 ,1,*, 李玉卓1,2, 朱金龙1, 吴红艳1, 徐坤1, 翟红1
,1,*, 李玉卓1,2, 朱金龙1, 吴红艳1, 徐坤1, 翟红1A Rapid, Non-destructive and Continuous Sampling Technique and DNA Extraction for Soybean Seed
Zhengjun Xia ,1,*, Yuzhuo Li1,2, Jinlong Zhu1, Hongyan Wu1, Kun Xu1, Hong Zhai1
,1,*, Yuzhuo Li1,2, Jinlong Zhu1, Hongyan Wu1, Kun Xu1, Hong Zhai1通讯作者: E-mail:xiazhj@iga.ac.cn
责任编辑: 孙冬花
收稿日期:2020-05-26接受日期:2020-10-5网络出版日期:2021-01-01
| 基金资助: |
Corresponding authors: *E-mail:xiazhj@iga.ac.cn
Received:2020-05-26Accepted:2020-10-5Online:2021-01-01

摘要
建立简便、快速和无损种子连续取样技术流程及基因型鉴定技术体系, 可节约种植成本及缩短鉴定周期, 提高基因功能研究和育种效率。该研究利用微型电钻和空气泵等简单装置设计了一种连续且无损钻取大豆(Glycine max)种子组织的方法, 并优化了利用384深孔微孔板高通量提取DNA及基因型鉴定技术体系。该方法也可用于水稻(Oryza sativa)和玉米(Zea mays)等多种主要作物的种子取样及基因型鉴定。
关键词:
Abstract
Establishment of a simple, quick, non-destructive, and continuous sampling procedure, can save planting cost and accelerate gene functional analysis and breeding procedures. In this study, we created a rapid, non-destructive, and continuous sampling technique using micro electric driller and air pump. We also optimized the high throughput DNA extraction method for the 384 deep-well plate and genotyping method. Furthermore, this technique could be applied in rice, maize and other crops for seed sampling and high throughput genotyping.
Keywords:
PDF (6168KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
夏正俊, 李玉卓, 朱金龙, 吴红艳, 徐坤, 翟红. 快速、无损大豆种子连续取样技术及其DNA制备. 植物学报, 2021, 56(1): 56-61 doi:10.11983/CBB20095
Xia Zhengjun, Li Yuzhuo, Zhu Jinlong, Wu Hongyan, Xu Kun, Zhai Hong.
随着后基因组时代的到来, 主要农作物种子纯度及品种鉴别、基因组功能研究和分子育种均需进行大样本的基因型鉴定。最初, 人们采用整体粉碎种子的方法鉴定种子的基因型。后来, 有专家尝试用手术刀将小麦(Triticum aestivum)种子胚乳端切下约50%, 然后提取DNA (即半种子法) (McCarthy et al., 2002); King等(2014)则用剃须刀片将十分之一的种子收集至96孔板中。但无论是用手术刀切取种子还是用剃须刀片收集样品效率均较低。Von Post等(2003)及 Kamiya和Kiguchi (2003)发明了钻孔法(适用于体积相对较大的植物种子), 并在大麦(Hordeum vulgare)和大豆(Glycine max)中加以应用, 但因不能连续取样, 影响了其整体效率。近年来, 随着大豆等作物基因功能的研究及分子设计育种的发展(薛勇彪等, 2015), 建立大容量、快速且无损种子钻取方法及其基因型鉴定技术体系势在必行。本研究利用微型电钻、移液枪头及空气泵等简单装置, 设计了一种快速且无损大豆种子的连续钻取方法及后续DNA制备技术, 可用于高通量基因型鉴定等下游工作。此外, 应用该技术取样后的大豆等作物种子能够正常发芽和生长, 可节省田间种植空间与时间, 提高育种效率。
1 植物材料
实验材料包括大豆(Glycine max L.) Harosoy近等基因系Harosoy-E1 (L68-694、E1e2E3E4e5和PI547-707)和Harosoy (e1) (L58-266、e1e2E3E4e5和PI547-676), 及其建立的F2代遗传和衍生群体。2 仪器设备
包括电钻(钻头直径约0.9-1.3 mm, EYELA mini DC stirrer 3S, 东京理化器械株式会社, 日本)、稳定气压空气压缩泵(奥突斯1100W-40L型, 浙江省台州市)、离心机(DD-5M, 湘仪)、回旋式振荡器(HY-5, 荣华仪器制造有限公司)、PCR仪(SimpliAmp, Applied Biosystems)、水浴锅(HH600, 茂祥仪器设备厂)、聚丙烯384透明底白色深孔方型微孔板(每孔容积约为240 μL) (Corning)和普通5 mL移液器吸头。3 试剂
包括CTAB (十六烷基三甲基溴化铵, 上海生工)溶液、1.5% CTAB、1.4 mol·L-1 NaCl、100 mmol·L-1 Tris-HCl (pH8.0)、20 mmol·L-1 EDTA (4 mL 0.5 mol Stock·(100 mL)-1)、1% PVP-40和CIA溶液(在氯仿中加入1/24异戊醇)。4 大豆种子取样和基因型鉴定方法
4.1 取样技术流程
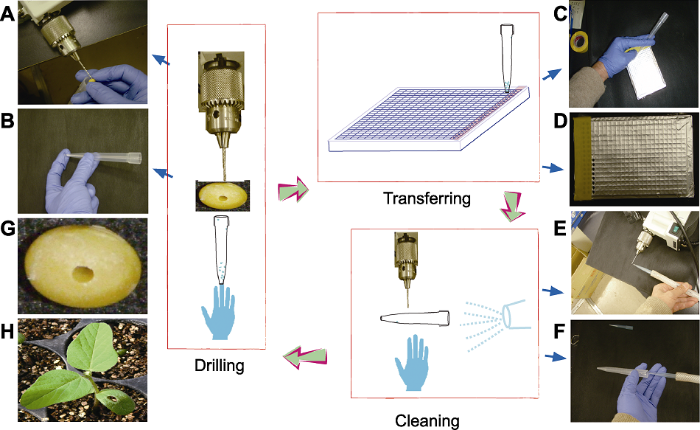
快速、无损大豆种子钻取流程如图1所示。为减少样品交叉污染, 先在深孔方型微孔板上覆盖1层锡箔纸, 然后轻压以凸现微孔位置。再将微型电钻固定在水平台面上, 左手持5 mL移液枪头(用无名指抵住枪头底部), 右手拿种子, 调好种子的种胚与钻头的相对位置, 以免在钻取胚乳组织时导致种胚部分受到损伤(图1A)。钻取时, 将转速调至每分钟180-200转, 待钻头深入至种子胚乳部分时, 左手将移液枪头的粗端移置钻头下, 用枪头管收集5.85-9.75 mg的大豆胚乳组织。收集完成后, 将5 mL移液枪头近水平放置(图1B), 移液枪头的顶端对准384深孔板的相应孔后, 用枪头刺破锡箔纸, 轻弹枪头数下, 使胚乳组织落入384深孔板中(图1C)。一列样品取样完成后, 用胶带将锡箔纸上的孔贴上, 防止放置下一列样品时交叉污染(图1D)。重复上述步骤, 直至完成所有种子取样。注意每完成1个种子取样均需用高压气流清除钻头、移液枪头及手套上残留的样品(图1E, F)。将钻取后的种子编号或顺序放入96孔板的培养板中。1个384样品板取样完成后, 低速离心, 使样品沉降于底部。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1种子钻孔和样品采集过程
(A) 种子组织的钻取; (B) 移液枪头收集种子组织; (C) 钻取种子组织并转移至384深孔板中; (D) 一列取样完成后贴胶带保护; (E) 钻头的清洁; (F) 移液枪头和手套的清洁; (G) 钻孔后的大豆种子; (H) 钻孔大豆种子播种后14天的幼苗。中间部分为整体流程简图, 种子取样经钻取、转移和清洁3个步骤, 再进行下一个种子的钻取。
Figure 1Procedure for seed drilling and sample collection
(A) Drilling of seed tissue; (B) Dilled tissue was collected by a pipette tip; (C) Transferring into a 384 deep-well plate; (D) When all wells in a row of the 384 deep-well plate were filled, the row will be sealed with a sticky tape; (E) Cleaning of the driller; (F) Cleaning of the tip and gloves; (G) Appearance of drilled seed; (H) Seedling of drilled seed (14 days after being sown). In the middle panel, the whole procedure is shown, in which seed sampling is performed by drilling, transferring and cleaning before proceeding to the next one.
4.2 DNA提取
揭开已完成取样的384深孔板表面的锡箔纸, 用连续加样器向每孔加入85 μL CTAB溶液, 低速离心, 使植物样品与溶液均沉降于底部, 并去除气泡, 贴上锡箔封板膜。在摇床(每分钟60转)中60°C恒温水浴30分钟, 半小时后取出至60°C带有水平振荡装置的恒温箱中, 低速振荡1.5小时。取出并冷却至室温后加入75 μL CIA (氯仿:异戊醇=24:1, (v/v)), 室温下在水平摇床上摇2小时; 2 500 ×g离心45分钟。用8连枪或12连枪吸取50 μL上清液至384深孔板, 加入30 μL异丙酮; 2 500 ×g离心60分钟; 150 ×g倒置离心数秒; 之后, 加入10 μL 70%无水乙醇; 150 ×g倒置离心数秒。室温放置5-10分钟, 直至沉淀晾干; 加入约50 μL (视沉淀大小而定)含RNase的TE溶液, 完全溶解DNA。
4.3 PCR反应及电泳
DNA模板准备: 用8连移液枪吸取1 μL 384深孔板中的DNA至384 PCR板中, 每孔的PCR反应液包括: 0.8 μL 10×不含镁离子的PCR缓冲液(TaKaRa, Japan), 0.8 μL dNTP (TaKaRa, Japan), 0.3 μL MgCl2溶液(TaKaRa, Japan), 第1对PCR引物(含10 μmol·L-1 Satt557正向引物以及10 μmol·L-1 Satt557反向引物) 0.3 μL, 第2对PCR引物(含10 μmol·L-1 S8正向引物以及10 μmol·L-1 S8反向引物) 0.3 μL, 0.1 μL TaKaRa ExTaq酶(TaKaRa, Japan)。加入灭菌超纯水(ddH2O)至8 μL。PCR反应条件: 95°C预变性5分钟; 95°C变性1分钟, 52°C退火1分钟, 68°C延伸1分钟, 32个循环; 72°C延伸5分钟; 4°C保存。
电泳及显色: 凝胶(分离胶浓度为13%, 浓缩胶浓度为5%)用Vistra Green (Amersham Pharmacia Biotech, UK)染色后, 在FluorI-mager 585仪器(Amersham Pharmacia Biotech)上, 使用HEGS (high- efficiency genome scanning)系统(Xia et al., 2007)扫描成像。
5 应用实例
5.1 大豆生育期基因克隆
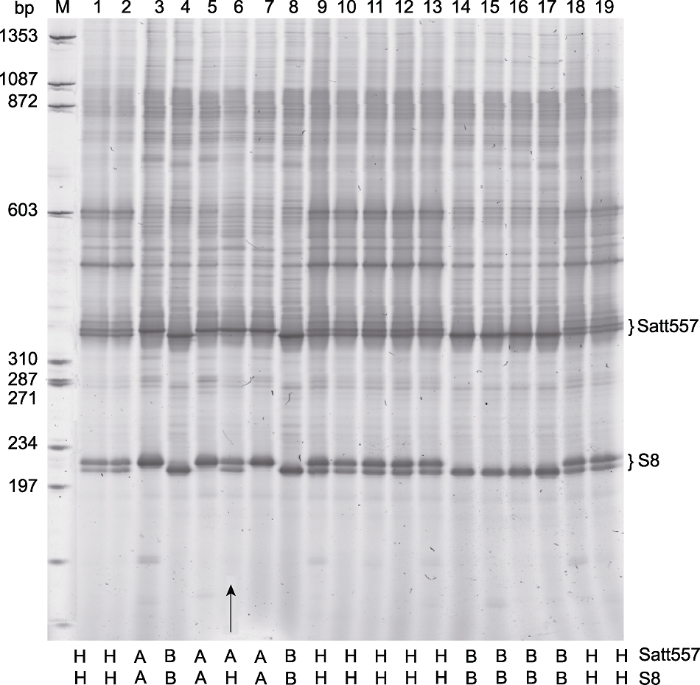
本研究建立的取样方法只需利用简单的设备即可实现连续钻取和收集种子组织样品, 同时保持种子的发芽势。钻取的种子组织无须粉碎, 可直接用于高通量DNA提取与基因型鉴定(图1)。与现有钻孔法(Kamiya and Kiguchi, 2003; 程文等, 2016)相比, 钻取效率明显提高。使用该方法, 每人每日可累计钻取大豆种子组织样品384样品板4-6块, 相当于1 500-2 300粒大豆种子。采用图位克隆法分离生育期基因E1时, 需要从大量个体中筛选出遗传重组体。E1基因位于着丝点附近, 重组率很低。若能够在种子阶段明确大豆的基因型, 筛选E1区域的重组体则可节省大量田间种植时间与空间。按照上述方法, 我们采集了36块384孔板, 约13 824粒种子的组织样品; 使用CTAB抽提法提取种子DNA, 获得了13 761粒种子的基因型信息, 成功鉴定出了10个遗传重组体。两对引物的单管式(one-tube) PCR结果见图2。在高通量鉴定系统中, 两引物相互间无影响。采用Satt557扩增的近等基因系Harosoy-E1和Harosoy (e1)间只有3个SNP差异, 在基因型鉴定系统中也能很好地区分开来。采用S8扩增的PCR产物较Satt557短, 故可根据1个样品两引物间的基因型迅速确定重组体。播种使用种子法鉴定后的种子, 对所获植株叶片进行取样, 分别验证Satt557和S8, 结果与种子基因型鉴定一致(图3)。在所验证的600个个体中成功率达100%; 并根据鉴定出的10个重组体, 成功克隆了E1基因(Xia et al., 2012)。
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2大豆遗传群体经种子钻取及DNA制备后的基因型鉴定
利用Satt557和S8两对引物进行单管PCR能准确鉴定两分子标记间的重组个体(箭头)。M: 分子质量标准品ΦX174 HaeIII; 1-19: Harosoy-E1与Harosoy (e1)杂交的F2群体(在每个泳道底部标注有每个个体2个分子标记的基因型)。
Figure 2Genotyping of a soybean genetic population through seed-drilling and thereafter DNA extraction
Accurate identification of recombinant occuring between two markers, Satt557 and S8, using one tube PCR (arrow). M: Molecular weight marker ΦX174 HaeIII; 1-19: F2 population that were derived from Harosoy-E1 × Harosoy (e1) (the genotypes of each individual were indicated at the bottom of each lane).
图3
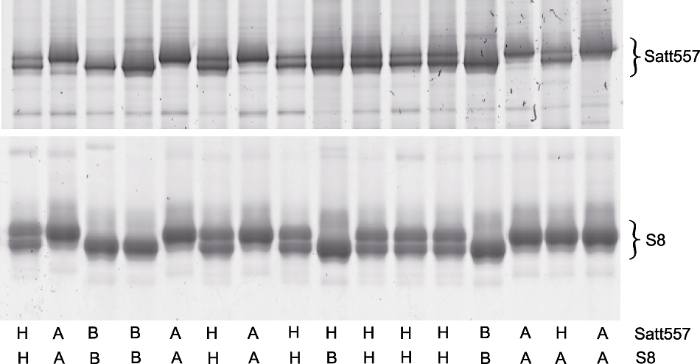 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3种子钻孔法鉴定结果的验证
钻孔的大豆种子出苗后, 提取其叶片DNA进行相关引物的基因型鉴定, 以验证种子基因型鉴定结果的准确性。上部和下部凝胶图分别为Satt557和S8的验证结果(底部标注了2个分子标记的基因型)。
Figure 3Verification of genotyping results obtained through seed-drilling
DNA were extracted from leaves of the plants that were developed from the drilled seeds, thereafter genotyping of Satt557 and S8 were performed to verify the accuracy of genotyping data of seed. The upper and the bottom gels are shown the genotyping result of Satt557 and S8, respectively (the genotypes of each individual were indicated at the bottom of each lane).
5.2 水稻、大豆及玉米种子与钻头的选配
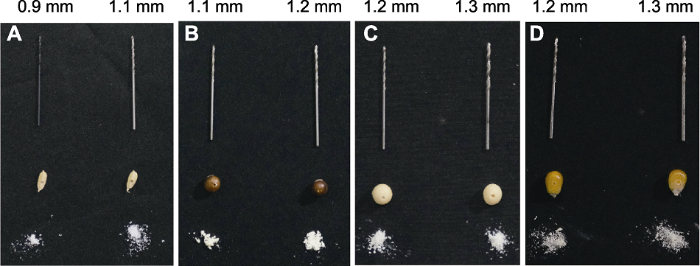
本方法可用于水稻(Oryza sativa)、小粒大豆农家品种和玉米(Zea mays)等多种作物种子的取样。基于我们的试验, 水稻和小粒大豆分别宜选用直径为0.9-1.1和1.1 mm的钻头; 大粒大豆和玉米则宜选用直径为1.2-1.3 mm的钻头(图4)。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4水稻、大豆和玉米种子大小及对应钻头型号
(A) 水稻种子(平均钻孔深度为(1.52±0.12) mm, 直径0.9 mm的钻头获取的平均组织重量为(1.54±0.12) mg, 直径1.1 mm的钻头获取的平均组织重量为(2.30±0.18) mg); (B) 小粒大豆种子(平均钻孔深度为(3.88±0.37) mm, 直径1.1 mm的钻头获取的平均组织重量为(5.85±0.99) mg, 直径1.2 mm的钻头获取的平均组织重量为(6.96±0.66) mg); (C) 大粒大豆种子(平均钻孔深度为(4.63±0.43) mm, 直径1.2 mm的钻头获取的平均组织重量为(8.31±0.79) mg, 直径1.3 mm的钻头获取的平均组织重量为(9.75±0.92) mg)。(D) 玉米种子(平均钻孔深度为(3.78±0.46) mm, 直径1.2 mm的钻头获取的平均组织重量为(6.79±0.83) mg, 直径1.3 mm的钻头获取的平均组织重量为(7.97±0.98) mg)。
Figure 4The seed dimensions of rice, soybean and maize and the selection of their appropriate drillers
(A) Rice seed (average drilling depth is (1.52±0.12) mm; average acquired tissue weight is (1.54±0.12) mg for the 0.9 mm diameter driller; average acquired tissue weight is (2.30±0.18) mg for the 1.1 mm diameter driller). (B) Soybean seed (small size) (average drilling depth is (3.88±0.37) mm; average acquired tissue weight is (5.85±0.99) mg for the 1.1 mm diameter driller; average acquired tissue weight is (6.96±0.66) mg for the 1.2 mm diameter driller). (C) Soybean seed (large size) (average drilling depth is (4.63±0.43) mm; average acquired tissue weight is (8.31±0.79) mg for the 1.2 mm diameter driller; average acquired tissue weight is (9.75±0.92) mg for the 1.3 mm diameter driller). (D) Maize seed (average drilling depth is (3.78±0.46) mm; average acquired tissue weight is (6.79±0.83) mg for the 1.2 mm diameter driller; average acquired tissue weight is (7.97±0.98) mg for the 1.3 mm diameter driller.
5.3 钻取样本的DNA提取
本方法所需设备简单, 一般研究室或育种单位均可配备; 并可根据样本容量和下游应用途径选择使用384孔深孔板或96孔深孔板。取样后的种子的发芽率和生长率与正常种子相似。对于特殊的种子, 为防止其吸水过快和萌发过程中病原菌入侵可用蜡封闭钻孔口(将蜡烛在孔口摩擦即可, 不要点燃蜡烛)。如需大量和高质量的DNA, 可适当加大钻取的植物组织量, 用每孔为2 mL的96孔深孔板取样和DNA提取(使用含有蛋白酶K的提取液(能使植物组织中的蛋白质完全消化)) (Kamiya and Kiguchi, 2003)。6 注意事项
(1) 钻取过程中可能出现静电, 影响组织的钻取和转移。此时, 可选用新的枪头或将移液枪头与较大体积的金属接触, 释放静电。(2) 为避免样品污染, 不收集种皮组织; 并且每钻取完1粒种子, 用空气泵中的气流清除钻头、移液枪头和手套上残留的组织样品, 或用乙醇擦拭上述物品。此外, 选用适量扩增效果好的引物, PCR反应的循环数不超过30。
(3) 用CTAB溶液等不含蛋白酶的提取液提取DNA时, 因钻取样品较多, 会出现少量白色胶状物(主要是蛋白质), 一定程度上影响样品的转移效率, 使个别样品的基因型鉴定失败。此时, 可适当减少钻取的组织量。在克隆大豆生育期基因过程中, 我们鉴定的36块384孔板的13 824粒大豆种子中, 有61粒的基因型鉴定失败, 占比约0.44%, 不影响整体基因型的鉴定效果。
(4) 下游的DNA提取及PCR均使用384孔板(或96孔板), 以提高基因型的鉴定效率。
(5) 将钻取的种子按顺序放置在样品板中, 可在基因型鉴定后迅速找出所需种子。
(6) 在钻取其它植物种子时, 需先对钻头直径、转速和钻孔深度进行预试验, 并根据试验目的选择相应的DNA提取和基因型鉴定技术。
(责任编辑: 孙冬花)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
URLPMID:24863292 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/dnares/dsm027URLPMID:18192280 [本文引用: 1]

Soybean [Glycine max (L.) Merrill] is the most important leguminous crop in the world due to its high contents of high-quality protein and oil for human and animal consumption as well as for industrial uses. An accurate and saturated genetic linkage map of soybean is an essential tool for studies on modern soybean genomics. In order to update the linkage map of a F2 population derived from a cross between Misuzudaizu and Moshidou Gong 503 and to make it more informative and useful to the soybean genome research community, a total of 318 AFLP, 121 SSR, 108 RFLP, and 126 STS markers were newly developed and integrated into the framework of the previously described linkage map. The updated genetic map is composed of 509 RFLP, 318 SSR, 318 AFLP, 97 AFLP-derived STS, 29 BAC-end or EST-derived STS, 1 RAPD, and five morphological markers, covering a map distance of 3080 cM (Kosambi function) in 20 linkage groups (LGs). To our knowledge, this is presently the densest linkage map developed from a single F2 population in soybean. The average intermarker distance was reduced to 2.41 from 5.78 cM in the earlier version of the linkage map. Most SSR and RFLP markers were relatively evenly distributed among different LGs in contrast to the moderately clustered AFLP markers. The number of gaps of more than 25 cM was reduced to 6 from 19 in the earlier version of the linkage map. The coverage of the linkage map was extended since 17 markers were mapped beyond the distal ends of the previous linkage map. In particular, 17 markers were tagged in a 5.7 cM interval between CE47M5a and Satt100 on LG C2, where several important QTLs were clustered. This newly updated soybean linkage map will enable to streamline positional cloning of agronomically important trait locus genes, and promote the development of physical maps, genome sequencing, and other genomic research activities.
DOI:10.1073/pnas.1117982109URLPMID:22619331 [本文引用: 1]

The complex and coordinated regulation of flowering has high ecological and agricultural significance. The maturity locus E1 has a large impact on flowering time in soybean, but the molecular basis for the E1 locus is largely unknown. Through positional cloning, we delimited the E1 locus to a 17.4-kb region containing an intron-free gene (E1). The E1 protein contains a putative bipartite nuclear localization signal and a region distantly related to B3 domain. In the recessive allele, a nonsynonymous substitution occurred in the putative nuclear localization signal, leading to the loss of localization specificity of the E1 protein and earlier flowering. The early-flowering phenotype was consistently observed in three ethylmethanesulfonate-induced mutants and two natural mutations that harbored a premature stop codon or a deletion of the entire E1 gene. E1 expression was significantly suppressed under short-day conditions and showed a bimodal diurnal pattern under long-day conditions, suggesting its response to photoperiod and its dominant effect induced by long day length. When a functional E1 gene was transformed into the early-flowering cultivar Kariyutaka with low E1 expression, transgenic plants carrying exogenous E1 displayed late flowering. Furthermore, the transcript abundance of E1 was negatively correlated with that of GmFT2a and GmFT5a, homologues of FLOWERING LOCUS T that promote flowering. These findings demonstrated the key role of E1 in repressing flowering and delaying maturity in soybean. The molecular identification of the maturity locus E1 will contribute to our understanding of the molecular mechanisms by which a short-day plant regulates flowering time and maturity.
一种快速、无损大豆种子DNA提取方法的建立和应用
1
2016
... 本研究建立的取样方法只需利用简单的设备即可实现连续钻取和收集种子组织样品, 同时保持种子的发芽势.钻取的种子组织无须粉碎, 可直接用于高通量DNA提取与基因型鉴定(
开启中国设计育种新篇章——“分子模块设计育种创新体系”战略性先导科技专项进展
1
2015
... 随着后基因组时代的到来, 主要农作物种子纯度及品种鉴别、基因组功能研究和分子育种均需进行大样本的基因型鉴定.最初, 人们采用整体粉碎种子的方法鉴定种子的基因型.后来, 有专家尝试用手术刀将小麦(Triticum aestivum)种子胚乳端切下约50%, 然后提取DNA (即半种子法) (
Rapid DNA extraction method from soybean seeds
3
2003
... 随着后基因组时代的到来, 主要农作物种子纯度及品种鉴别、基因组功能研究和分子育种均需进行大样本的基因型鉴定.最初, 人们采用整体粉碎种子的方法鉴定种子的基因型.后来, 有专家尝试用手术刀将小麦(Triticum aestivum)种子胚乳端切下约50%, 然后提取DNA (即半种子法) (
... 本研究建立的取样方法只需利用简单的设备即可实现连续钻取和收集种子组织样品, 同时保持种子的发芽势.钻取的种子组织无须粉碎, 可直接用于高通量DNA提取与基因型鉴定(
... 本方法所需设备简单, 一般研究室或育种单位均可配备; 并可根据样本容量和下游应用途径选择使用384孔深孔板或96孔深孔板.取样后的种子的发芽率和生长率与正常种子相似.对于特殊的种子, 为防止其吸水过快和萌发过程中病原菌入侵可用蜡封闭钻孔口(将蜡烛在孔口摩擦即可, 不要点燃蜡烛).如需大量和高质量的DNA, 可适当加大钻取的植物组织量, 用每孔为2 mL的96孔深孔板取样和DNA提取(使用含有蛋白酶K的提取液(能使植物组织中的蛋白质完全消化)) (
Non-toxic and efficient DNA extractions for soybean leaf and seed chips for high-throughput and large-scale genotyping
1
2014
... 随着后基因组时代的到来, 主要农作物种子纯度及品种鉴别、基因组功能研究和分子育种均需进行大样本的基因型鉴定.最初, 人们采用整体粉碎种子的方法鉴定种子的基因型.后来, 有专家尝试用手术刀将小麦(Triticum aestivum)种子胚乳端切下约50%, 然后提取DNA (即半种子法) (
Rapid identification of transformed wheat using a half- seed PCR assay
1
2002
... 随着后基因组时代的到来, 主要农作物种子纯度及品种鉴别、基因组功能研究和分子育种均需进行大样本的基因型鉴定.最初, 人们采用整体粉碎种子的方法鉴定种子的基因型.后来, 有专家尝试用手术刀将小麦(Triticum aestivum)种子胚乳端切下约50%, 然后提取DNA (即半种子法) (
A high-throughput DNA extraction method for barley seed
1
2003
... 随着后基因组时代的到来, 主要农作物种子纯度及品种鉴别、基因组功能研究和分子育种均需进行大样本的基因型鉴定.最初, 人们采用整体粉碎种子的方法鉴定种子的基因型.后来, 有专家尝试用手术刀将小麦(Triticum aestivum)种子胚乳端切下约50%, 然后提取DNA (即半种子法) (
An integrated high-density linkage map of soybean with RFLP, SSR, STS, and AFLP markers using a single F2 population
1
2007
... 电泳及显色: 凝胶(分离胶浓度为13%, 浓缩胶浓度为5%)用Vistra Green (Amersham Pharmacia Biotech, UK)染色后, 在FluorI-mager 585仪器(Amersham Pharmacia Biotech)上, 使用HEGS (high- efficiency genome scanning)系统(
Positional cloning and characterization reveal the molecular basis for soybean maturity locus E1 that regulates photoperiodic flowering
1
2012
... 采用图位克隆法分离生育期基因E1时, 需要从大量个体中筛选出遗传重组体.E1基因位于着丝点附近, 重组率很低.若能够在种子阶段明确大豆的基因型, 筛选E1区域的重组体则可节省大量田间种植时间与空间.按照上述方法, 我们采集了36块384孔板, 约13 824粒种子的组织样品; 使用CTAB抽提法提取种子DNA, 获得了13 761粒种子的基因型信息, 成功鉴定出了10个遗传重组体.两对引物的单管式(one-tube) PCR结果见
