of transcription factors, which are related by a high degree of amino acid sequence identity within their zinc finger DNA-binding domains, each show distinct but overlapping patterns of tissue-restricted expression. Although GATA-1, -2, and -3 have been shown to recognize a consensus sequence derived from regulatory elements in erythroid cell-specific genes, WGATAR (in which W indicates A/T and R indicates A/G), the potential for more subtle differences in the binding preferences of each factor has not been previously addressed. By employing a binding selection and polymerase chain reaction amplification scheme with randomized oligonucleotides, we have determined the binding-site specificities of bacterially expressed chicken GATA-1, -2, and -3 transcription factors. Whereas all three GATA factors bind an AGATAA erythroid consensus motif with high affinity, a second, alternative consensus DNA sequence, AGATCTTA, is also recognized well by GATA-2 and GATA-3 but only poorly by GATA-1. These studies suggest that all three GATA factors are capable of mediating transcriptional effects via a common erythroid consensus DNA-binding motif. Furthermore, GATA-2 and GATA-3, because of their distinct expression patterns and broader DNA recognition properties, may be involved in additional regulatory processes beyond those of GATA-1. The definition of an alternative GATA-2-GATA-3 consensus sequence may facilitate the identification of new target genes in the further elucidation of the roles that these transcription factors play during development.
URLPMID:23142663 [本文引用: 2]

GATA transcription factors regulate transcription by recognizing distinct GATA sites and by mediating long-range DNA looping. However, the molecular basis of these processes is not well understood. Chen and colleagues determined crystal structures of the DNA-binding domain (DBD) of the GATA3 protein. Strikingly, they find that the GATA3 DBD bridges two separate DNA fragments. These findings provide insights into the structure and function of GATA proteins and shed light on the molecular basis of long-range gene regulation.
URLPMID:25234600 [本文引用: 1]

Transcription factor GATA-6 plays essential roles in developmental processes and tissue specific functions through regulation of gene expression. GATA-6 mRNA utilizes two Met-codons in frame as translational initiation codons. Deletion of the nucleotide sequence encoding the PEST sequence (Glu(31)-Cys(46)) between the two initiation codons unusually reduced the protein molecular size on SDS-polyacrylamide gel-electrophoresis, and re-introduction of this sequence reversed this change. The long-type (L-type) GATA-6 containing this PEST sequence self-associated similarly to the short-type (S-type) GATA-6, as determined on co-immunoprecipitation of Myc-tagged GATA-6 with HA-tagged GATA-6. The L-type and S-type GATA-6 also interacted mutually. The L-type GATA-6 without the PEST sequence also self-associated and interacted with the S-type GATA-6. The transcriptional activation potential of L-type GATA-6 is higher than that of S-type GATA-6. When the PEST sequence (Glu(31)-Cys(46)) was inserted into the L-type GATA-6 without Arg(13)-Gly(101), the resultant recombinant protein showed significantly higher transcriptional activity, while the construct with an unrelated sequence exhibited lower activity. These results suggest that the Glu(31)-Cys(46) segment plays an important role in the transcriptional activation, although it does not participate in the self-association.
URL [本文引用: 2]

Abstract The liver performs a large number of essential synthetic and regulatory functions that are acquired during fetal development and persist throughout life. Their disruption underlies a diverse group of heritable and acquired diseases that affect both pediatric and adult patients. Although experimental analyses used to study liver development and disease are typically performed in cell culture models or rodents, the zebrafish is increasingly used to complement discoveries made in these systems. Forward and reverse genetic analyses over the past two decades have shown that the molecular program for liver development is largely conserved between zebrafish and mammals, and that the zebrafish can be used to model heritable human liver disorders. Recent work has demonstrated that zebrafish can also be used to study the mechanistic basis of acquired liver diseases. Here, we provide a comprehensive summary of how the zebrafish has contributed to our understanding of human liver development and disease. 漏 2013 American Physiological Society.
URL [本文引用: 1]

Review on GATA6 (GATA binding protein 6), with data on DNA, on the protein encoded, and where the gene is implicated.
URL [本文引用: 1]
URLPMID:1061656 [本文引用: 4]

Several lines of evidence suggest that GATA6 has an integral role in controlling development of the mammalian liver. Unfortunately, this proposal has been impossible to address directly because mouse embryos lacking GATA6 die during gastrulation. Here we show that the early embryonic deficiency associated with GATA6-knockout mice can be overcome by providing GATA6-null embryos with a wild-type extraembryonic endoderm with the use of tetraploid embryo complementation. Analysis of rescued Gata6-/- embryos revealed that, although hepatic specification occurs normally, the specified cells fail to differentiate and the liver bud does not expand. Although GATA6 is expressed in multiple tissues that impact development of the liver, including the heart, septum transversum mesenchyme, and vasculature, all are relatively unaffected by loss of GATA6, which is consistent with a cell-autonomous requirement for GATA6 during hepatogenesis. We also demonstrate that a closely related GATA factor, GATA4, is expressed transiently in the prehepatic endoderm during hepatic specification and then lost during expansion of the hepatic primordium. Our data support the proposal that GATA4 and GATA6 are functionally redundant during hepatic specification but that GATA6 alone is available for liver bud growth and commitment of the endoderm to a hepatic cell fate.
URL [本文引用: 1]

Single GATA-6 (G6(gcko)), GATA-4 (G4(gcko)), and double GATA-4/6 (G4/6(gcko)) granulosa cell-specific knockout mice were generated to further investigate the role of GATA transcription factors in ovarian function in vivo. No reproductive defects were found in G6(gcko) animals. G4(gcko) animals were subfertile as indicated by the reduced number of pups per litter and the release of significantly fewer oocytes at ovulation. In marked contrast, G4/6(gcko) females fail to ovulate and are infertile. Furthermore, G4/6(gcko) females had irregular estrous cycles, which correlate with the abnormal ovarian histology found in unstimulated adult G4/6(gcko) females showing lack of follicular development and increased follicular atresia. Moreover, treatment with exogenous gonadotropins did not rescue folliculogenesis or ovulation in double-knockout G4/6(gcko) mice. In addition, ovary weight and estradiol levels were significantly reduced in G4(gcko) and G4/6(gcko) animals when compared with control and G6(gcko) mice. Aromatase, P450scc, and LH receptor expression was significantly lower in G4(gcko) and G4/6(gcko) mice when compared with control animals. Most prominently, FSH receptor (FSHR) protein was undetectable in granulosa cells of G4(gcko) and G4/6(gcko). Accordingly, gel shift and reporter assays revealed that GATA-4 binds and stimulates the activity of the FSHR promoter. These results demonstrate that GATA-4 and GATA-6 are needed for normal ovarian function. Our data are consistent with a role for GATA-4 in the regulation of the FSHR gene and provide a possible molecular mechanism to explain the fertility defects observed in animals with deficient GATA expression in the ovary.
URLPMID:27246709 [本文引用: 4]

Distinct combinations of transcription factors are necessary to elicit cell fate changes in embryonic development. Yet within each group of fate-changing transcription factors, a subset called 'pioneer factors' are dominant in their ability to engage silent, unmarked chromatin and initiate the recruitment of other factors, thereby imparting new function to regulatory DNA sequences. Recent studies have shown that pioneer factors are also crucial for cellular reprogramming and that they are implicated in the marked changes in gene regulatory networks that occur in various cancers. Here, we provide an overview of the contexts in which pioneer factors function, how they can target silent genes, and their limitations at regions of heterochromatin. Understanding how pioneer factors regulate gene expression greatly enhances our understanding of how specific developmental lineages are established as well as how cell fates can be manipulated.
URLPMID:3697763 [本文引用: 1]

Molecular programs that mediate normal cell differentiation are required for oncogenesis and tumor cell survival in certain cancers. How cell-lineage-restricted genes specifically influence metastasis is poorly defined. In lung cancers, we uncovered a transcriptional program that is preferentially associated with distal airway epithelial differentiation and lung adenocarcinoma (ADC) progression. This program is regulated in part by the lineage transcription factors GATA6 and HOPX. These factors can cooperatively limit the metastatic competence of ADC cells, by modulating overlapping alveolar differentiation and invasogenic target genes. Thus, GATA6 and HOPX are critical nodes in a lineage-selective pathway that directly links effectors of airway epithelial specification to the inhibition of metastasis in the lung ADC subtype.
URLPMID:4650575 [本文引用: 5]

Members of the GATA protein family play important roles in lineage specification and transdifferentiation. Previous reports show that some members of the GATA protein family can also induce pluripotency in somatic cells by substituting for Oct4, a key pluripotency-associated factor. However, the mechanism linking lineage-specifying cues and the activation of pluripotency remains elusive. Here, we report that all GATA family members can substitute for Oct4 to induce pluripotency. We found that all members of the GATA family could inhibit the overrepresented ectodermal-lineage genes, which is consistent with previous reports indicating that a balance of different lineage-specifying forces is important for the restoration of pluripotency. A conserved zinc-finger DNA-binding domain in the C-terminus is critical for the GATA family to induce pluripotency. Using RNA-seq and ChIP-seq, we determined that the pluripotency-related gene Sall4 is a direct target of GATA family members during reprogramming and serves as a bridge linking the lineage-specifying GATA family to the pluripotency circuit. Thus, the GATA family is the first protein family of which all members can function as inducers of the reprogramming process and can substitute for Oct4. Our results suggest that the role of GATA family in reprogramming has been underestimated and that the GATA family may serve as an important mediator of cell fate conversion.
URLPMID:28504705 [本文引用: 1]

Abstract The epidermis is maintained by multiple stem cell populations whose progeny differentiate along diverse, and spatially distinct, lineages. Here we show that the transcription factor Gata6 controls the identity of the previously uncharacterized sebaceous duct (SD) lineage and identify the Gata6 downstream transcription factor network that specifies a lineage switch between sebocytes and SD cells. During wound healing differentiated Gata6 + cells migrate from the SD into the interfollicular epidermis and dedifferentiate, acquiring the ability to undergo long-term self-renewal and differentiate into a much wider range of epidermal lineages than in undamaged tissue. Our data not only demonstrate that the structural and functional complexity of the junctional zone is regulated by Gata6, but also reveal that dedifferentiation is a previously unrecognized property of post-mitotic, terminally differentiated cells that have lost contact with the basement membrane. This resolves the long-standing debate about the contribution of terminally differentiated cells to epidermal wound repair.
URLPMID:2413204 [本文引用: 1]

Pancreatobiliary cancers have among the highest mortality rates of any cancer type. Discovering the full spectrum of molecular genetic alterations may suggest new avenues for therapy. To catalogue genomic alterations, we carried out array-based genomic profiling of 31 exocrine pancreatic cancers and 6 distal bile duct cancers, expanded as xenografts to enrich the tumor cell fraction. We identified numerous focal DNA amplifications and deletions, including in 19% of pancreatobiliary cases gain at cytoband 18q11.2, a locus uncommonly amplified in other tumor types. The smallest shared amplification at 18q11.2 included GATA6, a transcriptional regulator previously linked to normal pancreas development. When amplified, GATA6 was overexpressed at both the mRNA and protein levels, and strong immunostaining was observed in 25 of 54 (46%) primary pancreatic cancers compared to 0 of 33 normal pancreas specimens surveyed. GATA6 expression in xenografts was associated with specific microarray gene-expression patterns, enriched for GATA binding sites and mitochondrial oxidative phosphorylation activity. siRNA mediated knockdown of GATA6 in pancreatic cancer cell lines with amplification led to reduced cell proliferation, cell cycle progression, and colony formation. Our findings indicate that GATA6 amplification and overexpression contribute to the oncogenic phenotypes of pancreatic cancer cells, and identify GATA6 as a candidate lineage-specific oncogene in pancreatobiliary cancer, with implications for novel treatment strategies.
[本文引用: 1]
[本文引用: 1]
URLPMID:4185421 [本文引用: 1]

Tissue-resident macrophages are heterogeneous as a consequence of anatomical niche-specific functions. Many populations self-renew independently of bone marrow in the adult, but the molecular mechanisms of this are poorly understood. We determined a transcriptional profile for the major self-renewing population of peritoneal macrophages in mice. These cells specifically expressed the transcription factor Gata6. Selective deficiency of Gata6 in myeloid cells caused substantial alterations in the transcriptome of peritoneal macrophages. Gata6 deficiency also resulted in dysregulated peritoneal macrophage proliferative renewal during homeostasis and in response to inflammation, which was associated with delays in the resolution of inflammation. Our investigations reveal that the tissue macrophage phenotype is under discrete tissue-selective transcriptional control and that this is fundamentally linked to the regulation of their proliferation renewal.
URLPMID:4867229 [本文引用: 1]

Abstract Liver disease is a major cause of illness and death worldwide. In China alone, liver diseases, primarily viral hepatitis (predominantly hepatitis B virus [HBV]), nonalcoholic fatty liver disease, and alcoholic liver disease, affect approximately 300 million people. The establishment of the Expanded Program on Immunization in 1992 has resulted in a substantial decline in the number of newly HBV-infected patients; however, the number of patients with alcoholic and nonalcoholic fatty liver diseases is rising at an alarming rate. Liver cancer, one of the most deadly cancers, is the second-most common cancer in China. Approximately 383,000 people die from liver cancer every year in China, which accounts for 51% of the deaths from liver cancer worldwide. Over the past 10 years, China has made some significant efforts to shed its "leader in liver diseases" title by investing large amounts of money in funding research, vaccines, and drug development for liver diseases and by recruiting many Western-trained hepatologists and scientists. Over the last two decades, hepatologists and scientists in China have made significant improvements in liver disease prevention, diagnosis, management, and therapy. They have been very active in liver disease research, as shown by the dramatic increase in the number of publications in Hepatology. Nevertheless, many challenges remain that must be tackled collaboratively. In this review, we discuss the epidemiology and characteristics of liver diseases and liver-related research in China. 脗漏 2014 by the American Association for the Study of Liver Diseases.
URLPMID:3093159 [本文引用: 1]

Abstract There is significant overlap in the genes and pathways that control liver development and those that regulate liver regeneration, hepatic progenitor cell expansion, response to injury, and cancer. Additionally, defects in liver development may underlie some congenital and perinatal liver diseases. Thus, studying hepatogenesis is important for understanding not only how the liver forms, but also how it functions. Elegant work in mice has uncovered a host of transcription factors and signaling molecules that govern the early steps of hepatic specification; however, the inherent difficulty of studying embryogenesis in utero has driven developmental biologists to seek new systems. The rapidly developing vertebrate zebrafish is a favorite model for embryology. The power of forward genetic screens combined with live real-time imaging of development in transparent zebrafish embryos has highlighted conserved processes essential for hepatogenesis and has uncovered some exciting new players. This review presents the advantages of zebrafish for studying liver development, underscoring how studies in zebrafish and mice complement each other. In addition to their value for studying development, zebrafish models of hepatic and biliary diseases are expanding, and using these small, inexpensive embryos for drug screening has become de rigueur . Zebrafish provide a shared platform for developmental biology and translational research, offering innovative methods for studying liver development and disease. The story of hepatogenesis has something for everyone. It involves transcriptional regulation, cell-cell interaction, signaling pathways, control of cell proliferation and apoptosis, plus morphogenic processes that sculpt vasculature, parenchymal cells, and mesenchyme to form the multifaceted liver. Decades of research on liver development in mice and other vertebrates offer valuable lessons in how the multipotent endoderm is programmed to form a functional liver. Of equal importance are insights that have illuminated the mechanisms by which hepatic progenitors are activated in a damaged liver, how the adult liver regenerates, and, possibly, the basis for engineering liver cells in vitro for cell transplantation to sustain patients with liver failure. Moreover, processes that are key to liver development are often co-opted during pathogenesis. Therefore, reviewing hepatogenesis is informative for both basic and translational researchers. In this review, we bring to light the many advantages offered by the tropical freshwater vertebrate zebrafish ( Danio rerio ) in studying hepatogenesis. By comparing zebrafish and mice, we highlight how work in each system complements the other and emphasize novel paradigms that have been uncovered using zebrafish. Finally, we highlight exciting efforts using zebrafish to model hepatobiliary diseases. (H EPATOLOGY 2009.)
URLPMID:26970006 [本文引用: 5]

The early specification, rapid growth and morphogenesis, and conserved functions of the embryonic liver across diverse model organisms have made the system an experimentally facile paradigm for understanding basic regulatory mechanisms that govern cell differentiation and organogenesis. This essay highlights concepts that have emerged from studies of the discrete steps of foregut endoderm development into the liver bud, as well as from modeling the steps via embryonic stem cell differentiation. Such concepts include understanding the chromatin basis for the competence of progenitor cells to develop into specific lineages; the importance of combinatorial signaling from different sources to induce cell fates; the impact of inductive signaling on preexisting chromatin states; the ability of separately specified domains of cells to merge into a common tissue; and the marked cell biological dynamics, including interactions with the developing vasculature, which establish the initial morphogenesis and patterning of a tissue. The principles gleaned from these studies, focusing on the 2 days it takes for the endoderm to develop into a liver bud, should be instructive for many other organogenic systems and for manipulating tissues in regenerative contexts for biomedical purposes.
[本文引用: 6]
URLPMID:16079152 [本文引用: 3]

We have developed a loss-of-function model for Gata4 in zebrafish, in order to examine broadly its requirement for organogenesis. We show that the function of Gata4 in zebrafish heart development is well conserved with that in mouse, and that, in addition, Gata4 is required for development of the intestine, liver, pancreas and swim bladder. Therefore, a single transcription factor regulates the formation of many organs. Gata6 is a closely related transcription factor with an overlapping expression pattern. We show that zebrafish depleted of Gata6 show defects in liver bud growth similar to mouse Gata6 mutants and zebrafish Gata4 morphants, and that zebrafish embryos depleted of both Gata4 and Gata6 display an earlier block in liver development, and thus completely lack liver buds. Therefore, Gata4 and Gata6 have distinct non-redundant functions in cardiac morphogenesis, but are redundant for an early step of liver development. In addition, both Gata4 and Gata6 are essential and non-redundant for liver growth following initial budding.
URLPMID:26109048 [本文引用: 1]

Abstract Transcription factor-mediated reprograming is a powerful method to study cell fate changes. In this study, we demonstrate that the transcription factor Gata6 can initiate reprograming of multiple cell types to induced extraembryonic endoderm stem (iXEN) cells. Intriguingly, Gata6 is sufficient to drive iXEN cells from mouse pluripotent cells and differentiated neural cells. Furthermore, GATA6 induction in human embryonic stem (hES) cells also down-regulates pluripotency gene expression and up-regulates extraembryonic endoderm (ExEn) genes, revealing a conserved function in mediating this cell fate switch. Profiling transcriptional changes following Gata6 induction in mES cells reveals step-wise pluripotency factor disengagement, with initial repression of Nanog and Esrrb, then Sox2, and finally Oct4, alongside step-wise activation of ExEn genes. Chromatin immunoprecipitation and subsequent high-throughput sequencing analysis shows Gata6 enrichment near pluripotency and endoderm genes, suggesting that Gata6 functions as both a direct repressor and activator. Together, this demonstrates that Gata6 is a versatile and potent reprograming factor that can act alone to drive a cell fate switch from diverse cell types. 漏 2015 Wamaitha et al.; Published by Cold Spring Harbor Laboratory Press.
URLPMID:16162334 [本文引用: 2]

Abstract The formation of the primitive endoderm covering the inner cell mass of early mouse embryos can be simulated in vitro by the differentiation of mouse embryonic stem (ES) cells in culture following either aggregation of suspended cells or stimulation of cell monolayers with retinoic acid. The developmentally regulated transcription factors GATA-4 and GATA-6 have determining role in mouse extraembryonic endoderm development. We analyzed the in vitro differentiation of mouse embryonic stem cells deficient of GATA factors and conclude that GATA-4 is required for ES cells to perceive a cell positioning (cell aggregation) signal and GATA-6 is required to sense morphogenic (retinoic acid) signal. The collaboration between GATA-6 and GATA-4, or GATA-6 and GATA-5 which can substitute for GATA-4, is involved in the perception of differentiation cues by embryonic stem cells in their determination of endoderm lineage. This study indicates that the lineage differentiation of ES cells can be manipulated by the expression of GATA factors.
URLPMID:25209243 [本文引用: 1]

During blastocyst formation, inner cell mass (ICM) cells differentiate into either epiblast (Epi) or primitive endoderm (PrE) cells, labeled by Nanog and Gata6, respectively, and organized in a salt-and-pepper pattern. Previous work in the mouse has shown that, in absence of Nanog, all ICM cells adopt a PrE identity. Moreover, the activation or the blockade of the Fgf/RTK pathway biases cell fate specification towards either PrE or Epi, respectively. We show that, in absence of Gata6, all ICM cells adopt an Epi identity. Furthermore, the analysis of Gata6(+/-) embryos reveals a dose-sensitive phenotype, with fewer PrE-specified cells. These results and previous findings have enabled the development of a mathematical model for the dynamics of the regulatory network that controls ICM differentiation into Epi or PrE cells. The model describes the temporal dynamics of Erk signaling and of the concentrations of Nanog, Gata6, secreted Fgf4 and Fgf receptor 2. The model is able to recapitulate most of the cell behaviors observed in different experimental conditions and provides a unifying mechanism for the dynamics of these developmental transitions. The mechanism relies on the co-existence between three stable steady states (tristability), which correspond to ICM, Epi and PrE cells, respectively. Altogether, modeling and experimental results uncover novel features of ICM cell fate specification such as the role of the initial induction of a subset of cells into Epi in the initiation of the salt-and-pepper pattern, or the precocious Epi specification in Gata6(+/-) embryos.
URLPMID:24835466 [本文引用: 1]

The transcription factor GATA6 is essential for primitive endoderm (PrE) formation. Schrode et聽al. show that GATA6 governs PrE cell fate choice by mediating the response to FGF signaling and that GATA6 levels regulate the rate of lineage commitment and the proportion of PrE and epiblast within the inner cell mass.
URLPMID:23349011 [本文引用: 1]

Summary Top of page Summary LINEAGE SPECIFICATION IN THE PREIMPLANTATION EMBRYO FIRST FATE DECISION: TE VERSUS ICM CHOICE THE SECOND FATE DECISION: CHOICE BETWEEN EPIBLAST AND PRIMITIVE ENDODERM CONCLUDING REMARKS: DEVELOPMENTAL BIASES AND PLASTICITIES DURING BLASTOCYST FORMATION ACKNOWLEDGMENTS REFERENCES The preimplantation period of mouse early embryonic development is devoted to the specification of two extraembryonic tissues and their spatial segregation from the pluripotent epiblast. During this period two cell fate decisions are made while cells gradually lose their totipotency. The first fate decision involves the segregation of the extraembryonic trophectoderm (TE) lineage from the inner cell mass (ICM); the second occurs within the ICM and involves the segregation of the extraembryonic primitive endoderm (PrE) lineage from the pluripotent epiblast (EPI) lineage, which eventually gives rise to the embryo proper. Multiple determinants, such as differential cellular properties, signaling cues and the activity of transcriptional regulators, influence lineage choice in the early embryo. Here, we provide an overview of our current understanding of the mechanisms governing these cell fate decisions ensuring proper lineage allocation and segregation, while at the same time providing the embryo with an inherent flexibility to adjust when perturbed. genesis 51:219鈥233. 漏 2013 Wiley Periodicals, Inc.
URLPMID:26932670 [本文引用: 1]

Abstract GATA4 and GATA6 are zinc finger transcription factors that have important functions in several mesodermal and endodermal organs, including heart, liver and pancreas. In humans, heterozygous mutations of either factor are associated with pancreatic agenesis; however, homozygous deletion of both Gata4 and Gata6 is necessary to disrupt pancreas development in mice. In this study, we demonstrate that arrested pancreatic development in Gata4fl/fl; Gata6fl/fl; Pdx1:Cre (pDKO) embryos is accompanied by the transition of ventral and dorsal pancreatic fates into intestinal or stomach lineages, respectively. These results indicate that GATA4 and GATA6 play essential roles in maintaining pancreas identity by regulating foregut endodermal fates. Remarkably, pancreatic anlagen derived from pDKO embryos also display a dramatic upregulation of hedgehog pathway components, which are normally absent from the presumptive pancreatic endoderm. Consistent with the erroneous activation of hedgehog signaling, we demonstrate that GATA4 and GATA6 are able to repress transcription through the sonic hedgehog (Shh) endoderm-specific enhancer MACS1 and that GATA-binding sites within this enhancer are necessary for this repressive activity. These studies establish the importance of GATA4/6-mediated inhibition of hedgehog signaling as a major mechanism regulating pancreatic endoderm specification during patterning of the gut tube.
URLPMID:16651540 [本文引用: 1]

A conserved molecular pathway has emerged controlling endoderm formation in Xenopus zebrafish and mice. Key genes in this pathway include Nodal ligands and transcription factors of the Mix-like paired homeodomain class, Gata4-6 zinc-finger factors and Sox17 HMG domain proteins. Although a linear epistatic pathway has been proposed, the precise hierarchical relationships between these factors and their downstream targets are largely unresolved. Here, we have used a combination of microarray analysis and loss-of-function experiments to examine the global regulatory network controlling Xenopus endoderm formation. We identified over 300 transcripts enriched in the gastrula endoderm, including most of the known endoderm regulators and over a hundred uncharacterized genes. Surprisingly only 10% of the endoderm transcriptome is regulated as predicted by the current linear model. We find that Nodal genes, Mixer and Sox17 have both shared and distinct sets of downstream targets, and that a number of unexpected autoregulatory loops exist between Sox17 and Gata4-6, between Sox17 and Bix1/Bix2/Bix4, and between Sox17 and Xnr4. Furthermore, we find that Mixer does not function primarily via Sox17 as previously proposed. These data provides new insight into the complexity of endoderm formation and will serve as valuable resource for establishing a complete endoderm gene regulatory network.
URL [本文引用: 2]
[本文引用: 1]
URLPMID:28196690 [本文引用: 1]

Abstract Induced pluripotent stem cells were created from a pancreas agenesis patient with a mutation in GATA6. Using genome-editing technology, additional stem cell lines with mutations in both GATA6 alleles were generated and demonstrated a severe block in definitive endoderm induction, which could be rescued by re-expression of several different GATA family members. Using the endodermal progenitor stem cell culture system to bypass the developmental block at the endoderm stage, cell lines with mutations in one or both GATA6 alleles could be differentiated into β-like cells but with reduced efficiency. Use of suboptimal doses of retinoic acid during pancreas specification revealed a more severe phenotype, more closely mimicking the patient's disease. GATA6 mutant β-like cells fail to secrete insulin upon glucose stimulation and demonstrate defective insulin processing. These data show that GATA6 plays a critical role in endoderm and pancreas specification and β-like cell functionality in humans. Copyright 08 2017 The Authors. Published by Elsevier Inc. All rights reserved.
URLPMID:12490293 [本文引用: 1]

Abstract Over the last decade significant advances have been made in our understanding of the molecular mechanisms that control early aspects of mammalian liver development. Studies using tissue explant cultures and molecular biology techniques as well as the analysis of transgenic and knockout mice have identified signaling molecules and transcription factors that are necessary for the onset of hepatogenesis. This review presents an overview of these studies and discusses the role of individual factors during hepatic development.
URLPMID:14647040 [本文引用: 2]

Hepatology. 2003 Dec;38(6):1331-47. Research Support, U.S. Gov't, P.H.S.; Review
URLPMID:19056973 [本文引用: 1]

Abstract Liver and pancreas progenitors develop from endoderm cells in the embryonic foregut. Shortly after their specification, liver and pancreas progenitors rapidly acquire markedly different cellular functions and regenerative capacities. These changes are elicited by inductive signals and genetic regulatory factors that are highly conserved among vertebrates. Interest in the development and regeneration of the organs has been fueled by the intense need for hepatocytes and pancreatic beta cells in the therapeutic treatment of liver failure and type I diabetes. Studies in diverse model organisms have revealed evolutionarily conserved inductive signals and transcription factor networks that elicit the differentiation of liver and pancreatic cells and provide guidance for how to promote hepatocyte and beta cell differentiation from diverse stem and progenitor cell types.
URLPMID:27555287 [本文引用: 3]

The liver is the second largest organ in the human body and is responsible for several functions that directly contribute to homeostasis. Hepatocytes are the main parenchymal liver cells that regulate multiple biochemical and metabolic functions and the synthesis of substances important to the body. Mesenchymal stem cells (MSCs) are a group of stem cells derived from the mesoderm, which can be obtained from various tissues. Under certain conditions, MSCs can differentiate into several cell types, including hepatocytes. Post-transcriptional regulations of liver development signalling and hepatocyte differentiation have been demonstrated. At the post-transcriptional level, microRNAs have emerged as precursors for determining cell fate during differentiation. MicroRNAs (miRNAs) are small non-coding RNAs involved in the post-transcriptional regulation of gene expression. They can determine the stem cell fate by repressing the translation of target mRNAs. In this review, we outline signalling pathways involved in stem cell differentiation to hepatocytes and its interplay with liver development. Hepatic differentiation models in two-dimensional and three-dimensional cultures used to analyse signalling mechanisms will be described. We also highlight the possible miRNAs involved in this process and the transdifferentiation signalling mechanisms present in hepatocytes.
URLPMID:11485993 [本文引用: 1]

Mesodermal signaling is critical for patterning the embryonic endoderm into different tissue domains. Classical tissue transplant experiments in the chick and recent studies in the mouse indicated that interactions with the cardiogenic mesoderm are necessary and sufficient to induce the liver in the ventral foregut endoderm. Using molecular markers and functional assays, we now show that septum transversum mesenchyme cells, a distinct mesoderm cell type, are closely apposed to the ventral endoderm and contribute to hepatic induction. Specifically, using a mouse Bmp4 null mutation and an inhibitor of BMPs, we find that BMP signaling from the septum transversum mesenchyme is necessary to induce liver genes in the endoderm and to exclude a pancreatic fate. BMPs apparently function, in part, by affecting the levels of the GATA4 transcription factor, and work in parallel to FGF signaling from the cardiac mesoderm. BMP signaling also appears critical for morphogenetic growth of the hepatic endoderm into a liver bud. Thus, the endodermal domain for the liver is specified by simultaneous signaling from distinct mesodermal sources.
URLPMID:22855527 [本文引用: 1]

GATA-6 is a zinc-finger transcription factor essential for early embryogenesis. Ablation of GATA-6 in mice impairs endoderm differentiation and causes apoptosis of epiblast cells. The endoderm defects have been attributed to the loss of HNF4, disabled-2, and GATA-4. However, the mechanisms underlying epiblast apoptosis are unclear. In this study we used mouse embryonic stem cell-derived embryoid bodies (EBs) as a model for peri-implantation development and found that ablation of GATA-6 causes massive apoptosis during EB differentiation. Endoderm grafting experiments and ectopic basement membrane (BM) assembly suggest that both BM and non-BM factors contribute to cell survival. Furthermore, the increased cell death in mutant EBs is accompanied by reduced expression of bone morphogenetic protein 2 (BMP-2). Chromatin immunoprecipitation reveals direct binding of GATA-6 to the Bmp2 promoter. Treatment of the mutant EBs with BMP-2 markedly suppresses apoptosis, whereas stable overexpression of the BMP antagonist noggin or a dominant-negative BMP receptor in normal EBs leads to increased apoptosis. Last, activation of SMAD1/5 by phosphorylation is significantly inhibited in the absence of GATA-6, and this is reversed by exogenous BMP-2. Treatment of normal EBs with SMAD phosphorylation inhibitor increases apoptosis. Collectively these results suggest that GATA-6 promotes cell survival by regulating endoderm expression of BMP-2 and BM during embryonic epithelial morphogenesis.
URLPMID:12094228 [本文引用: 1]

Abstract Genetic analysis, embryonic tissue explantation and in vivo chromatin studies have together identified the distinct regulatory steps that are necessary for the development of endoderm into a bud of liver tissue and, subsequently, into an organ. In this review, I discuss the acquisition of competence to express liver-specific genes by the endoderm, the control of early hepatic growth, the coordination of hepatic and vascular development and the cell differentiation that is necessary to generate a functioning liver. The regulatory mechanisms that underlie these phases are common to the development of many organ systems and might be recapitulated or disrupted during stem-cell differentiation and adult tissue pathogenesis.
URLPMID:27312502 [本文引用: 2]

The endoderm is the innermost embryonic germ layer, and in zebrafish, it gives rise to the lining of the gut, the gills, liver, pancreas, gallbladder, and derivatives of the pharyngeal pouch. These organs form the gastrointestinal tract and are involved with the absorption, delivery, and metabolism of nutrients. The liver has a central role in regulating these processes because it controls carbohydrate and lipid metabolism, protein synthesis, and breakdown of endogenous and xenobiotic products. Liver dysfunction frequently leads to significant morbidity and mortality; however, in most settings of organ injury, the liver exhibits remarkable regenerative capacity. In this chapter, we review the principal mechanisms of endoderm and liver formation and provide protocols to assess liver formation and liver regeneration.
URLPMID:11267302 [本文引用: 2]

Rossi M, De Simone P, Peritore D, Iappelli M, Pretagostini R, Lonardo MT, Cancrini C, Novelli G, Nudo F, De Blasis V, Donadio R, Berloco P, Cortesini R.
URLPMID:17507405 [本文引用: 2]

Based on data from in vitro tissue explant and ex vivo cell/bead implantation experiments, Bmp and Fgf signaling have been proposed to regulate hepatic specification. However, genetic evidence for this hypothesis has been lacking. Here, we provide in vivo genetic evidence that Bmp and Fgf signaling are essential for hepatic specification. We utilized transgenic zebrafish that overexpress dominant-negative forms of Bmp or Fgf receptors following heat-shock induction. These transgenes allow one to bypass the early embryonic requirements for Bmp and Fgf signaling, and also to completely block Bmp or Fgf signaling. We found that the expression of hhex and prox1, the earliest liver markers in zebrafish, was severely reduced in the liver region when Bmp or Fgf signaling was blocked just before hepatic specification. However, hhex and prox1 expression in adjacent endodermal and mesodermal tissues appeared unaffected by these manipulations. Additional genetic studies indicate that the endoderm maintains competence for Bmp-mediated hepatogenesis over an extended window of embryonic development. Altogether, these data provide the first genetic evidence that Bmp and Fgf signaling are essential for hepatic specification, and suggest that endodermal cells remain competent to differentiate into hepatocytes for longer than anticipated.
[本文引用: 1]
URLPMID:12912923 [本文引用: 1]

Abstract Top of page Abstract Introduction Results Discussion Materials and methods Acknowledgements References Supporting Information GATA-6 is expressed in presumptive cardiac mesoderm before gastrulation, but its role in heart development has been unclear. Here we show that Xenopus and zebrafish embryos, injected with antisense morpholino oligonucleotides designed specifically to knock-down translation of GATA-6 protein, are severely compromised for heart development. Injected embryos express greatly reduced levels of contractile machinery genes and, at the same stage, of regulatory genes such as bone morphogenetic protein-4 (BMP-4) and the Nkx2 family. In contrast, initial BMP and Nkx2 expression is normal, suggesting a maintenance role for GATA-6. Endoderm is critical for heart formation in several vertebrates including Xenopus , and separate perturbation of GATA-6 expression in the deep anterior endoderm and in the overlying heart mesoderm shows that GATA-6 is required in both for cardiogenesis. The GATA-6 requirement in cardiac mesoderm was confirmed in zebrafish, an organism in which endoderm is thought not to be necessary for heart formation. We therefore conclude that proper maturation of cardiac mesoderm requires GATA-6, which functions to maintain BMP-4 and Nkx2 expression.
URLPMID:12606287 [本文引用: 1]

Transcription factors GATA-4, -5, and -6 constitute an evolutionary conserved subfamily of vertebrate zinc finger regulators highly expressed in the developing heart and gut. Genetic evidence suggests that each protein is essential for embryonic development, but their exact functions are not fully elucidated. Moreover, because all three proteins share similar transcriptional properties in vitro, and because transcripts for two or more GATA genes are present in similar tissues, the molecular basis underlying in vivo specificity of GATA factors remains undefined. Knowledge of the exact cell types expressing each protein and identification of downstream targets would greatly help define their function. We have used high-resolution immunohistochemistry to precisely determine the cellular distribution of the GATA-4, -5, and -6 proteins in murine embryogenesis. The results reveal novel sites of expression in mesodermal and ectodermal cells. In particular, GATA-4 and -6 expression was closely associated with yolk sac vasculogenesis and early endoderm鈥搈esoderm signaling. Additionally, GATA-6 was strongly expressed in the embryonic ectoderm, neural tube, and neural crest-derived cells. This pattern of expression closely paralled that of BMP-4, and the BMP-4 gene was identified as a direct downstream target for GATA-4 and -6. These findings offer new insight into the function of GATA-4 and -6 during early stages of embryogenesis and reveal the existence of a positive cross-regulatory loop between BMP-4 and GATA-4. They also raise the possibility that part of the early defects in GATA-4 and/or GATA-6 null embryos may be due to impaired BMP-4 signaling.
URLPMID:20564202 [本文引用: 1]

Stress-induced Sapk/Jnk signaling is involved in cell survival and apoptosis. Recent studies have increased our understanding of the physiological roles of Jnk signaling in embryonic development. However, still unclear is the precise function of Jnk signaling during gastrulation, a critical step in the establishment of the vertebrate body plan. Here we use morpholino-mediated knockdown of the zebrafish orthologs of the Jnk activators Mkk4 and Mkk7 to examine the effect of Jnk signaling abrogation on early vertebrate embryogenesis. Depletion of zebrafish Mkk4b led to abnormal convergent extension (CE) during gastrulation, whereas Mkk7 morphants exhibited defective somitogenesis. Surprisingly, Mkk4b morphants displayed marked upregulation of wnt11, which is the triggering ligand of CE and stimulates Jnk activation via the non-canonical Wnt pathway. Conversely, ectopic activation of Jnk signaling by overexpression of an active form of Mkk4b led to wnt11 downregulation. Mosaic lineage tracing studies revealed that Mkk4b-Jnk signaling suppressed wnt11 expression in a non-cell-autonomous manner. These findings provide the first evidence that wnt11 itself is a downstream target of the Jnk cascade in the non-canonical Wnt pathway. Our work demonstrates that Jnk activation is indispensable for multiple steps during vertebrate body plan formation. Furthermore, non-canonical Wnt signaling may coordinate vertebrate CE movements by triggering Jnk activation that represses the expression of the CE-triggering ligand wnt11.
[本文引用: 1]
URLPMID:18635606 [本文引用: 1]

Mouse liver induction occurs via the acquisition of ventral endoderm competence to respond to inductive signals from adjacent mesoderm, followed by hepatic specification. Little is known about the regulatory circuit involved in these processes. Through the analysis of vHnf1 (Hnf1b)-deficient embryos, generated by tetraploid embryo complementation, we demonstrate that lack of vHNF1 leads to defective hepatic bud formation and abnormal gut regionalization. Thickening of the ventral hepatic endoderm and expression of known hepatic genes do not occur. At earlier stages, hepatic specification of vHnf1-/- ventral endoderm is disrupted. More importantly, mutant ventral endoderm cultured in vitro loses its responsiveness to inductive FGF signals and fails to induce the hepatic-specification genes albumin and transthyretin. Analysis of liver induction in zebrafish indicates a conserved role of vHNF1 in vertebrates. Our results reveal the crucial role of vHNF1 at the earliest steps of liver induction: the acquisition of endoderm competence and the hepatic specification.
URLPMID:12808453 [本文引用: 2]

Abstract Although advances have been made in understanding cell differentiation, only rudimentary knowledge exists concerning how differentiated cells form tissues and organs. We studied liver organogenesis because the cell and tissue architecture of this organ is well defined. Approximately 60% of the adult liver consists of hepatocytes that are arranged as single-cell anastomosing plates extending from the portal region of the liver lobule toward the central vein. The basal surface of the hepatocytes is separated from adjacent sinusoidal endothelial cells by the space of Disse, where the exchange of substances between serum and hepatocytes takes place. The hepatocyte's apical surface forms bile canaliculi that transport bile to the hepatic ducts. Proper liver architecture is crucial for hepatic function and is commonly disrupted in disease states, including cirrhosis and hepatitis. Here we report that hepatocyte nuclear factor 4alpha (Hnf4alpha) is essential for morphological and functional differentiation of hepatocytes, accumulation of hepatic glycogen stores and generation of a hepatic epithelium. We show that Hnf4alpha is a dominant regulator of the epithelial phenotype because its ectopic expression in fibroblasts induces a mesenchymal-to-epithelial transition. Most importantly, the morphogenetic parameters controlled by Hnf4alpha in hepatocytes are essential for normal liver architecture, including the organization of the sinusoidal endothelium.
URLPMID:21852396 [本文引用: 1]

The availability of pluripotent stem cells offers the possibility of using such cells to model and . With this in mind, we previously established a protocol that facilitates the differentiation of both stem cells and induced pluripotent stem cells into cells that share many characteristics with hepatocytes. The use of highly defined culture conditions and the avoidance of feeder cells or embryoid bodies allowed synchronous and reproducible differentiation to occur. The differentiation towards a hepatocyte-like fate appeared to recapitulate many of the developmental stages normally associated with the formation of hepatocytes in vivo. In the current study, we addressed the feasibility of using pluripotent stem cells to probe the molecular mechanisms underlying hepatocyte differentiation. We demonstrate (1) that stem cells express a number of that characterize each stage in the differentiation process, (2) that gene expression can be efficiently depleted throughout the differentiation time course using shRNAs expressed from lentiviruses and (3) that the nuclear hormone receptor is essential for specification of hepatic progenitor cells by establishing the expression of the network of factors that controls the onset of hepatocyte cell fate.
URLPMID:3961246 [本文引用: 1]

Human embryonic stem cells (hESCs) could provide a major window into human developmental biology, because the differentiation methods from hESCs mimic human embryogenesis. We previously reported that the overexpression of hematopoietically expressed homeobox (HHEX) in the hESC-derived definitive endoderm (DE) cells markedly promotes hepatic specification. However, it remains unclear how HHEX functions in this process. To reveal the molecular mechanisms of hepatic specification by HHEX, we tried to identify the genes directly targeted by HHEX. We found that HHEX knockdown considerably enhanced the expression level of eomesodermin (EOMES). In addition, HHEX bound to the HHEX response element located in the first intron of EOMES. Loss-of-function assays of EOMES showed that the gene expression levels of hepatoblast markers were significantly upregulated, suggesting that EOMES has a negative role in hepatic specification from the DE cells. Furthermore, EOMES exerts its effects downstream of HHEX in hepatic specification from the DE cells. In conclusion, the present results suggest that HHEX promotes hepatic specification by repressing EOMES expression.
URL [本文引用: 1]

Transcription factors are adaptor molecules that detect regulatory sequences in the DNA and target the assembly of protein complexes that control gene expression. Yet much of the DNA in the eukaryotic cell is in nucleosomes and thereby occluded by histones, and can be further occluded by higher-order chromatin structures and repressor complexes. Indeed, genome-wide location analyses have revealed that, for all transcription factors tested, the vast majority of potential DNA-binding sites are unoccupied, demonstrating the inaccessibility of most of the nuclear DNA. This raises the question of how target sites at silent genes become bound de novo by transcription factors, thereby initiating regulatory events in chromatin. Binding cooperativity can be sufficient for many kinds of factors to simultaneously engage a target site in chromatin and activate gene expression. However, in cases in which the binding of a series of factors is sequential in time and thus not initially cooperative, special "pioneer transcription factors" can be the first to engage target sites in chromatin. Such initial binding can passively enhance transcription by reducing the number of additional factors that are needed to bind the DNA, culminating in activation. In addition, pioneer factor binding can actively open up the local chromatin and directly make it competent for other factors to bind. Passive and active roles for the pioneer factor FoxA occur in embryonic development, steroid hormone induction, and human cancers. Herein we review the field and describe how pioneer factors may enable cellular reprogramming.
URLPMID:22555599 [本文引用: 1]

Understanding the basis for multipotency, whereby stem cells and other progenitors can differentiate into certain tissues and not others, provides insights into the mechanism of cell programming in development, homeostasis, and disease. We recently reported a screen of diverse chromatin marks to obtain clues about chromatin states in the multipotent embryonic endoderm. Genetic and pharmacologic tests of certain marks090005 function demonstrated that the relevant chromatin modifying factors modulate the fate choice for liver or pancreas induction in the endoderm. The information about chromatin states from embryonic studies can be used to predict lineage-specific developmental potential and chromatin modifiers to enhance particular cell fate transitions from stem cells.
URLPMID:20614624 [本文引用: 4]

The liver is the largest internal organ and it provides many essential metabolic, exocrine and endocrine functions. Hepatocytes are the principal cell type in the liver and these along with biliary epithelial cells are derived from the embryonic endoderm. Embryological experiments in animal models have demonstrated that liver development occurs through a progressive series of reciprocal tissue interactions between the embryonic endoderm and nearby mesoderm. In the last ten years many of the genes and molecular pathways that regulate hepatogenesis have been identified. Recently application of this knowledge has enabled researchers to produce “hepatic-like” tissue from embryonic stem (ES) cells in vitro, which may ultimately lead to therapeutically useful tissue for transplantation. This review summarizes the current understanding of the molecular pathways controlling liver and biliary system development focusing on studies in the mouse embryo where this process is best understood.
URLPMID:11397003 [本文引用: 1]

Members of both the bone morphogenetic protein (Bmp) and EGF-CFC families have been implicated in vertebrate myocardial development. Zebrafish swirl ( swr ) encodes Bmp2b, a member of the Bmp family required for patterning the dorsoventral axis. Zebrafish one-eyed pinhead ( oep ) encodes a maternally and zygotically expressed member of the EGF-CFC family essential for Nodal signaling. Both swr/bmp2b and oep mutants exhibit severe defects in myocardial development. swr/bmp2b mutants exhibit reduced or absent expression of nkx2.5 , an early marker of the myocardial precursors. Embryos lacking zygotic oep ( Zoep mutants) display cardia bifida and, as we show here, also display reduced or absent nkx2.5 expression. Recently, we have demonstrated that the zinc finger transcription factor Gata5 is an essential regulator of nkx2.5 expression. In this paper, we investigate the relationships between bmp2b , oep , gata5 , and nkx2.5 . We show that both swr/bmp2b and Zoep mutants exhibit defects in gata5 expression in the myocardial precursors. Forced expression of gata5 in swr/bmp2b and Zoep mutants restores robust nkx2.5 expression. Moreover, overexpression of gata5 in Zoep mutants restores expression of cmlc1 , a myocardial sarcomeric gene. These results indicate that both Bmp2b and Oep regulate gata5 expression in the myocardial precursors, and that Gata5 does not require Bmp2b or Oep to promote early myocardial differentiation. We conclude that Bmp2b and Oep function at least partly through Gata5 to regulate nkx2.5 expression and promote myocardial differentiation. We integrate these and other data to propose a pathway of the molecular events regulating early myocardial differentiation in zebrafish.
URLPMID:24367609 [本文引用: 2]

Abstract GATA transcription factors and their Friend of Gata (FOG) cofactors control the development of diverse tissues. GATA4 and GATA6 are essential for the expansion of the embryonic liver bud, but their expression patterns and functions in the adult liver are unclear. We characterized the expression of GATA and FOG factors in whole mouse liver and purified hepatocytes. GATA4, GATA6, and FOG1 are the most prominently expressed family members in whole liver and hepatocytes. GATA4 chromatin immunoprecipitation followed by high throughput sequencing (ChIP-seq) identified 4409 occupied sites, associated with genes enriched in ontologies related to liver function, including lipid and glucose metabolism. However, hepatocyte-specific excision of Gata4 had little impact on gross liver architecture and function, even under conditions of regenerative stress, and, despite the large number of GATA4 occupied genes, resulted in relatively few changes in gene expression. To address possible redundancy between GATA4 and GATA6, both factors were conditionally excised. Surprisingly, combined Gata4,6 loss did not exacerbate the phenotype resulting from Gata4 loss alone. This points to the presence of an unusually robust transcriptional network in adult hepatocytes that ensures the maintenance of liver function.
URLPMID:16364283 [本文引用: 1]

Little is known about the mechanism by which embryonic liver, lung, and pancreas progenitor cells emerge from the endodermal epithelium to initiate organogenesis. Understanding this process and its genetic control provides insight into ontogeny, developmental abnormalities, and tissue regeneration. We find that shortly after hepatic endoderm cells are specified, they undergo a transition from a columnar, gut morphology to a pseudostratified morphology, with concomitant "interkinetic nuclear migration" (INM) during cell division. INM is a hallmark of pseudostratified epithelia and the process used by neural progenitors to emerge from the neural epithelium. We find that the transition of the hepatic endoderm, but not the neural epithelium, to a pseudostratified epithelium is dependent upon the cell-autonomous activity of the homeobox gene Hex. In the absence of Hex, hepatic endoderm cells survive but maintain a columnar, simple epithelial phenotype and ectopically express Shh and other genes characteristic of the midgut epithelium. Thus, Hex promotes endoderm organogenesis by promoting the transition to a pseudostratified epithelium, which in turn allows hepatoblasts to emerge into the stromal environment and continue differentiating.
URLPMID:17828556 [本文引用: 1]

The homeobox transcription factor Prox1 is expressed in embryonic hepatoblasts and remains expressed in adult hepatocytes. Prox1-null mice show severe deficiencies in liver development, although the underlying mechanisms are unknown. We have studied the effects of Prox1 on the transcriptional profile of met-murine hepatocytes (MMH) obtained on embryonic day 14 (ED14). These immortalized murine hepatoblasts express numerous hepatoblast markers, but not Prox1. We have performed stable transfection with Prox1 cDNA, analyzed the transcriptome with Agilent mouse whole-genome microarrays, and validated genes by quantitative reverse transcription/polymerase chain reaction. We have observed the up-regulation of 22 genes and the down-regulation of 232 genes, by more than 12-fold. Many of these genes are involved in metabolic hepatocyte functions and may be regulated by Prox1 directly or indirectly, e.g., by the down-regulation of hepatocyte nuclear factor 4伪. Prox1 induces the down-regulation of transcription factors that are highly expressed in neighboring endodermal organs, suggesting a function during hepatoblast commitment. Prox1 does not influence the proliferative activity of MMH but regulates genes involved in liver morphogenesis. We have observed the up-regulation of both type-IV伪3 procollagen and functionally active matrix metalloproteinase-2 (MMP-2), an observation that places Prox1 at the center of liver matrix turnover. This is consistent with MMP-2 expression in hepatoblasts during liver development and with the persistence of a basal lamina around the liver bud in Prox1-deficient mice. Our studies suggest that Prox1 is a multifunctional regulator of liver morphogenesis and of hepatocyte function and commitment.
.
URLPMID:20430021 [本文引用: 1]

Abstract Kr眉ppel-like factor 6 (Klf6; copeb in zebrafish) is a zinc-finger transcription factor and tumor suppressor gene. Klf6(-)(/)(-) mice have defects in hematopoiesis and angiogenesis and do not form a liver. However, the vascular abnormalities in Klf6(-/-) mice obfuscate its role in liver development since these two processes are linked in mammals. We utilized zebrafish and mouse ES cells to investigate the role of copeb in endoderm specification and hepatogenesis separate from its function in angiogenesis. During zebrafish development, copeb expression is enriched in digestive organs. Morpholino knockdown of copeb blocks expansion of the liver, pancreas and intestine, but does not affect their specification, differentiation or the vascularization of the liver. Decreased hepatocyte proliferation in copeb morphants is accompanied by upregulation of the cell cycle inhibitor, cdkn1a, a Copeb transcriptional target. A cell autonomous role for Klf6 in endoderm and hepatic development was investigated by manipulating Klf6 expression in mouse ES cells driven to differentiate along the hepatic lineage. Expression of the endoderm markers Hnf3beta, Gata4, Sox17, and CxCr4 is not induced in Klf6(-/-) cells but is upregulated in ES cells over-expressing Klf6. Collectively, these findings indicate that copeb/Klf6 is essential for the development of endoderm-derived organs. Copyright (c) 2010 Elsevier Inc. All rights reserved.
URLPMID:19140222 [本文引用: 1]

Abstract Top of page Abstract Materials and Methods Results Discussion Acknowledgements References Supporting Information After specification of the hepatic endoderm, mammalian liver organogenesis progresses through a series of morphological stages that culminate in the migration of hepatocytes into the underlying mesenchyme to populate the hepatic lobes. Here, we show that in the mouse the transcriptional repressor Tbx3, a member of the T-box protein family, is required for the transition from a hepatic diverticulum with a pseudo-stratified epithelium to a cell-emergent liver bud. In Tbx3 -deficient embryos, proliferation in the hepatic epithelium is severely reduced, hepatoblasts fail to delaminate, and cholangiocyte rather than hepatocyte differentiation occurs. Molecular analyses suggest that the primary function of Tbx3 is to maintain expression of hepatocyte transcription factors, including hepatic nuclear factor 4a (Hnf4a) and CCAAT/enhancer binding protein (C/EBP), alpha (Cebpa), and to repress expression of cholangiocyte transcription factors such as Onecut1 (Hnf6) and Hnf1b. Conclusion : Tbx3 controls liver bud expansion by suppressing cholangiocyte and favoring hepatocyte differentiation in the liver bud. (H EPATOLOGY 2009.)
URLPMID:23799566 [本文引用: 1]

Abstract The liver is derived from the ventral foregut endoderm. After hepatic specification, liver progenitor cells delaminate from the endoderm and invade the septum transversum mesenchyme to form the liver bud. In addition to proliferation and expansion, liver progenitor cells differentiate into two epithelial cell types, each arranged into unique structures with distinctive function. Growth, morphogenesis, and differentiation during liver development are regulated by a variety of factors that are expressed in a spatially and temporally specific manner. A comprehensive understanding of the regulatory mechanisms underlying the liver development has influenced the diagnosis of liver diseases and further progress will be critical for future advances in therapy. This review highlights some of the best understood steps of liver development, summarizing progress in our understanding of the molecular mechanisms that underlie differentiation, morphogenesis, and functional integration of the liver. Copyright 漏 2012 Wiley Periodicals, Inc.
[本文引用: 1]
URLMagsci [本文引用: 1]
URLMagsci [本文引用: 1]
URLPMID:26732624 [本文引用: 3]

Human induced pluripotent stem cells (hiPSCs) have potential for personalized and regenerative medicine. While most of the methods using these cells have focused on deriving homogenous populations of specialized cells, there has been modest success in producing hiPSC-derived organotypic tissues or organoids. Here we present a novel approach for generating and then co-differentiating hiPSC-derived progenitors. With a genetically engineered pulse of GATA-binding protein 6 (GATA6) expression, we initiate rapid emergence of all three germ layers as a complex function of GATA6 expression levels and tissue context. Within 2 weeks we obtain a complex tissue that recapitulates early developmental processes and exhibits a liver bud-like phenotype, including haematopoietic and stromal cells as well as a neuronal niche. Collectively, our approach demonstrates derivation of complex tissues from hiPSCs using a single autologous hiPSCs as source and generates a range of stromal cells that co-develop with parenchymal cells to form tissues. There has been limited success in generating tissues from human induced pluripotent stem cells (hiPSCs). Here, the authors genetically engineer expression of the transcription factor Gata6 in a single isogenic hiPSC population resulting in complex tissue structures that exhibit liver bud-like properties.
URLPMID:4409934 [本文引用: 1]

Pioneer transcription factors (TFs) access silent chromatin and initiate cell-fate changes, using diverse types of DNA binding domains (DBDs). FoxA, the paradigm pioneer TF, has a winged helix DBD that resembles linker histone and thereby binds its target sites on nucleosomes and in compacted chromatin. Herein, we compare the nucleosome and chromatin targeting activities of Oct4 (POU DBD), Sox2 (HMG box DBD), Klf4 (zinc finger DBD), and c-Myc (bHLH DBD), which together reprogram somatic cells to pluripotency. Purified Oct4, Sox2, and Klf4 proteins can bind nucleosomes invitro, and invivo they preferentially target silent sites enriched for nucleosomes. Pioneer activity relates simply to the ability of a given DBD to target partial motifs displayed on the nucleosome surface. Such partial motif recognition can occur by coordinate binding between factors. Our findings provide insight into how pioneer factors can target naive chromatin sites.
URLPMID:26826681 [本文引用: 1]

Among the diverse transcription factors that are necessary to elicit changes in cell fate, both in embryonic development and in cellular reprogramming, a subset of factors are capable of binding to their target sequences on nucleosomal DNA and initiating regulatory events in silent chromatin. Such ‘pioneer transcription factors’ initiate cooperative interactions with other regulatory proteins to elicit changes in local chromatin structure. As a consequence of pioneer factor binding, the local chromatin can either become open and competent for activation, closed and repressed, or transcriptionally active. Understanding how pioneer factors initiate chromatin dynamics and how such can be blocked at heterochromatic sites provides insights into controlling cell fate transitions at will.
URLPMID:24582926 [本文引用: 1]

Obtaining fully functional cell types is a major challenge for drug discovery and regenerative medicine. Currently, a fundamental solution to this key problem is still lacking. Here, we show that functional human induced hepatocytes (hiHeps) can be generated from fibroblasts by overexpressing the hepatic fate conversion factors HNF1A , HNF4A , and HNF6 along with the maturation factors ATF5 , PROX1 , and CEBPA . hiHeps express a spectrum of phase I and II drug-metabolizing enzymes and phase III drug transporters. Importantly, the metabolic activities of CYP3A4, CYP1A2, CYP2B6, CYP2C9, and CYP2C19 are comparable between hiHeps and freshly isolated primary human hepatocytes. Transplanted hiHeps repopulate up to 30% of the livers of Tet-uPA/Rag2 61/61 /γc 61/61 mice and secrete more than 300μg/ml human ALBUMIN invivo. Our data demonstrate that human hepatocytes with drug metabolic function can be generated by lineage reprogramming, thus providing a cell resource for pharmaceutical applications.
URLPMID:24582927 [本文引用: 1]

Abstract The generation of large numbers of functional human hepatocytes for cell-based approaches to liver disease is an important and unmet goal. Direct reprogramming of fibroblasts to hepatic lineages could offer a solution to this problem but so far has only been achieved with mouse cells. Here, we generated human induced hepatocytes (hiHeps) from fibroblasts by lentiviral expression of FOXA3, HNF1A, and HNF4A. hiHeps express hepatic gene programs, can be expanded in聽vitro, and display functions characteristic of mature hepatocytes, including cytochrome P450 enzyme activity and biliary drug clearance. Upon transplantation into mice with concanavalin-A-induced acute liver failure and fatal metabolic liver disease due to fumarylacetoacetate dehydrolase (Fah) deficiency, hiHeps restore the liver function and prolong survival. Collectively, our results demonstrate successful lineage conversion of nonhepatic human cells into mature hepatocytes with potential for biomedical and pharmaceutical applications. Copyright 漏 2014 Elsevier Inc. All rights reserved.
URLPMID:21562492 [本文引用: 1]

The generation of functional hepatocytes independent of donor liver organs is of great therapeutic interest with regard to regenerative medicine and possible cures for liver disease. Induced hepatic differentiation has been achieved previously using embryonic stem cells or induced pluripotent stem cells. Particularly, hepatocytes generated from a patient's own induced pluripotent stem cells could theoretically avoid immunological rejection. However, the induction of hepatocytes from induced pluripotent stem cells is a complicated process that would probably be replaced with the arrival of improved technology. Overexpression of lineage-specific transcription factors directly converts terminally differentiated cells into some other lineages, including neurons, cardiomyocytes and blood progenitors; however, it remains unclear whether these lineage-converted cells could repair damaged tissues in vivo. Here we demonstrate the direct induction of functional hepatocyte-like (iHep) cells from mouse tail-tip fibroblasts by transduction of Gata4, Hnf1伪 and Foxa3, and inactivation of p19(Arf). iHep cells show typical epithelial morphology, express hepatic genes and acquire hepatocyte functions. Notably, transplanted iHep cells repopulate the livers of fumarylacetoacetate-hydrolase-deficient (Fah(-/-)) mice and rescue almost half of recipients from death by restoring liver functions. Our study provides a novel strategy to generate functional hepatocyte-like cells for the purpose of liver engineering and regenerative medicine.
URLPMID:20801124 [本文引用: 1]

姝ACKGROUND AIMS:Hepatocyte-like cells can be derived from pluripotent stem cells such as embryonic stem(ES) cells,but ES cell-derived hepatic cells with extensive capacity to repopulate liver have not been identified. We aimed to identify and purify ES cell-derived hepatoblast-like progenitor cells and to explore their capacity for liver repopulation in mice after in vitro expansion.METHODS:Unmanipulated mouse ES cells were cultured under defined conditions and allowed to undergo stepwise hepatic differentiation.The derived hepatic cells were examined by morphologic,fluorescence-activated cell sorting,gene expression,and clonal expansion analyses.The capacities of ES cell-derived hepatic progenitor cells to repopulate liver were investigated in mice that were deficient in fumarylacetoacetate hydrolase(Fah)(a model of liver injury).RESULTS:Mouse ES cells were induced to differentiate into a population that contained hepatic progenitor cells;this population included cells that expressed epithelial cell adhesion molecule(EpCAM) but did not express c-Kit.Clonal hepatic progenitors that arose from single c-Kit~-EpCAM~+ cells could undergo long-term expansion and maintain hepatoblast-like characteristics.Enriched c-KitEpCAM~+ cells and clonally expanded hepatic progenitor cells repopulated the livers of Fah-deficient mice without inducing tumorigenesis.CONCLUSIONS:ES cell-derived c-Kit~-EpCAM~+ cells contain a population of hepatoblast-like progenitor cells that can repopulate livers of mice.
URLPMID:24457331 [本文引用: 1]

Generation of functional and vascularized organs from human induced pluripotent stem cells (iPSCs) will facilitate our understanding of human developmental biology and disease modeling, hopefully offering a drug-screening platform and providing novel therapies against end-stage organ failure. Here we describe a protocol for the in vitro generation of a 3D liver bud from human iPSC cultures and the monitoring of further hepatic maturation after transplantation at various ectopic sites. iPSC-derived specified hepatic cells are dissociated and suspended with endothelial cells and mesenchymal stem cells. These mixed cells are then plated onto a presolidified matrix, and they form a 3D spherical tissue mass termed a liver bud (iPSC-LB) in 1-2 d. To facilitate additional maturation, 4-d-old iPSC-LBs are transplanted in the immunodeficient mouse. Live imaging has identified functional blood perfusion into the preformed human vascular networks. Functional analyses show the appearance of multiple hepatic functions in a chronological manner in vivo.
URLPMID:27997988 [本文引用: 1]

In contrast to all other organs, liver-to-body-weight ratio needs to be maintained always at 100% of what is required for body homeostasis. Adjustment of liver size to 100% of what is required for homeostasis has been called "hepatostat." Removal of a portion of any other organ is followed with local regeneration of a limited degree, but it never attempts to reach 100% of the original size. The complex mechanisms involved in this uniquely hepatic process encompass a variety of regenerative pathways that are specific to different types of injury. The most studied form of liver regeneration (LR) is that occurring after loss of hepatocytes in a single acute injury, such as rodent LR after two-thirds partial hepatectomy or administration of damaging chemicals (CCl4 , acetaminophen, etc.). Alternative regenerative pathways become activated when normal regeneration is thwarted and trigger the appearance of "progenitor" cells. Chronic loss of hepatocytes is associated with regenerative efforts characterized by continual hepatocyte proliferation and often has adverse consequences (development of cirrhosis or liver cancer). Even though a very few hepatocytes proliferate at any given time in normal liver, the mechanisms involved in the maintenance of liver weight by this slow process in the absence of liver injury are not as well understood. (Hepatology 2016).
.
URLPMID:25031271 [本文引用: 1]

Despite the tremendous hurdles presented by the complexity of the liver's structure and function, advances in liver physiology, stem cell biology and reprogramming, and the engineering of tissues and devices are accelerating the development of cell-based therapies for treating liver disease and liver failure. This State of the Art Review discusses both the near- and long-term prospects for such cell-based therapies and the unique challenges for clinical translation.
URLPMID:27908934 [本文引用: 1]

Abstract Cell proliferation is essential to rapid tissue growth and repair, but can result in replication-associated genome damage. Here, we implicate the transcription factor Gata6 in adult mouse hair follicle regeneration where it controls the renewal of rapidly proliferating epithelial (matrix) progenitors and hence the extent of production of terminally differentiated lineages. We find that Gata6 protects against DNA damage associated with proliferation, thus preventing cell cycle arrest and apoptosis. Furthermore, we show that in0002vivo Gata6 stimulates EDA-receptor signaling adaptor Edaradd level and NF-0202B pathway activation, known to be important for DNA damage repair and stress response in general and for hair follicle growth in particular. In cultured keratinocytes, Edaradd rescues DNA damage, cell survival, and proliferation of Gata6 knockout cells and restores MCM10 expression. Our data add to recent evidence in embryonic stem and neural progenitor cells, suggesting a model whereby developmentally regulated transcription factors protect from DNA damage associated with proliferation at key stages of rapid tissue growth. Our data may add to understanding why Gata6 is a frequent target of amplification in cancers. 0008 2016 The Authors.
URLPMID:2562713 [本文引用: 1]

Abstract Epithelial organs, including the lung, are known to possess regenerative abilities through activation of endogenous stem cell populations, but the molecular pathways regulating stem cell expansion and regeneration are not well understood. Here we show that Gata6 regulates the temporal appearance and number of bronchioalveolar stem cells (BASCs) in the lung, its absence in Gata6-null lung epithelium leading to the precocious appearance of BASCs and concurrent loss in epithelial differentiation. This expansion of BASCs was the result of a pronounced increase in canonical Wnt signaling in lung epithelium upon loss of Gata6. Expression of the noncanonical Wnt receptor Fzd2 was downregulated in Gata6 mutants and increased Fzd2 or decreased beta-catenin expression rescued, in part, the lung epithelial defects in Gata6 mutants. During lung epithelial regeneration, canonical Wnt signaling was activated in the niche containing BASCs and forced activation of Wnt signaling led to a large increase in BASC numbers. Moreover, Gata6 was required for proper lung epithelial regeneration, and postnatal loss of Gata6 led to increased BASC expansion and decreased differentiation. Together, these data demonstrate that Gata6-regulated Wnt signaling controls the balance between progenitor expansion and epithelial differentiation required for both lung development and regeneration.
