 ,1*1
,1*1 2
3
Bryophyte-cyanobacteria symbioses and their nitrogen fixation capacity—A review
PI Chun-Yan1,2, LIU Xin1, WANG Zhe3, BAO Wei-Kai ,1*1
,1*1 2
3
通讯作者: (baowk@cib.ac.cn)
编委: 程晓莉
责任编辑: 李敏
| 基金资助: |
Online:2018-04-20
| Fund supported: |

摘要
苔藓-蓝藻共生体(BCS)能固氮, 是养分贫瘠地区森林氮输入的不可忽视的来源。BCS关系与固氮能力研究为科学认识生态系统氮输入与氮循环过程和机理提供了新的视角和有效途径, 具有重要的理论价值。然而, BCS关系、固氮作用与机理的研究迄今未受到足够关注, 报道较少, 认识仍然是零星而片段化的。基于系统查阅的相关文献, 该文综述了BCS的种类组成与共生关系类型、固氮能力及所固定氮的去向及其影响因素和作用机理, 指出了存在的问题及需要深入关注和亟待突破的4个研究方向。
关键词:
Abstract
Bryophyte-cyanobacteria symbiosis (BCS) is a key source of nitrogen input into ecosystems in nutrient-poor regions. Investigating BCS relationships and the nitrogen fixation capacity can be a new pathway and window to explore the process and mechanism of nitrogen input and nitrogen cycling. However, BCS relationships and nitrogen fixation/cycling processes and mechanisms remain poorly studied, and most of these studies have only focused on the boreal forest, with no report from Chinese forests. Based on systematic literature search and analysis, this review provides a summary on BCS relationships, the nitrogen fixation capability of BCS, the fate of fixed nitrogen, as well as the environmental factors and driving mechanisms of BCS. Firstly, we synthesized different types of BCS, the mechanisms by which the fixed nitrogen is transferred to and used by other plants within the forest, the rate of fixed nitrogen, the factors influencing the rate of nitrogen fixation. Moreover we point out the existing problems that need to pay close attention to and at least four research directions need to break through. Furthermore, the theoretical basis of BCS is provided for further research, promote and deepen the cognition of BCS and nitrogen-fixing research.
Keywords:
PDF (1626KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
皮春燕, 刘鑫, 王喆, 包维楷. 苔藓-蓝藻共生体关系与固氮能力研究进展. 植物生态学报[J], 2018, 42(4): 407-418 DOI:10.17521/cjpe.2017.0191
PI Chun-Yan, LIU Xin, WANG Zhe, BAO Wei-Kai.
生态系统净初级生产力常常受到可利用氮的限制(Tamm, 1991)。生物固氮是自然生态系统最主要的氮来源( Stewart, 1966 ; Cleveland et al., 1999), 但在许多生态系统, 如分布在高海拔和高纬度地区的亚高山针叶林和北方针叶林中, 往往缺乏能够与固氮根瘤菌形成共生关系的物种(如豆科植物), 那么生态系统的氮来源问题究竟是如何解决的呢?后来发现以蓝藻类(或蓝细菌, cyanobacteria)为主的固氮微生物是这些生态系统中氮的主要来源(Deluca, 2002b; Reed et al., 2011)。蓝藻是一类自养原核生物(Rai et al., 2000), 能够独立营自养生存, 也能以附生(epiphyte)或内生(endophyte/entophyte)等形式与真菌和苔藓等形成共生体(Meeks, 1990, 2007; Rai et al., 2000; Adams & Duggan, 2008), 其生物固氮作用主要靠固氮酶进行, 且固氮酶集中于异形胞(heterocyst)中, 所以氮固定常发生在蓝藻体内的异形胞中, 而异形胞的数量与蓝藻生境有密切关系(Adams & Duggan, 2008)。
苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(图1), 盖度达60%以上(刘俊华等, 2005; Liu & Bao, 2014), 生物量可达3 t·hm-2以上, 占森林总初级生产量的1/3 (Deluca et al., 2002b)。林下苔藓层在调节微气候方面发挥着重要作用, 直接或间接地影响森林生态系统中养分元素的生物地球化学循环过程(Cornelissen et al., 2007)。其中一个有趣的但目前所知甚少的途径就是苔藓能够主动释放特定化学物质促进蓝藻与其形成共生关系(Bay et al., 2013), 苔藓-蓝藻共生体(bryophyte-cyanobacteria symbiosis)能在森林地表进行更高效的氮固定和氮转运。已发现得益于苔藓供给碳水化合物等营养物质及苔藓层所提供的相对稳定的温度、水分、湿度等微环境条件, 苔藓-蓝藻类共生体中的蓝藻异形胞数量与固氮能力均显著高于独立营自养生存的蓝藻。比如, 共生体中蓝藻体内异形胞数量可达到独立生存时的6-10倍, 固氮速率则可增加至7倍(Adams & Duggan, 2008), 另外也发现共生体固定的氮转移至苔藓植物可使苔藓植物生物量增加(Berg et al., 2012)。苔藓-蓝藻共生体在北方森林和极地环境中广泛存在(Zackrisson et al., 2004), 由于其巨大的生物量, 林下苔藓-蓝藻共生体在亚高山针叶林和北方针叶林生态系统中的固氮速率可达1-2 kg·hm-2·a-1, 而后通过凋落物分解、淋溶等形式释放到外界环境中, 被其他植物利用, 是森林生态系统重要的氮汇和氮源(Wilson & Coxson, 1999; Deluca et al, 2002b; Gerber et al., 2010; Gundale et al, 2011), 对各生态系统乃至全球氮输入与氮循环具有不容忽视的生态学意义。因此, 系统深入认识苔藓-蓝藻类共生体关系及其固氮效应与机制对于揭示森林养分循环过程与森林管理具有重要的理论价值。
图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1青藏高原东部亚高山冷杉老龄林林下苔藓层片。苔藓层主要由塔藓、锦丝藓、毛梳藓和赤茎藓等构成。
Fig. 1An old-growth Abies fargesii var. faxoniana forest site in eastern Qinghai-Xizang Plateau with a moss dominated understory. Dominate moss species are Hylocomium splendens, Actinothuidium hookeri, Ptilium crista-castrensis, and Pleurozium schreberi.
然而苔藓-蓝藻共生体关系及效应的相关研究目前仍然贫乏, 已有认识还处于零散和片段化状态, 这一显著影响森林生态系统氮循环的关键途径及其过程仍未引起人们的广泛兴趣和重视。因此, 系统地分析、梳理和归纳已有对苔藓-蓝藻共生体固氮研究的进展和科学认识是十分必要的。本文作者系统查阅了过去50年来苔藓-蓝藻共生体及其相关研究的文献报道, 试图综述如下方面的研究现状: (1)苔藓-蓝藻共生体的类群、物种组成与共生关系; (2)苔藓-蓝藻共生体的固氮能力及其所固定氮的去向; (3)影响共生体关系及其固氮能力的因素与作用机理; 进一步梳理出当前已有的结论与需要聚焦的方向, 为苔藓-蓝藻共生体关系及其效应的深入研究提供基础。
1苔藓-蓝藻共生体类群及共生关系
1.1 苔藓-蓝藻共生体类群与组成物种
从现有报道来看, 依据苔藓类群可将苔藓-蓝藻共生体分为3类, 即角苔-蓝藻共生体(hornwort- cyanobacteria symbiosis)、苔-蓝藻共生体(liverwort-cyanobacteria symbiosis)和藓-蓝藻共生体(moss-?cyanobacteria symbiosis)。迄今已知能与蓝藻共生固氮的苔藓共35属41种(表1), 在全球已知苔藓种类中仅占很小比例。
角苔类在角苔属(Anthoceros)、树角苔属(Dendroceros)、短角苔属(Notothylas)和黄角苔属(Phaeoceros) 4个属的物种与蓝藻有共生固氮关系(Meeks, 1990), 但尚未明确鉴定到具体种类; 发现苔类中有6属的物种能够与蓝藻共生, 其中仅2属明确至种, 即Anastrophyllum involutifolium和Chiloscyphus leptanthus (Rippka et al., 1979; Meeks, 1990; West & Adams, 1997; Houle et al., 2006; Arróniz- Crespo et al., 2014)。
目前发现能与蓝藻共生固氮的藓类种类相对而言最多, 有25属31种。其中仍有5属(真藓属(Bryum)、镰刀藓属(Drepanocladus)、Dupontia、紫萼藓属(Grimmia)和毛梳藓(Ptilium))未见明确的物种报道; 仅4属(黑藓属(Andreaea)、砂藓属(Racomitrium)、泥炭藓属(Sphagnum)和镰刀藓属(Drepanocladus))有1个以上的物种能够与蓝藻共生, 其中已在砂藓属的4个种中发现共生关系, 分别是Racomitrium subcrispipilum、R. laevigatum、R. lanuginosum和R.
didymium。在亚高山针叶林和北方针叶林林下常见的藓类塔藓(Hylocomium splendens)和赤茎藓(Pleurozium schreberi)均具有与蓝藻共生固氮的能力, 但在波叶曲尾藓(Dicranum polysetum)和金发藓(Polytrichum commune)中却未发现共生固氮现象(Bay et al., 2013)。
目前已知能与苔藓形成共生关系的固氮蓝藻有8属(表1)。其中, 除念珠藻属(Nostoc)的灰色念珠藻(Nostoc muscorum)外, 其他7个属未明确鉴定到具体种类(Granhall & Selander, 1973; Rippka et al., 1979; Reddy & Giddens, 1981; Davey & Marchant, 1983; West & Adams, 1997; Houle et al., 2006; Arróniz-?Crespo et al., 2014)。
Table 1
表1
表1苔藓-蓝藻共生体类型及共生形式
Table 1
| 类型 Type | 苔藓种类及与蓝藻关系 Bbryophyte and the relationship with cyanobacteria (En or Ep) | 蓝藻种类 Cyanobacteria | 参考文献 Reference |
|---|---|---|---|
| 角苔-藻共生体 Hornwort-cyanobacteria symbiosis | 角苔属 Anthoceros sp. (En 1) 树角苔属 Dendroceros sp. (En 1) 短角苔属 Notothylas sp. (En 1) 黄角苔属 Phaeoceros sp. (En 1) | 1 念珠藻属 Nostoc sp. | Rippka et al., 1979; Meeks, 1990; West & Adams, 1997; Houle et al., 2006; Arróniz-Crespo et al., 2014 |
| 苔-藻共生体 Liverwort-cyanobacteria symbiosis | Anastrophyllum involutifolium (Ep1) 壶苞苔属 Blasia sp. (Ep 1, 2, 4; En 1) Cavicularia sp. (En 1) Chiloscyphus leptanthus (Ep 1) 地钱属 Marchantia sp. (Ep 1) 光萼苔 Porella sp. (Ep 1) | 1 念珠藻属 Nostoc sp. 2 眉藻属 Calothrix sp. 3 真枝藻属 Stigonema sp. 4 Chlorogloeopsis sp. | Rippka et al., 1979; Meeks, 1990; West & Adams, 1997; Houle et al., 2006; Arróniz-Crespo et al., 2014 |
| 藓-藻共生体 Moss-cyanobacteria symbiosis | Acroschisma wilsonii (Ep 1) Andreaea alpine (Ep 1) Andreaea laxifolia (Ep 1) 皱缩藓 Aulacomnium palustre (Ep 1) Blepharidophyllum densifolium (Ep 1) 真藓属 Bryum sp. (Ep 1, 5, 6 7) Calliergon richardsonii (Ep 1) 角齿藓 Ceratodon purpureus (Ep 5, 6, 7) Clasmatocolea humilis (Ep 1) Cryptochila grandiflora (Ep 1) Dendroligotrichum squamosum (Ep 1) Dicranoloma chilense (Ep1) Ditrichum cylindricarpum (Ep 1) 镰刀藓属 Drepanocladus sp. (Ep 1, 2, 4) Drepanocladus exannulatus (Ep 1) Dupontia sp. (Ep 1) 紫萼藓属 Grimmia sp. (Ep 1) Heteroscyphus magellanicus (Ep 1) 塔藓 Hylocomium splendens (Ep 1, 3) 沼寒藓 Paludella squarrosa (Ep 1) 赤茎藓 Pleurozium schreberi (Ep 1, 3) 毛梳藓 Ptilium sp. (Ep 1, 3) Racomitrium Subcrispipilum (Ep 1) Racomitrium laevigatum (Ep 1) 白毛砂藓 Racomitrium lanuginosum (Ep 1) Racomitrium didymium (Ep 1) 三洋藓 Sanionia uncinata (EP 1) Sphagnum lindebergii (Ep 1; En 1.1) 岸生泥炭藓 Sphagnum riparium (Ep 1; En 1.1) 毛青藓 Tomentypnum nitens (Ep 1) 小石藓 Weisia controversa (Ep 5, 6, 7) | 1 念珠藻属 Nostoc sp. 1.1 灰色念珠藻 Nostoc muscorum 2 眉藻属 Calothrix sp. 3 真枝藻属 Stigonema sp. 4 伪枝藻属 Scytonema sp. 5 Anobena sp. 6 颤藻属Oscillatoria sp. 7 鞘丝藻属 Lyngbya sp. | Granhall & Selander, 1973b; Rippka et al., 1979; Reddy & Giddens, 1981; Davey & Marchant, 1983; Meeks, 1990; West & Adams, 1997; Gentili et al., 2005; Houle et al., 2006; Gavazov et al., 2010; Arróniz-Crespo et al., 2014 |
| 小计 Total | 41 | 8 |
新窗口打开|下载CSV
1.2 苔藓-蓝藻共生体的共生关系类型
苔藓和蓝藻的共生关系有2种: 蓝藻内生和附生于苔藓。角苔与蓝藻共生体均为内生型关系。在苔-蓝藻共生关系中, 蓝藻的内生位置至少有2种报道。一种是有关念珠藻属(Nostoc)物种生长于苔类配子体的耳廓(auricles)中(Rippka et al., 1979; Meeks, 1990; West & Adams, 1997; Houle et al., 2006), 另一种是生长于配子体腹面的黏液腔(slime cavities)中(Meeks, 1990), 如壶苞苔属(Blasia)和Cavicularia属能够与念珠藻形成的内生关系。内生型的藓-蓝藻共生体目前仅发现泥炭藓属中的Sphagnum lindebergii和岸生泥炭藓(Sphagnum riparium)与Nostoc muscorum形成的共生体(Granhall & Selander, 1973)。附生型的共生关系多见于地钱属(Marchantia)、光萼苔属(Porella)、裂萼苔属(Chiloscyphu)、毛叶苔属(Ptilidium)和挺叶苔属(Anastrophyllum)的苔-藻共生体以及各种藓-藻共生体中(表1)。除上述泥炭藓属2种与念珠藻形成内生型的共生外, 其余藓类蓝藻共生体均为附生形式。其中, Anobena、Oscillatoria和Lyngbya属的蓝藻可以附生于真藓类角齿藓(Ceratodon purpureus)和小石藓(Weisia controversa)中(Reddy & Giddens, 1981), 念珠藻属蓝藻可以附生的位置一般在多种藓类的叶片内弯部(Deluca et al., 2002b)。值得注意的是, 藓类在附生型的共生体中占据主导地位, 而同一苔藓与蓝藻是否能同时具有内生与附生型共生关系均不清楚。Bay等(2013)发现, 在氮缺乏时, 分布广泛的赤茎藓和塔藓会释放特定的引诱剂吸引念珠藻附生, 并促进念珠藻产生藻殖段和异形胞提高固氮能力; 但当藓类对氮的需求下降时(例如孢子体被移除时), 共生体的固氮速率也下降。遗憾的是, 苔藓如何释放特定的引诱剂及相关作用机制尚未见报道。
2 固氮能力
苔藓-蓝藻共生固氮能力的定量研究到目前为止仅聚焦于藓-藻共生体, 而缺乏角苔-藻与苔-藻共生体的报道。归纳已有的报道发现, 各苔藓-蓝藻共生体的固氮能力介于0.25-1.7 kg·hm-2·a-1, 如图2所示, 其中固氮量最高的地区为Deluca等(2002b)报道的27个地区(瑞典、挪威和芬兰), 固氮量平均值为1.7 kg·hm-2·a-1; 瑞典的Hornavan湖和Uddjaure湖的平均固氮量最低, 为0.25 kg·hm-2·a-1 (Lagerstorm et al., 2007); 我们注意到, 有限的藓-蓝藻共生体的固氮能力研究主要聚焦在北欧与北美高纬度北方针叶林或北极圈附近地区(Rosén & Lindberg, 1980; Deluca et al., 2008), 其他地区(包括中国)的案例研究非常贫乏。关于藓-蓝藻共生体的固氮能力动态变化的报道仅见Deluca等(2002b)的工作, 报道了瑞典Reivo森林中赤茎藓-蓝藻共生体半年(5-11月)的动态固氮率变化, 发现在苔藓旺盛生长季的6月、8月底和10月出现固氮率峰值, 未见年固氮量动态变化规律以及其他种类的苔藓-蓝藻共生体固氮能力变化的报道。目前报道的苔藓物种仅针对以上区域森林中的优势种类, 如赤茎藓、塔藓和泥炭藓, 缺少对其他苔藓种类的研究。就有限案例的比较而言, 赤茎藓-蓝藻共生体(0.85 kg·hm-2·a-1)和泥炭藓-蓝藻共生体(0.81 kg·hm-2·a-1)的固氮量平均值高于塔藓-蓝藻共生体(0.64 kg·hm-2·a-1), 混合苔藓-蓝藻共生体的固氮量最低(0.53 kg·hm-2·a-1)(图2)。森林中的苔藓植物种类繁多, 研究森林中的不同种类苔藓-蓝藻共生体的固氮能力, 能更准确地评估森林中由苔藓-蓝藻固定的氮的输入量, 可为森林中的氮循环研究提供更准确的科学依据。
图2
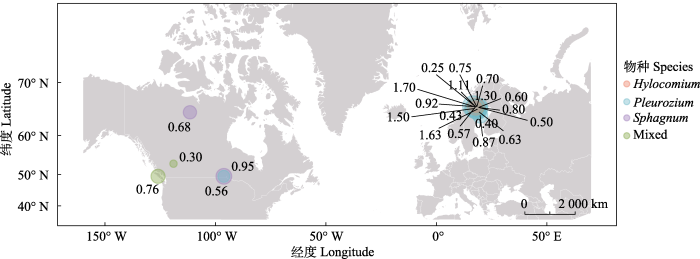 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2不同区域苔藓-蓝藻共生体固氮能力(kg·hm-2·a-1)。图中数据来源于: Blundon & Dale, 1990; Deluca et al., 2002b; Lagerstorm et al., 2007; Markham, 2009; Zackrisson et al., 2009; Gundale et al., 2011; Lindo & Whiteley, 2011; Stewart et al., 2011a; Rousk et al., 2013b; Stuiver et al., 2015。
Fig. 2Nitrogen fixation capacities (kg·hm-2·a-1) of bryophyte-cyanobacteria symbioses in different sites. All the data in the figure are from: Blundon & Dale, 1990; Deluca et al., 2002b; Lagerstorm et al., 2007; Markham, 2009; Zackrisson et al., 2009; Gundale et al., 2011; Lindo & Whiteley, 2011; Stewart et al., 2011a; Rousk et al., 2013b; Stuiver et al., 2015.
3 苔藓-蓝藻共生体所固定氮的去向
有研究发现, 藓-藻共生体所固定的氮只有20%留存于藻类, 其他的转移至苔藓植物内(Meeks et al., 2002; Adams & Duggan, 2012; Rousk et al., 2016f)。转移至苔藓植物体内的氮能通过菌根真菌送至其他高等植物的根部被利用(Carleton & Read,2011)。目前对苔藓植物体内的氮转移方式除菌根真菌方式外, 还有受到干扰(如火烧)后氮释放至土壤中, 但速率很慢(Rousk et al., 2013c)。由于多数苔藓与蓝藻之间以附生的形式共生, 两者之间没有特化的结构用于转移固定的氮, 推测蓝藻固定的氮未被宿主苔藓吸收而直接释放到环境中的可能性亦存在, 特别是在降水频繁的季节(Coxson, 1991; Wilson & Coxson, 1999)。然而, 目前针对泥炭藓属少数物种的研究认为大部分共生体固定的氮最终都转移到了苔藓体内(Rai et al., 2002; Rousk et al., 2016f)。总体而言, 定量地研究苔藓-蓝藻共生体所固定氮的去向的报道很少, 共生体所固定氮的去向与氮转移过程及其机理仍然很不清楚。
转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用。(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(Turetsky et al., 2008)。苔藓凋落物的分解速率低于其他高等植物凋落物(杨林等, 2015), 因此通过苔藓凋落物分解释放氮将是速度慢且周期长的过程。(2)苔藓体内部分养分和碳水化合物将在干湿交替和冻融交替的过程中淋失(Coxson, 1991; Wilson & Coxson, 1999)。但迄今为止, 苔藓氮淋失占苔藓体内氮的比例, 以及氮淋失在生态系统氮循环中的作用仍然有待进一步量化的研究。(3)苔藓体内的氮通过菌根真菌被邻近其他植物利用。放射性同位素标记实验表明, 菌根真菌将磷和氮转运到欧洲赤松(Pinus sylvestris)的根系中(Carleton & Read, 1991)。虽然这一过程也可能是经过苔藓的养分淋失实现的, 但有研究证实, 苔藓植株确实能够受菌根真菌侵染(Zhang & Guo, 2007)。
4 影响苔藓-蓝藻共生体固氮的因素及其作用机理
4.1 水分、温度、光照及其综合影响
苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制。因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大。水分增加能使苔藓-蓝藻共生体的固氮能力加强(Gundale et al., 2009, 2012b; Jackson et al., 2011)。因为苔藓是敏感的变水植物, 水分含量变化能显著改变其生理生化过程(李佑稷等, 2004; Rousk et al., 2013d), 因而可能会导致依存苔藓的蓝藻的生长发生改变。已经发现, 连续干燥3天后苔藓-蓝藻共生体失去固氮能力(小于0.2 μmol·m-2·h-1), 虽在复水过程中能缓慢恢复, 但需5天左右才能恢复原有固氮水平(Proctor, 2001; Rousk et al., 2013d), 可见水对蓝藻的固氮能力影响很大, 所以水是苔藓-蓝藻共生体固氮过程中的关键因子(Gundale et al., 2012b)。两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(Granhall & Selander, 1973; Gentili et al., 2005 ); 赤茎藓和塔藓与蓝藻的共生体均在温度高于30.3 ℃时固氮速率明显下降, 但比较而言赤茎藓-蓝藻共生体比塔藓-蓝藻共生体对温度的耐受强度更高(Gundale et al., 2012a)。北方森林中年平均温度的升高会使苔藓-蓝藻共生体的固氮率增加(Houlton et al., 2008), 然而过高的温度(32 ℃)会影响苔藓-蓝藻共生体的氮固定速率(Zielke et al., 2002); 将分离出的与苔藓共生的念珠藻(Nostoc sp.)和眉藻(Calothrix sp.)在不同温度下的固氮活性比较发现, 两者的固氮速率迥异。在低温(5 ℃)下, 念珠藻具有低的固氮活性, 而眉藻在此温度下无固氮能力; 在高温(30 ℃)下, 眉藻却具有最高的固氮活性, 几乎是同等温度下念珠藻固氮活性的3倍(Gentili et al., 2005), 蓝藻的不同最适固氮温度可能是导致苔藓-蓝藻共生体固氮作用季节差异的原因(Deluca et al., 2002b; Zackrisson et al., 2004)。此外, 温度升高亦使得固氮酶的启动推迟, 使蓝藻的固氮速率降低(Gundale et al., 2009; Stewart et al., 2011b)。在水分不受限的情况下, 0-10 ℃时共生体固氮率较低; 高于10 ℃时温度升高会使固氮速率快速提高(Vitousek et al., 2002; 苏延桂等, 2011; Rousk et al., 2017b), 因为固氮酶能耐受的最高温在25 ℃左右, 所以固氮最高温在25 ℃左右。Zielke等(2002)发现自由生长的蓝藻固氮最高温为25 ℃, 但和苔藓共生后的共生体其固氮最高温为32 ℃。因此, 温度对蓝藻固氮能力有较大影响, 而与苔藓形成共生关系更有利于氮固定, 可能是因为与苔藓共生后, 改变了蓝藻固氮适宜温度范围。Rousk等(2017b)对赤茎藓和毛青藓的蓝藻共生体进行水分(0、25%、100%)和温度(10、20、30 ℃)双因素对共生体固氮能力的影响研究, 发现毛青藓-蓝藻共生体固氮速率受水分含量的影响较大, 且两种共生体在高的水分含量下具有更大的固氮酶活性, 固氮最适温度在20-30 ℃, 与苏延桂等(2011)对荒漠土壤生物结皮固氮酶活性的研究及Vitousek等(2002)的结果类似。
蓝藻固氮作用的过程对光具有依赖性。光可以通过光合作用影响其固氮过程中的电子供应链, 或是影响固氮必需的高能量物质的产生。蓝藻固氮酶的活性也受到光的调节, 不同的光照强度下固氮活性呈现相应的高低差异, 且光照可缓解因添加结合态氮对固氮作用的抑制效应(陈因和方大惟, 1983)。自由生长的蓝藻的固氮作用不能离开光照, 与苔藓附生的藓-蓝藻共生体的固氮作用同样受到光的较大影响。Basilier等(1978)发现泥炭藓(Sphagnum sp.)和镰刀藓-蓝藻共生体的固氮活性受到光的强烈影响, 但内生于苔藓体内的蓝藻是否可以通过苔藓的光合系统供应其固氮所需的电子, 从而使其对光的依赖性降低, 尚未见相关报道。
在最适温度下, 提高光照强度会增加共生体固氮能力。Gentili等(2005)发现, 同在13 ℃时, 光照强度160 μmol·m-2·s-1下的共生体固氮能力大于光照强度为80 μmol·m-2·s-1时的固氮能力。目前已发现赤茎藓-蓝藻共生体的最适温度-光照综合值为 23 ℃、130 μmol·m-2·s-1 (Gentili et al., 2005)和22℃、500 μmol·m-2·s-1, 而塔藓-蓝藻共生体最适温度-光照综合值为16.3 ℃、500 μmol·m-2·s-1 (Gundale et al., 2012a)。两研究中赤茎藓的最适温度-光照值中温度值相似, 但是光照值迥异, Gentili等(2005)未设置23 ℃下更高的光照强度, 未能明确更高光照是否增加其固氮能力, 这可能是两研究结果差异的原因。明确温度和光照等非生物因素的综合作用对生物固氮的影响, 可以预测和模拟森林环境中氮的循环, 及森林冠层下的苔藓层氮输入在气候变化中引起的潜在反馈效应(Sorensen & Michelsen, 2011), 但是目前对非生物因子作用于苔藓-蓝藻共生体固氮能力的综合影响的研究十分贫乏。
4.2 氮、磷及金属元素的影响
苔藓为蓝藻提供了适宜的生存环境, 苔藓的生物量决定的苔藓种群结构直接影响到蓝藻的生存环境, 进而影响到苔藓-蓝藻共生体的固氮作用。大气沉降的养分是苔藓养分的主要来源, 因此养分对苔藓-蓝藻共生体固氮作用的影响值得特别关注。目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响。氮沉降量的增加能减少苔藓生物量(Gundale et al., 2013; Du et al., 2014), 共生体的固氮能力相应下降(Gundale et al., 2013; Stuiver et al., 2015)。另外, 苔藓对氮沉降的敏感程度因物种而异(Rousk & Michelsen, 2016)。长期实验发现, 塔藓-蓝藻共生体的生物量和固氮能力受氮沉降影响的程度高于赤茎藓-蓝藻共生体(Gundale et al., 2013)。苔藓-蓝藻共生体对氮沉降的响应存在明显的剂量效应(dose-effect)关系。在氮沉降量较低(<3 kg·hm-2)时, 苔藓-蓝藻共生体的固氮能力与对照(无额外氮沉降)无明显差异(Zackrisson et al., 2009; Gundale et al., 2011); 氮沉降量在3-12 kg·hm-2之间时, 苔藓-蓝藻共生体的固氮速率出现不同程度的下降, 苔藓植物体表面附生的蓝藻数量也呈现相应的下降趋势(Gundale et al., 2011; Rousk et al., 2013e); 氮沉降在较高水平(> 12 kg·hm-2)下, 固氮作用停滞, 苔藓植物体表面几乎没有蓝藻附生(Jones et al., 2004; Gundale et al., 2011; Rousk et al., 2013a, 2013e)。也有研究发现氮添加量为10 kg·hm-2·a-1时, 赤茎藓共生体的固氮作用并没有受到限制(Rousk, 2013a), 但当氮沉降大于10 kg·hm-2·a-1时, 其固氮作用受到显著抑制(Rousk et al., 2013b)。表明苔藓-蓝藻共生体的固氮速率确实受到氮沉降量的影响, 但具体阈值以及阈值的种间差异仍然有待进一步研究。
苔藓-蓝藻共生体对磷的响应, 或者说磷对苔藓-蓝藻共生体固氮能力的影响, 目前还难有确切定论, 相关结果仍然是矛盾的。Chapin等(1991)发现, 添加磷时北极柳(Salix arctica)环境下的苔藓体固氮能力均增加, 塔藓和赤茎藓-蓝藻固氮能力亦增加(Rousk et al., 2017a); 但Zackrisson等(2004, 2009)对赤茎藓和塔藓-蓝藻共生体的磷添加(5 kg·hm-2·a-1)实验表明, 磷的添加对其固氮能力并无影响; Smith (1984)将Brachythecium subplicatum-蓝藻共生体置于磷浓度为2.5 μg·mL-1的液体中培养, 发现磷的添加抑制其固氮能力, 此种培养方式与土壤培养方式的不同, 可能是导致结果差异的原因, 且磷添加对共生体固氮作用亦可能存在种间差异及阈值, 但目前尚未见明确报道。上述养分冲突性结果表明, 未来需要更多且更深入的苔藓-蓝藻共生体对磷的响应的研究。此外, 最近Rousk等(2017a)发现, 添加重金属钼(Mo)增加了塔藓-蓝藻共生体和赤茎藓-蓝藻共生体的固氮能力, 且单独添加钼比单独添加磷和磷及钼的混合添加能更好地提升共生体中的固氮酶活性, 他们指出苔藓-蓝藻共生体的固氮作用受到重金属钼的限制, 且钼对蓝藻的生物量有影响。
4.3 pH值的影响
已经发现环境pH值能显著影响藓-蓝藻共生关系及其固氮能力。当pH值为3.8时, 3种泥炭藓(Sphagnum balticum、锈色泥炭藓(S. fuscum)和垂枝泥炭藓(S. jensenii))完全没有藻类与之共生; 在pH值为4.2和4.9的环境中, S. lindebergii和岸生泥炭藓有藻类内生; 在pH值更高的环境中, 藻类附生或自由生长(Granhall & Selander, 1973); 蓝藻在自由生长时的最适环境pH值为5-10, 且在培养过程中酸性环境pH值上升, 而碱性环境pH值未发生较大的变化(罗伟和沈健英, 2007; 郑云普等, 2010), 说明蓝藻自由生长时更适宜碱性环境, 但是和泥炭藓共生后, 能在更低pH值的环境中生存且固氮。Smith (1984)亦发现Brachythecium subplicatum-藻类共生体于水体培养时最适固氮pH值为5.9-6.2。由此, 蓝藻和泥炭藓共生后能适应更严酷的环境条件。上述研究结果来自于室内培养实验与野外调查, 但野外自然条件下的实验研究贫乏, pH对苔藓-蓝藻共生体的形成以及固氮能力的影响程度及其机理还不清楚。4.4 森林垂直冠层和演替阶段的影响
苔藓群落组成与生物量在森林的空间差异及其随森林发育演替的变化是明显的, 必然引起苔藓-蓝藻共生体关系及其固氮能力的变化。明确这样的时空变化特点与规律十分必要。Lindo和Whiteley (2011)发现, 在海岸温带雨林中, 地表层为生态系统提供的有效氮较冠层附生苔藓-蓝藻共生体少。从该森林中30、15 m冠层与地表的苔藓-蓝藻共生体固氮量和总的蓝藻(伪枝藻属)细胞数目的比较都显示, 冠层的藻类数目最多, 固氮量最高, 而地表的氮固定量最低(Lindo & Whiteley, 2011)。受水分、风速和光强的影响, 森林冠层的苔藓植物种类和生物量都较亚冠层的苔藓植物种类少, 而氮固定量低(Newmaster et al., 2003; Han et al., 2010)。苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(Zackrisson et al., 2004; Stuiver et al., 2015), 原因比较复杂。目前认识到的原因有4个: 一是演替后期虽然整个系统的氮量很大, 但可被苔藓直接吸收的可利用氮量却很少(DeLuca et al., 2002a)。 二是幼林中地表苔藓层覆盖率比老林的少1/3以上, 老林不管是冠层还是地表都具有对苔藓-蓝藻共生体生长更有利的环境条件(Newmaster et al., 2003; Lagerstrom et al., 2007; Han et al., 2010)。三是因为演替早期受到苔藓植物和蓝藻数量的限制, 其固氮能力低(DeLuca et al., 2007); 也有人发现, 林木生物量的增加和林下透光率的减少使苔藓-蓝藻共生体(塔藓-蓝藻)的固氮能力下降(Stuiver et al., 2015)。但是Arróniz-Crespo等(2014)对智利西南部达尔文山脉(Tierra del Fuego)冰消区域植被不同发育时期(即演替过程中)苔藓-蓝藻共生体固氮能力的研究显示, 在较为湿润的冰川消退早期(4-7年, 植被主要为石生苔藓植物), 共生体固氮能力最强, 为1.656-1.780 kg·kg-1·a-1; 但是在较干燥的冰川消退中期(26-66年, 植被主要为10 m左右高的灌丛或乔木), 苔藓-蓝藻共生体的固氮能力次之, 为0.338-0.511 kg·kg-1·a-1; 晚期(80年)时, 其固氮能力最低, 仅为0.288 kg·kg-1·a-1。上述结果表明, 森林垂直层次或演替阶段对苔藓-蓝藻共生体的形成及其固氮能力影响的差异性取决于微环境条件以及苔藓-蓝藻共生体相互作用的强度与过程, 机理相当复杂。
4.5 生物因素影响
苔藓-蓝藻共生体关系显然受到形成共生体的苔藓与蓝藻种类的影响, 可以推测也受其他可能影响其微生境与关系的生物类群特性如乔木层类群、灌草类群的影响。已发现, 由于蓝藻自身的固氮能力差异及其对环境适应能力不同, 苔藓-蓝藻共生体的固氮能力有差异(Zielke et al., 2009)。念珠藻是目前所知藻类中能与多种苔藓共生固氮的种类, 能与苔藓形成共生体的其他藻类数量较少, 而共生体固氮能力因藻类不同种类的差异性及其机理尚不清楚。苔藓能通过促使藻类异形胞数量的增加而增加共生体的固氮率, 也能产生一种毒素物质抑制藻类生长(Rousk, 2013a), 不同苔藓促进藻类异形胞数量的能力不可能一样。因此, 共生苔藓种类也影响与之共生的藻类种类及其固氮能力(前面已有论述)。Sanna等(2015)发现共生体固氮率大小取决于苔藓种类, 泥炭藓-藻类共生体较森林中的其他藓-藻共生体固氮能力强。目前尚缺乏严谨的实验去深入揭示苔藓与藻类共生体的关系及其可能的固氮能力如何受苔藓与藻类的影响, 更缺乏其他生物因素对苔藓-蓝藻共生体关系及其功能影响的研究。5 结论和展望
5.1 结论和认识
从过去50余年来苔藓-蓝藻共生体及其可能的固氮能力研究可以归纳出一些初步结论和认识。(1)目前已知能形成苔藓-蓝藻共生体的苔藓有35属41种(表1), 蓝藻有8属, 占全球已知苔藓和蓝藻种类的比例很小。即使如此, 其中的大部分仍然未能准确识别到物种水平。因此, 苔藓-蓝藻共生体可能是广泛现象, 因为大多数已知藓种(如塔藓、赤茎藓)是广泛分布的种类, 但就苔藓与蓝藻的物种多样性而言, 苔藓-蓝藻共生体的形成是否是普遍的还不清楚。
(2)苔藓-蓝藻共生体可根据苔藓类群差异简单分为3个类群: 角苔-蓝藻共生体、苔-蓝藻共生体和藓-蓝藻共生体, 存在内生和附生两种共生关系; 角苔与苔类和蓝藻的共生关系主要以内生为主, 而藓类与蓝藻的共生关系以附生为主; 共生关系中苔藓为蓝藻不仅提供了更稳定的微生境, 也有可能提供营养物质(如碳水化合物)以启动或维持藻类定居与生长, 从而改善蓝藻的固氮能力。
(3)藓-蓝藻共生体具有固氮能力, 而且比蓝藻单独生长时的固氮能力更强, 但并不是所有藓-蓝藻共生体均具有固氮能力; 角苔-蓝藻与苔-蓝藻共生体固氮能力的强弱目前仍未有确切报道; 内生与附生共生体的固氮能力差异性也不清楚。苔藓-蓝 藻共生固氮能力研究目前仅聚焦于藓-蓝藻类型, 而缺乏角苔-蓝藻与苔-蓝藻的共生体固氮能力的 报道。
(4)仍然有限的藓-蓝藻共生体的固氮能力及其变化研究主要聚焦于北欧与北美高纬度北方针叶林或北极圈附近地区的植被, 其他地区(包括中国)的案例研究非常贫乏。已报道的藓-蓝藻共生体固氮能力为0.25-1.7 kg·hm-2·a-1, 因区域差异与苔藓种类不同而差异较大, 固氮能力变化研究的工作十分缺乏。
(5)苔藓-蓝藻共生体关系及其固氮能力受共生的藓与蓝藻的种类, 光、温、水等环境因素以及氮、磷和钼等元素的影响, 但作用机制尚不清楚。
5.2 需要聚焦的研究方向与科学问题
苔藓-蓝藻共生体及其互作关系、固氮能力、影响因素及其作用机理的认识仍然是粗浅的, 很多关键问题仍不清楚, 需要更多更深入的研究去加深对苔藓蓝藻共生体关系、效应与机制的科学认识, 深化或突破我们对生物多样性维持机制与养分循环机制的已有认识。现有工作和认识明显不足, 需要聚焦。5.2.1 苔藓-蓝藻共生关系、动态变化及其互作机制
苔藓与蓝藻间存在共生关系是明确的, 但如何形成的?苔藓释放的何种特定物质如何促进共生体的形成?共生关系是否在苔藓多样性中是普遍而广泛的?多数附生型苔藓-蓝藻共生体并没有特化的附生结构, 但与藓类附生却大大加强了蓝藻固氮能力, 这种附生形式的共生体是否广泛存在于苔藓物种与固氮蓝藻之间?这些基础性问题并不清楚。因此, 加强苔藓-蓝藻共生关系、动态变化及其互作机制的研究是必要的, 不仅有助于强化对苔藓-蓝藻共生体形成、演变与机理的规律性与一般性的深入认识, 也能形成和推动跨植物类群多样性维持机制的创新性认识。现有苔藓-蓝藻共生体关系的研究主要是采用形态学手段进行初步的静态观察, 藓-藻共生体关系的定量的分子生物学研究证据缺乏必要的应用。采取严谨的生态学实验方法, 开展苔藓-蓝藻共生关系及其互作机制的分子生物学基础研究是当前亟待展开的重要突破方向。
5.2.2 苔藓-蓝藻共生体的固氮效应及其动态变化与驱动机制
对苔藓-蓝藻共生体的固氮效应及其动态变化的认识严重不足。苔藓如何调控共生体的固氮作用?内生固氮和附生固氮的固氮效率是否有明显的差异?共生体关系及其固氮效应如何变化?互动关系如何相互驱动?共生体所固定的氮如何转移?转移途径、方式及其数量怎样变化?这些基础性理论问题大大制约着苔藓-蓝藻共生体固氮效应与机理研究的深入。
5.2.3 苔藓-蓝藻共生体固氮能力与生态环境变化的关系与作用机理
苔藓-蓝藻共生体关系及其固氮能力对环境(气候)变化十分敏感, 逐渐成为全球气候变化研究的重要对象(Gundale et al., 2012a)。在北欧所开展的几个生态学实验包括大气N沉降、增温以及降水变化的模拟实验研究, 获得了一些新的事实依据, 但大多仅针对少数单个因子的效应认识, 缺乏对多环境因子综合作用效应的案例研究。全球气候变化的区域差异性相当明显, 因此, 从气候变化角度获得的那些基本认识在全球范围内的普遍性以及区域差异性仍然需要更多的研究进一步证实和完善, 以加深对气候变化程度与苔藓-蓝藻共生体固氮能力的定量关系的理论认识。森林对全球气候变化具有很强的调节作用, 全球变化通过森林调控而作用于林下苔藓-蓝藻共生体, 因此, 森林环境变化与全球气候变化至少在程度上是有些差异的, 揭示苔藓-蓝藻共生体固氮能力与林下生态环境变化的关系十分必要。更重要的是, 当前环境变化与苔藓-蓝藻共生体固氮能力的关系在生理学、生物学与生态及其化学方面的机理认识十分贫乏, 应该成为一个重要研究方向。在苔藓-蓝藻共生体固氮的关键区域(例如高山和亚高山森林生态系统中), 结合多种森林环境因子监控, 进行长期野外观测, 深入研究环境变化对苔藓-蓝藻共生体固氮的影响, 将有助于评估共生体固氮对该生态系统的实际贡献, 并可模拟和预测未来环境变化对生态系统氮循环的影响。
5.2.4 苔藓-蓝藻共生体固氮能力时空格局变化规律及其在森林生态系统氮循环中的贡献
到目前为止, 有限的研究为认识苔藓-蓝藻共生体固氮能力在生态系统氮输入中的重要性提供了明确的证据和新的视角, 无疑为生态系统氮循环过程及其机制的认识带来了新的希望。要充分认识贫瘠生态系统演替中养分驱动机制, 急需开展更多更广泛的苔藓-蓝藻共生体固氮研究, 以明确不同区域生态系统苔藓-蓝藻共生体固氮能力的时空格局变化规律, 厘清苔藓-蓝藻共生体固氮在森林生态系统氮循环中的贡献率。在自然条件下的实验生态学方法与长期定位研究方法的有机结合是这方面研究在方法上的必然选择, 亟待强化。此外, 鉴于苔藓在高山、亚高山生态系统, 北方针叶林以及北极圈生态系统中的优势程度, 这些区域苔藓-蓝藻共生体固氮能力的研究应该是重点关注的问题。在区域生态系统养分循环模型研究与应用中应充分考虑苔藓以及苔藓-蓝藻共生体在氮循环中的作用。强化苔藓-蓝藻共生体关系及其形成机制、固氮过程与环境变化的关系及其作用机理的研究将有助于准确预测森林的动态, 以及更充分地认识苔藓在森林生态系统氮循环中的作用。

扫码加入读者圈
听语音, 看问答
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 3]
[本文引用: 1]
DOIURLPMID [本文引用: 2]

Abstract Bryophyte establishment represents a positive feedback process that enhances soil development in newly exposed terrain. Further, biological nitrogen (N) fixation by cyanobacteria in association with mosses can be an important supply of N to terrestrial ecosystems, however the role of these associations during post-glacial primary succession is not yet fully understood. Here, we analyzed chronosequences in front of two receding glaciers with contrasting climatic conditions (wetter vs drier) at Cordillera Darwin (Tierra del Fuego) and found that most mosses had the capacity to support an epiphytic flora of cyanobacteria and exhibited high rates of N2 fixation. Pioneer moss-cyanobacteria associations showed the highest N2 fixation rates (4.60 and 4.96 g N g-1 bryo. d-1) very early after glacier retreat (4 and 7 years) which may help accelerate soil development under wetter conditions. In drier climate, N2 fixation on bryophyte-cyanobacteria associations was also high (0.94 and 1.42 g N g-1 bryo. d-1) but peaked at intermediate-aged sites (26 and 66 years). N2 fixation capacity on bryophytes was primarily driven by epiphytic cyanobacteria abundance rather than community composition. Most liverworts showed low colonization and N2 fixation rates, and mosses did not exhibit consistent differences across life forms and habitat (saxicolous vs terricolous). We also found a clear relationship between cyanobacteria genera and the stages of ecological succession, but no relationship was found with host species identity. Glacier forelands in Tierra del Fuego show fast rates of soil transformation which imply large quantities of N inputs. Our results highlight the potential contribution of bryophyte-cyanobacteria associations to N accumulation during post-glacial primary succession and further describe the factors that drive N2-fixation rates in post-glacial areas with very low N deposition.
DOIURL

Nitrogen fixation by blue-green algae associated with Sphagnum and Drepanocladus mosses growing in wet depressions and pools was studied in minerotrophic parts of the Stordalen mire in Swedish Lapland. Nitrogen fixation rates of 0.5-6.4 g m-2 yr-1 were estimated on Sphagnum riparium with the acetylene reduction method. Fixation varied considerably along moss plants and for Sphagnum was found to be lower on apical and non-green parts. Fixation was higher at the periphery of a moss community. Activity was strongly light dependent and seemed little affected by pH-variations between pH 4.3-6.8. A daily fixation maximum around noon, and an annual maximum around the middle of the growing season, were indicated. /// Фиксация азота сине-зелеными, вдорослямн, связанными с мхами Sphagnum и Drepanocladus, растущимми в мокрых понижениях и лужах, исследованы в минеротрофных частях Стордаленского болота в Шведской Лапландии. Интенсивность фиксации азота составляет 0,5 - 6,4 г/ M2 по измерениям на Sphagnum riparium методом восстановления ацетилена. Интенсивность фиксации сильно различается в разных частях мха и у Sphagnum уменьшается по направлению к апикальному концу и незеленьм частям. Фиксация повышается на периферии моховых ассоциаций. Активность сильно зависит от освещенности, но слабо нзменяется при колебаниях pH в пределах 4,3 - 6,8. Дневиой максимум фиксации наблюдается около полудня, а годовой максимум - примерно в середине вегетационного периода.
DOIURLPMID [本文引用: 2]

The mechanistic basis of feather moss cyanobacteria associations, a main driver of nitrogen (N) input into boreal forests, remains unknown. Here, we studied colonization by Nostoc sp. on two feather mosses that form these associations (Pleurozium schreberi and Hylocomium splendens) and two acrocarpous mosses that do not (Dicranum polysetum and Polytrichum commune). We also determined how N availability and moss reproductive stage affects colonization, and measured N transfer from cyanobacteria to mosses.The ability of mosses to induce differentiation of cyanobacterial hormogonia, and of hormogonia to then colonize mosses and re-establish a functional symbiosis was determined through microcosm experiments, microscopy and acetylene reduction assays. Nitrogen transfer between cyanobacteria and Pleurozium schreberi was monitored by secondary ion mass spectrometry (SIMS).All mosses induced hormogonia differentiation but only feather mosses were subsequently colonized. Colonization on Pleurozium schreberi was enhanced during the moss reproductive phase but impaired by elevated N. Transfer of N from cyanobacteria to their host moss was observed.Our results reveal that feather mosses likely secrete species-specific chemo-attractants when N-limited, which guide cyanobacteria towards them and from which they gain N. We conclude that this signalling is regulated by N demands of mosses, and serves as a control of N input into boreal forests.
DOIURL [本文引用: 1]

Despite the general assumption that nitrogen fixed by associated cyanobacteria will be readily utilised for growth by the Sphagnum, no empirical evidence is available in the literature. Therefore the effects of nitrogen transfer from cyanobacteria associated with S. riparium were investigated.Cultivation of S. riparium with and without cyanobacteria was performed under laboratory conditions for 57 days.We show that nitrogen fixation by cyanobacteria associated with Sphagnum mosses, influences moss growth by transfer of fixed nitrogen to the moss. More than 35 % of the nitrogen fixed by cyanobacteria was transferred to the newly formed moss biomass and resulted in an increase in the growth of Sphagnum biomass compared to the controls. The variation in the increase of nitrogen content explained 76 % of the biomass increment.Hence, nitrogen fixation will have immediate effect on the carbon fixation by Sphagnum. This shows that factors regulating nitrogen fixation will have a direct effect on the role of Sphagnum dominated ecosystems with respect to carbon cycling.
DOIURL

Dinitrogen fixation rates were investigated in a 200-yr-old chronosequence, using an in situ acetylene reduction technique. Three successional plant communities representative of three stages of Engelmann spruce (Picea engelmannii) forest development were sampled from a sequence of recessional moraines located in front of the Robson Glacier. The legume Hedysarum boreale var. mackenzii was considered the major nitrogen-fixing agent on all the moraines. Hedysarum boreale contributed 72% of the nitrogen input in the pioneer herb stage, 79% in the intermediate Dryas stage, and 88% in the spruce forest stage. Soil microorganisms (e.g., Gloeocapsa) added 26, 20, and 8% of the nitrogen input, respectively. The estimated annual nitrogen input from nitrogen fixation decreased approximately eight-fold over the 200 yr forest succession. It was determined that the input in the pioneer stage was 57.2, in the intermediate stage 41.6, and in the forest stage 7.3 mmol C2H4m-2yr-1. A marked decline in nitrogen fixation is characteristic of the late stage in forest succession and may be a mechanism which maintains the steady state in total nitrogen accumulation.
DOIURL [本文引用: 1]

ABSTRACT Conifer eather moss ecosystems dominate large areas of the boreal forest regions of the world, but the interrelations between these two components of the system are poorly understood. Mycorrhizal roots of the trees grow in close association with the mosses. The possibility that nutrients can be transferred from moss shoots to trees through mycorrhizal fungi was investigated using the feather moss Pleurozium schreberi and mycorrhizal seedlings of Pinus contorta. Shoots of the moss were divided into three categories, viz. green, senescent, and dead, and nutrient contents of aqueous leachates from the segments were measured before and after drying. Significant quantities of nitrogen and phosphorus were released from moss shoots especially after drying. Senescing segments consistently released more N than those that were dead and generally released more than did the green segments. All categories of segments released some protein nitrogen, and drying induced leakage of glucose, fructose, and sucrose. Leachates of entire moss shoots were capable of supporting growth of three mycorrhizal fungi in pure culture. Moss shoots added to chambers containing mycorrhizal plants were colonized by the fungal associates of the plants, particularly intensive growth occurring in the senescent region of the moss shoots. Phosphate (32P) and carbon (14C), previously fed to the moss shoots, was absorbed by mycorrhizal mycelia and transferred over distances of centimetres to infected roots of pine plants and then to their shoots. The significance of these uptake and transfer processes for nutrient cycling in boreal forest ecosystems is discussed. Key words: leachate, nitrogen, phosphorus, sugars, protein, radioisotope.
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]

Human activities have clearly caused dramatic alterations of the terrestrial nitrogen cycle, and analyses of the extent and effects of such changes are now common in the scientific literature. However, any attempt to evaluate N cycling processes within ecosystems, as well as anthropogenic influences on the N cycle, requires an understanding of the magnitude of inputs via biological nitrogen fixation (BNF). Although there have been many studies addressing the microbiology, physiology, and magnitude of N fixation at local scales, there are very few estimates of BNF over large scales. We utilized >100 preexisting published estimates of BNF to generate biome- and global-level estimates of biological N fixation. We also used net primary productivity (NPP) and evapotranspiration (ET) estimates from the Century terrestrial ecosystem model to examine global relationships between these variables and BNF as well as to compare observed and Century-modeled BNF. Our data-based estimates showed a strong positive relationship between ecosystem ET and BNF, and our analyses suggest that while the model's simple relationships for BNF predict broad scale patterns, they do not capture much of the variability or magnitude of published rates. Patterns of BNF were also similar to patterns of ecosystem NPP. Our “best estimate” of potential nitrogen fixation by natural ecosystems is 65195 Tg N yr611, with a range of 100–290 Tg N yr611. Although these estimates do not account for the decrease in natural N fixation due to cultivation, this would not dramatically alter our estimate, as the greatest reductions in area have occurred in systems characterized by relatively low rates of N fixation (e.g., grasslands). Although our estimate of BNF in natural ecosystems is similar to previously published estimates of terrestrial BNF, we believe that this study provides a more documented, constrained estimate of this important flux.
[本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 1]

We have recently shown that bimorph piezoelectric PVDF films induce formation of periosteal bone in vivo and attributed this phenomenon to a piezoelectric effect. In the present study films were implanted in rabbits to encircle the femoral diaphysis. Specimens obtained after 6 and 12 days were subjected to routine processing for electron microscopy as well as fixation using the Ka-pyroantimonate technique. The electron micrographs revealed that initial osteoblastic differentiation and formation of collagenous matrix were followed by Ca accumulation in mitochondria. Calcification of the matrix progressed with deposition of mineralizing nodules and their fusion to form larger calcified masses. This was associated with disappearance of the pyroantimonate positive material from mitochondria. These ultrastructural observations confirm that bimorph films induce bone formation and disclose some features of the calcification process of the osseous callus.
DOIURLPMID [本文引用: 1]

Biological feedback mechanisms regulate fundamental ecosystem processes and potentially regulate ecosystem productivity. To date, no studies have documented the down-regulation of terrestrial nitrogen (N) fixation via an ecosystem-level feedback mechanism. Herein, we demonstrate such a feedback in boreal forests. Rapid cycling of N in early secondary succession forests yielded greater throughfall N deposition, which in turn decreased N fixation by cyanobacterial associates in feather moss carpets that reside on the forest floor. The forest canopy exerts a tight control on biotic N input at a period of high productivity.
DOIURLPMID [本文引用: 1]

Scots pine (Pinus sylvestris L.) forests of northern Sweden are often considered to be N limited. This limitation may have been exacerbated by the elimination of wildfire as a natural disturbance factor in these boreal forests. Phenolic inhibition of N mineralization and nitrification (due to litter and exudates of ericaceous shrubs) has been proposed as a mechanism for N limitation of these forests, but this hypothesis remains largely untested. N mineralization rates, nitrification rates, and sorption of free phenolic compounds were assessed along a fire-induced chronosequence in northern Sweden. A total of 34 forest stands varying in age since the last fire were identified and characterized. Overstorey and understorey vegetative composition and depth of humus were analysed in replicated plots at all 34 sites. Eight of the forest stands aged 3-352 years since the last fire were selected for intensive investigation in which ten replicate ionic resin capsules (used to assess net N mineralization and nitrification) and non-ionic carbonaceous resin capsules (used to assess free phenolic compounds) were installed at the interface of humus and mineral soil. A highly significant correlation was observed between site age and net sorption of inorganic N to resin capsules. Net accumulation of NH6262 and NO6163 on resin capsules followed a linear decrease (R2=0.61, P<0.01) with time perhaps as a result of increased N immobilization with successional C loading. NO6163 sorption to resin capsules followed a logarithmic decrease (R2=0.80, P<0.01) that may be related to a logarithmic increase in dwarf shrub cover and decreased soil charcoal sorption potential along this chronosequence. A replicated field study was conducted at one of the late successional field sites to assess the influence of charcoal and an added labile N source on N turnover. Three rates of charcoal (0, 100, and 1,000 g$\text{M}^{-2}$) and two rates of glycine (0 and 50 g N as glycine$\text{M}^{-2}$) were applied in a factorial design to microplots in a randomized complete block pattern. Net ammonification (as assessed by NH6262 sorption to resins) was readily increased by the addition of a labile N source, but this increase in NH6262 did not stimulate nitrification. Nitrification was stimulated slightly by the addition of charcoal resulting in similar levels of resin-sorbed NO6163 as those found in early successional sites. Resin-sorbed polyphenol concentrations were decreased with charcoal amendments, but were actually increased with N amendments (likely due to decomposition of polyphenols). Net N mineralization appears to be limited by rapid NH6262 immobilization whereas nitrification is limited by the lack of an appropriate environment or by the presence of inhibitory compounds in late successional forests of northern Sweden.
[本文引用: 1]
DOIURLPMID [本文引用: 4]

Abstract Biological nitrogen (N) fixation is the primary source of N within natural ecosystems, yet the origin of boreal forest N has remained elusive. The boreal forests of Eurasia and North America lack any significant, widespread symbiotic N-fixing plants. With the exception of scattered stands of alder in early primary successional forests, N-fixation in boreal forests is considered to be extremely limited. Nitrogen-fixation in northern European boreal forests has been estimated at only 0.5 kg N ha(-1) yr(-1); however, organic N is accumulated in these ecosystems at a rate of 3 kg N ha(-1) yr(-1) (ref. 8). Our limited understanding of the origin of boreal N is unacceptable given the extent of the boreal forest region, but predictable given our imperfect knowledge of N-fixation. Herein we report on a N-fixing symbiosis between a cyanobacterium (Nostoc sp.) and the ubiquitous feather moss, Pleurozium schreberi (Bird) Mitt. that alone fixes between 1.5 and 2.0 kg N ha(-1) yr(-1) in mid- to late-successional forests of northern Scandinavia and Finland. Previous efforts have probably underestimated N-fixation potential in boreal forests.
DOIURLPMID [本文引用: 1]

61Effects of N deposition on moss biomass and stoichiometry were investigated.61N deposition reduced biomass ratios of green to brown moss tissues.61N deposition increased N and P contents in moss tissues.61N deposition caused significant changes of moss carbon and nutrient pools.
DOIURL

Dinitrogen fixation by cyanobacteria is of particular importance for the nutrient economy of cold biomes, constituting the main pathway for new N supplies to tundra ecosystems. It is prevalent in cyanobacterial colonies on bryophytes and in obligate associations within cyanolichens. Recent studies, applying interspecific variation in plant functional traits to upscale species effects on ecosystems, have all but neglected cryptogams and their association with cyanobacteria. Here we looked for species-specific patterns that determine cryptogam-mediated rates of N 2 fixation in the Subarctic. We hypothesised a contrast in N 2 fixation rates (1) between the structurally and physiologically different lichens and bryophytes, and (2) within bryophytes based on their respective plant functional types. Throughout the survey we supplied 15 N-labelled N 2 gas to quantify fixation rates for monospecific moss, liverwort and lichen turfs. We sampled fifteen species in a design that captures spatial and temporal variations during the growing season in Abisko region, Sweden. We measured N 2 fixation potential of each turf in a common environment and in its field sampling site, in order to embrace both comparativeness and realism. Cyanolichens and bryophytes differed significantly in their cyanobacterial N 2 fixation capacity, which was not driven by microhabitat characteristics, but rather by morphology and physiology. Cyanolichens were much more prominent fixers than bryophytes per unit dry weight, but not per unit area due to their low specific thallus weight. Mosses did not exhibit consistent differences in N 2 fixation rates across species and functional types. Liverworts did not fix detectable amounts of N 2 . Despite the very high rates of N 2 fixation associated with cyanolichens, large cover of mosses per unit area at the landscape scale compensates for their lower fixation rates, thereby probably making them the primary regional atmospheric nitrogen sink.
DOIURLPMID [本文引用: 2]

Cyanobacteria colonizing the feather moss Pleurozium schreberi were isolated from moss samples collected in northern Sweden and subjected to physiological and molecular characterization. Morphological studies of isolated and moss-associated cyanobacteria were carried out by light microscopy. Molecular tools were used for cyanobacteria identification, and a reconstitution experiment of the association between non-associative mosses and cyanobacteria was conducted. The influence of temperature on N2 fixation in the different cyanobacterial isolates and the influence of light and temperature on N2-fixation rates in the moss were studied using the acetylene reduction assay. Two different cyanobacteria were effectively isolated from P. schreberi: Nostoc sp. and Calothrix sp. A third genus, Stigonema sp. was identified by microscopy, but could not be isolated. The Nostoc sp. was found to fix N2 at lower temperatures than Calothrix sp. Nostoc sp. and Stigonema sp. were the predominant cyanobacteria colonizing the moss. The attempt to reconstitute the association between the moss and cyanobacteria was successful. The two isolated genera of cyanobacteria in feather moss samples collected in northern Sweden differ in their temperature optima, which may have important ecological implications.
DOIURL [本文引用: 1]

[1] Global anthropogenic changes in carbon (C) and nitrogen (N) cycles call for modeling tools that are able to address and quantify essential interactions between N, C, and climate in terrestrial ecosystems. Here we introduce a prognostic N cycle within the Princeton Geophysical Fluid Dynamic Laboratory (GFDL) LM3V land model. The model captures mechanisms essential for N cycling and their feedbacks on C cycling: N limitation of plant productivity, the N dependence of C decomposition and stabilization in soils, removal of available N by competing sinks, ecosystem losses that include dissolved organic and volatile N, and ecosystem inputs through biological N fixation. Our model captures many essential characteristics of C-N interactions and is capable of broadly recreating spatial and temporal variations in N and C dynamics. The introduced N dynamics improve the model's short-term NPP response to step changes in CO2. Consistent with theories of successional dynamics, we find that physical disturbance induces strong C-N feedbacks, caused by intermittent N loss and subsequent N limitation. In contrast, C-N interactions are weak when the coupled model system approaches equilibrium. Thus, at steady state, many simulated features of the carbon cycle, such as primary productivity and carbon inventories, are similar to simulations that do not include C-N feedbacks.
[本文引用: 4]
DOIURLPMID [本文引用: 3]

Abstract Bryophytes achieve substantial biomass and play several key functional roles in boreal forests that can influence how carbon (C) and nitrogen (N) cycling respond to atmospheric deposition of reactive nitrogen (Nr). They associate with cyanobacteria that fix atmospheric N090202, and downregulation of this process may offset anthropogenic Nr inputs to boreal systems. Bryophytes also promote soil C accumulation by thermally insulating soils, and changes in their biomass influence soil C dynamics. Using a unique large-scale (0.1 ha forested plots), long-term experiment (16 years) in northern Sweden where we simulated anthropogenic Nr deposition, we measured the biomass and N090202-fixation response of two bryophyte species, the feather mosses Hylocomium splendens and Pleurozium schreberi. Our data show that the biomass declined for both species; however, N090202-fixation rates per unit mass and per unit area declined only for H. splendens. The low and high treatments resulted in a 29% and 54% reduction in total feather moss biomass, and a 58% and 97% reduction in total N090202-fixation rate per unit area, respectively. These results help to quantify the sensitivity of feather moss biomass and N090202 fixation to chronic Nr deposition, which is relevant for modelling ecosystem C and N balances in boreal ecosystems.
[本文引用: 4]
DOIURL [本文引用: 2]

We conducted a pair of experiments to assess whether nitrogen (N) fixation by a feathermoss-cyanobacteria association was sensitive to moisture availability and quality of litter inputs, and whether sensitivity to these factors differed between young and old forests. In our first greenhouse experiment, we experimentally varied the frequency of water addition to Pleurozium schreberi (Brid.) Mitt...
DOIURLPMID [本文引用: 3]

090004Plant productivity is predicted to increase in northern latitudes as a result of climate warming; however, this may depend on whether biological nitrogen (N)-fixation also increases. We evaluated how the variation in temperature and light affects N-fixation by two boreal feather mosses, Pleurozium schreberi and Hylocomium splendens, which are the primary source of N-fixation in most boreal environments.090004We measured N-fixation rates 2 and 4 wk after exposure to a factorial combination of environments of normal, intermediate and high temperature (16.3, 22.0 and 30.300°C) and light (148.0, 295.7 and 517.3 0204mol m0908082 s0908081).090004Our results showed that P. schreberi achieved higher N-fixation rates relative to H. splendens in response to warming treatments, but that the highest warming treatment eventually caused N-fixation to decline for both species. Light strongly interacted with warming treatments, having positive effects at low or intermediate temperatures and damaging effects at high temperatures.090004These results suggest that climate warming may increase N-fixation in boreal forests, but that increased shading by the forest canopy or the occurrence of extreme temperature events could limit increases. They also suggest that P. schreberi may become a larger source of N in boreal forests relative to H. splendens as climate warming progresses.
DOIURLPMID [本文引用: 2]

Plant productivity is predicted to increase in boreal forests owing to climate change, but this may depend on whether N inputs from biological N-fixation also increases. We evaluated how alteration of climatic factors affects N input from a widespread boreal N-fixer, i.e. cyanobacteria associated with the feather moss Pleurozium schreberi. In each of 10 forest stands in northern Sweden, we established climate-change plots, including a control (ambient climate) plot and three plots experiencing a +2/C temperature increase, an approximately threefold reduction in precipitation frequency, and either 0.07, 0.29 or 1.16 times normal summer precipitation. We monitored N-fixation in these plots five times between 2007 and 2009, and three times in 2010 after climate treatments ended to assess their recovery. Warmer temperatures combined with less frequent precipitation reduced feather moss moisture content and N-fixation rates regardless of total precipitation. After climate treatments ended, recovery of N-fixation rates occurred on the scale of weeks to months, suggesting resilience of N-fixation to changes in climatic conditions. These results suggest that modelling of biological N-inputs in boreal forests should emphasize precipitation frequency and evaporative water loss in conjunction with elevated temperature rather than absolute changes in mean precipitation.
DOIURLPMID [本文引用: 2]

Epiphytic plants play an important role in the nutrient cycle of forest ecosystems. There had been fewer studies in subtropical regions than in other climate zones. Prior research showed that the canopy epiphyte could fix nitrogen combined with microorganism in tropical forest. The epiphytic plants enwrapping trees in canopy layer are very abundant in the subtropical mountainous cloud forest of Ailao Mountain (central and southern Yunnan Province, SW China). This forest lacks widespread nitrogen-fixing plants, and the nitrogen origin is elusive. Maybe there also exist such nitrogen-fixing systems in epiphyte community. Nitrogen-fixing potentials of canopy epiphytes increased greatly from dry season to wet season. There occurred an obvious difference on the epiphytic nitrogen fixation abilities between upper canopy layer and sub-canopy layer in alternant period between wet season and dry season. Epiphytic nitrogen-fixing potentials for the subtropical moist forest in Ailao Mountains ranged between 0.027 and 2.2402kg02ha 611 65year 611 . Our results indicate that the canopy epiphytes in the subtropical moist forest of Ailao Mountains can fix a significant amount of atmospheric nitrogen. This finding suggests a new nitrogen source for the subtropical forest ecosystem, thus can have profound impact on the studies of nitrogen cycling.
DOIURL [本文引用: 3]

The recent discovery that N2 fixation rates by the feather moss carpet of boreal Scandinavian forests increases with stand maturity has put into question the paradigm that Ni fixation is negligible in mature boreal forest. The Na fixation was attributed to a previously unknown association between Nostoc sp., a N2-fixing cyanobacteium and Pleurozium schreberi (Brid.) Mitt., a feather moss that is abundant worldwide in the boreal forest. Here we report for the first time that this association also exists in the Canadian boreal forest. We discovered, however, that Nostoc was found growing not only on Pleurozium but also on two other moss species (Hylocomnium splendens (Hedw.) Br. Eur. and Ptillium crista-cas-trensis (Hedw.) De Not.). In addition, the N2-fixing cyanobacterium Sligonema sp. was observed on the three moss species mentioned above, indicating the existence of six different associations. At least one of the six associations was found at 9 of 13 sites that are representative of a large areaof the Quebec boreal forest. These findings suggest possibilities for further research, aimed at measuring the unaccounted for Ni-fixing potential of the feather moss carpet in Canadian boreal forests.
DOIURLPMID [本文引用: 1]

Dinitrogen (N) fixation is widely recognized as an important process in controlling ecosystem responses to global environmental change, both today and in the past; however, significant discrepancies exist between theory and observations of patterns of Nfixation across major sectors of the land biosphere. A question remains as to why symbiotic N-fixing plants are more abundant in vast areas of the tropics than in many of the mature forests that seem to be nitrogen-limited in the temperate and boreal zones. Here we present a unifying framework for terrestrial Nfixation that can explain the geographic occurrence of Nfixers across diverse biomes and at the global scale. By examining trade-offs inherent in plant carbon, nitrogen and phosphorus capture, we find a clear advantage to symbiotic Nfixers in phosphorus-limited tropical savannas and lowland tropical forests. The ability of Nfixers to invest nitrogen into phosphorus acquisition seems vital to sustained Nfixation in phosphorus-limited tropical ecosystems. In contrast, modern-day temperatures seem to constrain Nfixation rates and N-fixing species from mature forests in the high latitudes. We propose that an analysis that couples biogeochemical cycling and biophysical mechanisms is sufficient to explain the principal geographical patterns of symbiotic Nfixation on land, thus providing a basis for predicting the response of nutrient-limited ecosystems to climate change and increasing atmospheric CO.
DOIURL [本文引用: 1]

Feather mosses in boreal forests form a dense ground-cover that is an important driver of both nutrient and carbon cycling. While moss growth is highly sensitive to moisture availability, little is known about how moss effects on nutrient and carbon cycling are affected by the dynamics of moisture input to the ecosystem. We experimentally investigated how rainfall regimes affected ecosystem processes driven by the dominant boreal feather moss Pleurozium schreberi by manipulating total moisture amount, frequency of moisture addition and moss presence/absence. Moisture treatments represented the range of rainfall conditions that occur in Swedish boreal forests as well as shifts in rainfall expected through climate change. We found that nitrogen (N) fixation by cyanobacteria in feather mosses (the main biological N input to boreal forests) was strongly influenced by both moisture amount and frequency, and their interaction; increased frequency had greater effects when amounts were higher. Within a given moisture amount, N fixation varied up to seven-fold depending on how that amount was distributed temporally. We also found that mosses promoted vascular litter decomposition rates, concentrations of litter nutrients, and active soil microbial biomass, and reduced N release into soil solution. These effects were usually strongest under low moisture amount and/or frequency, and revealed a buffering effect of mosses on the decomposer subsystem under moisture limitation. These results highlight that both the amount and temporal distribution of rainfall, determine the effect of feather mosses on ecosystem N input and the decomposer subsystem. They also emphasize the role of feather mosses in mediating moisture effects on decomposer processes. Finally, our results suggest that projected shifts in precipitation in the Swedish boreal forest through climate change will result in increased moss growth and N2 fixation but a reduced dependency of the decomposer subsystem on feather moss cover for moisture retention.
[本文引用: 1]
DOIURL [本文引用: 2]

1. Ecosystem retrogression occurs during the very long-term absence of major disturbances, and it is characterized by decreases in productivity, decomposition rates and nutrient availability. Ratios of total soil nitrogen (N) to phosphorus (P) also characteristically increase during retrogression, but the nature of N inputs to ecosystems undergoing retrogression has seldom been explored. 2. We studied a 5000-year-old chronosequence involving 30 islands that differed greatly in history of disturbance (wildfire through lightning strike), with increasing time since disturbance leading to ecosystem retrogression. For each island, we quantified N inputs through biological fixation by cyanobacteria hosted by each of two feather moss species that dominate the ground layer vegetation ( Pleurozium schreberi and Hylocomium splendens ), and compared these with N inputs through atmospheric deposition. 3. Both N 2 fixation per unit land area and fixation per unit moss mass increased significantly with increasing time since disturbance for both moss species. As retrogression progressed, the amount of total N input through biological fixation increased to levels comparable to that of input through atmospheric deposition. 4. Across the chronosequence, N has been accumulating in the humus layer at a rate of 100·8 kg ha 0908081 year 0908081 in the absence of fire during the past 5000 years. The added N input from biological fixation in this area of low atmospheric N deposition helps explain this relatively high rate of sequestration. 5. Our results show that, contrary to several claims in the literature, biological N 2 fixation is not only important in early-successional ecosystems but also in late-successional systems that have undergone retrogression. This fixation can contribute both to the elevated N : P ratios that occur during retrogression and to accumulation of N capital in the soil. However, much of this N may exist in forms that are relatively unavailable to co-existing plant species.
URL [本文引用: 1]

The net photosynthetic rate (Pn) of ),plant water content (PWC) and irradiance (Photosynthetically Active Radiation,PAR) in laboratory.The results showed that Pn was closely related to PAR,T and PWC.The response curve of Pn to PAR was a right angle hyperbola,and the parameters were affected by T and PWC.The Pn reduction was great when PWC was low and T was high,or when PWC was high and T was low.The peak Pn (P) was observed underweak light (PAR·m) and relatively low (50%~80%) PWC.Under higher T,P was higher,but decreased when T was higher than 25 ℃.The PWC range within which P occurred increased in accordance with the increase of PAR.When PAR was lower than 200 μmol·s·m,the T range within which P occurred was relatively high (20~25 ℃),and P increased with the increase of PWC.When PWC was higher than 80%,P decreased with the increase of PWC.The T range within which P occurred decreased in accordance with the increase of PAR.Under moderate light (230·m),the sensitivity of Pn response to T and PWC was higher than that under higher or lower light.The Pn response to T and PWC was saddle-like,and its parameters were altered according to the variation in PAR.
URL [本文引用: 1]

The net photosynthetic rate (Pn) of ),plant water content (PWC) and irradiance (Photosynthetically Active Radiation,PAR) in laboratory.The results showed that Pn was closely related to PAR,T and PWC.The response curve of Pn to PAR was a right angle hyperbola,and the parameters were affected by T and PWC.The Pn reduction was great when PWC was low and T was high,or when PWC was high and T was low.The peak Pn (P) was observed underweak light (PAR·m) and relatively low (50%~80%) PWC.Under higher T,P was higher,but decreased when T was higher than 25 ℃.The PWC range within which P occurred increased in accordance with the increase of PAR.When PAR was lower than 200 μmol·s·m,the T range within which P occurred was relatively high (20~25 ℃),and P increased with the increase of PWC.When PWC was higher than 80%,P decreased with the increase of PWC.The T range within which P occurred decreased in accordance with the increase of PAR.Under moderate light (230·m),the sensitivity of Pn response to T and PWC was higher than that under higher or lower light.The Pn response to T and PWC was saddle-like,and its parameters were altered according to the variation in PAR.
DOIURL [本文引用: 1]

Symbiotic cyanobacteria—bryophyte associations on the forest floor are shown to contribute significantly to stand-level nitrogen budgets through the process of biological nitrogen fixation (BNF), but few studies have considered the role of canopy bryophytes. Given the high biomass of epiphytic bryophytes in many tree species of the North American temperate rain forest, we suggest that canopy bryophytes may contribute substantially to stand-level N dynamics. We confirm the presence of cyanobacteria and measure rates of BNF at three heights (0, 15 and 3002m) in Sitka spruce trees across three watershed estuaries of Clayoquot Sound, British Columbia, Canada. This study is the first to report BNF by cyanobacteria associated with epiphytic and forest floor bryophytes in the coastal temperate rain forest of North America. Cyanobacteria density was significantly greater in epiphytic bryophytes compared to mosses on the forest floor, and rates of BNF were highest at 3002m in the canopy. The majority of total stand-level BNF (0.7602kg02N · ha -1 · yr -1 ) occurs in the canopy, rather than on the forest floor (0.2602kg02N · ha -1 · yr -1 ). We suggest that BNF by cyanobacterial-bryophyte associations in the canopy of coastal temperate rain forests is a unique source of ecosystem N, which is dependent on large, old trees with high epiphytic bryophyte biomass.
DOIURL [本文引用: 1]

引入并应用斑块概念对大渡河上游藓类-冷杉原始林下地表1 hm2面积的锦丝藓、大羽藓、赤茎藓、塔藓、锦丝-赤茎藓等五种地表苔藓斑块结构特征作了调查分析,并同时测定了斑块相关环境因子(空气温度、空气湿度、 基质湿度、光照强度、乔木盖度、灌木盖度、草本盖度及生物量、凋落物盖度).对数据进行综合分析发现,不同斑块类型在生物量、个体密度、层片厚度和物种丰 富度上都表现出一定的差异性,所调查环境因素各个方面在五种斑块之间都表现出较强的差异性,且各类斑块的结构特征与环境因子间分别表现出很强的相关性.影 响斑块结构特征的最主要因素是藓丛表面空气温度,空气湿度、光照强度和草本层盖度、灌木层的盖度等也对斑块特征产生重要影响,而草本层生物量、凋落物盖度 及乔木层盖度仅对个别斑块的结构特征产生影响,基质湿度并未对斑块特征产生显著影响,可能是因为不同藓类生长基质不同所致.
DOIURL [本文引用: 1]

引入并应用斑块概念对大渡河上游藓类-冷杉原始林下地表1 hm2面积的锦丝藓、大羽藓、赤茎藓、塔藓、锦丝-赤茎藓等五种地表苔藓斑块结构特征作了调查分析,并同时测定了斑块相关环境因子(空气温度、空气湿度、 基质湿度、光照强度、乔木盖度、灌木盖度、草本盖度及生物量、凋落物盖度).对数据进行综合分析发现,不同斑块类型在生物量、个体密度、层片厚度和物种丰 富度上都表现出一定的差异性,所调查环境因素各个方面在五种斑块之间都表现出较强的差异性,且各类斑块的结构特征与环境因子间分别表现出很强的相关性.影 响斑块结构特征的最主要因素是藓丛表面空气温度,空气湿度、光照强度和草本层盖度、灌木层的盖度等也对斑块特征产生重要影响,而草本层生物量、凋落物盖度 及乔木层盖度仅对个别斑块的结构特征产生影响,基质湿度并未对斑块特征产生显著影响,可能是因为不同藓类生长基质不同所致.
DOIURL [本文引用: 1]

understory vegetation; species groups; driving mechanism; physical disturbance; clear-cut logging; Tibetan Plateau
[本文引用: 1]
[本文引用: 1]
DOIURLPMID

Traditionally it has been thought that most boreal forest communities lack a significant input of biologically fixed nitrogen. Recent discoveries of nitrogen fixation by cyanobacteria associated with mosses have resulted in a re-evaluation of this view. While it is recognized that rates of nitrogen fixation in mosses can be highly variable, there is little understanding as to why this occurs. I monitored nitrogen fixation, using acetylene reduction, in wet lowland and dry upland boreal forest communities, in central Canada, over a growing season. At the peak of nitrogen fixation in mid summer, Sphagnum capillifolium had an 11 times higher rate of fixation than Pleurozium schreberi. Variation in canopy openness and precipitation had no effect on rates of fixation over the growing season. In P. schreberi fixation rates did not vary between sites. Temperature had a positive effect on fixation rates in both S. capillifolium and P. schreberi, but the effect was 4 times more pronounced in S. capillifolium. Seasonal rates of nitrogen fixation were estimated at 193 mg N m6305 for S. capillifolium and 23 mg N m6305 for P. schreberi. With moderate increases in climate warming, predicted increases in nitrogen fixation in S. capillifolium are sufficient to raise its decomposition rate. Increased temperatures may therefore act synergistically to change boreal systems from a sink to a source of carbon.
[本文引用: 5]
Vol. 8.
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]

Nitrogen (N) availability is thought to frequently limit terrestrial ecosystem processes, and explicit consideration of N biogeochemistry, including biological N2 fixation, is central to understanding ecosystem responses to environmental change. Yet, the importance of free-living N2 fixation—a process that occurs on a wide variety of substrates, is nearly ubiquitous in terrestrial ecosystems, and may often represent the dominant pathway for acquiring newly available N—is often underappreciated. Here, we draw from studies that investigate free-living N2 fixation from functional, physiological, genetic, and ecological perspectives. We show that recent research and analytical advances have generated a wealth of new information that provides novel insight into the ecology of N2 fixation as well as raises new questions and priorities for future work. These priorities include a need to better integrate free-living N2 fixation into conceptual and analytical evaluations of the N cycle's role in a variety of global change scenarios.
[本文引用: 3]
DOIURL [本文引用: 1]

An investigation on biological nitrogen fixation in the forest floor was carried out in five watershed areas in Central Sweden during the summers 1978 and 1979. Five different types of bottom layers with underlying soil horizons were investigated for nitrogen fixation. An inventory on the occurrence of potential nitrogen fixing lichens and alder trees was also carried out. Low fixation rates were determined in mesic-soil mosses, F/H,layers and mineral soil. Though two orders of magnitude higher than in mesic-soil mosses, the nitrogen fixation in the investigated Sphagnum mosses must be considered as low, probably due to absence of blue-green algal associations. The contribution to total nitrogen fixation by lichens is low, but Alnus incana may play an important role. The estimated biological nitrogen fixation calculated as a weighted mean over the five watersheds is in the order of 0.5 kg N ha -1 yr -1 .
DOIURLPMID [本文引用: 1]

Abstract Biological nitrogen fixation (BNF) performed by moss-associated cyanobacteria is one of the main sources of new nitrogen (N) input in pristine, high-latitude ecosystems. Yet, the nutrients that limit BNF remain elusive. Here, we tested whether this important ecosystem function is limited by the availability of molybdenum (Mo), phosphorus (P), or both. BNF in dominant mosses was measured with the acetylene reduction assay (ARA) at different time intervals following Mo and P additions, in both laboratory microcosms with mosses from a boreal spruce forest and field plots in subarctic tundra. We further used a (15) N2 tracer technique to assess the ARA to N2 fixation conversion ratios at our subarctic site. BNF was up to four-fold higher shortly after the addition of Mo, in both the laboratory and field experiments. A similar positive response to Mo was found in moss colonizing cyanobacterial biomass. As the growing season progressed, nitrogenase activity became progressively more P limited. The ARA : (15) N2 ratios increased with increasing Mo additions. These findings show that N2 fixation activity as well as cyanobacterial biomass in dominant feather mosses from boreal forests and subarctic tundra are limited by Mo availability.
DOIURLPMID [本文引用: 3]

Abstract Cyanobacteria-plant symbioses play an important role in many ecosystems due to the fixation of atmospheric nitrogen (N) by the cyanobacterial symbiont. The ubiquitous feather moss Pleurozium schreberi (Brid.) Mitt. is colonized by cyanobacteria in boreal systems with low N deposition. Here, cyanobacteria fix substantial amounts of N090202 and represent a potential N source. The feather moss appears to be resistant to decomposition, which could be partly a result of toxins produced by cyanobacteria. To assess how cyanobacteria modulated the toxicity of moss, we measured inhibition of bacterial growth. Moss with varying numbers of cyanobacteria was added to soil bacteria to test the inhibition of their growth using the thymidine incorporation technique. Moss could universally inhibit bacterial growth, but moss toxicity did not increase with N090202 fixation rates (numbers of cyanobacteria). Instead, we see evidence for a negative relationship between moss toxicity to bacteria and N090202 fixation, which could be related to the ecological mechanisms that govern the cyanobacteria-moss relationship. We conclude that cyanobacteria associated with moss do not contribute to the resistance to decomposition of moss, and from our results emerges the question as to what type of relationship the moss and cyanobacteria share.
DOIURL [本文引用: 1]

Background and aims The feather moss Pleurozium schreberi (Brid.) Mitt. is colonized by cyanobacteria, which fix substantial amounts of atmospheric nitrogen (N) in pristine and N-poor ecosystems. Cyanobacterial N 2 fixation is inhibited by N deposition. However, the threshold of N input that leads to the inhibition of N 2 fixation has not been adequately investigated. Further, the ability of N 2 fixation to recover in mosses from high N deposition areas has not been studied to date. Methods We conducted two laboratory studies in which we (1) applied a range of concentrations of N as NH 4 NO 3 to mosses from low N-deposition areas, and (2) we deprived mosses from a high N-deposition area of N to test their ability to recover N 2 fixation. Results Higher addition rates (up to 1002kg02N02ha 611 ) did not systematically inhibit N 2 fixation in P. schreberi . Conversely, upon weeks of N deprivation of mosses from a high N environment, N 2 fixation rates increased. Conclusions The threshold of total N deposition above which N 2 fixation in P. schreberi is inhibited is likely to be > 1002kg02N02ha 611 . Further, cyanobacteria are able to recover from high N inputs and are able to fix atmospheric N 2 after a period of N deprivation.
DOIURLPMID [本文引用: 1]

The biological fixation of atmospheric nitrogen (N) is a major pathway for available N entering ecosystems. In N-limited boreal forests, a significant amount of N2is fixed by cyanobacteria living in association with mosses, contributing up to 50% to the total N input. In this review, we synthesize reports on the drivers of N2fixation in feather moss-cyanobacteria associations to gain a deeper understanding of their role for ecosystem-N-cycling. Nitrogen fixation in moss-cyanobacteria associations is inhibited by N inputs and therefore, significant fixation occurs only in low N-deposition areas. While it has been shown that artificial N additions in the laboratory as well as in the field inhibit N2fixation in moss-cyanobacteria associations, the type, as well as the amounts of N that enters the system, affect N2fixation differently. Another major driver of N2fixation is the moisture status of the cyanobacteria-hosting moss, wherein moist conditions promote N2fixation. Mosses experience large fluctuations in their hydrological status, undergoing significant natural drying and rewetting cycles over the course of only a few hours, especially in summer, which likely compromises the N input to the system via N2fixation. Perhaps the most central question, however, that remains unanswered is the fate of the fixed N2in mosses. The cyanobacteria are likely to leak N, but whether this N is transferred to the soil and if so, at which rates and timescales, is unknown. Despite our increasing understanding of the drivers of N2fixation, the role moss-cyanobacteria associations play in ecosystem-N-cycling remains unresolved. Further, the relationship mosses and cyanobacteria share is unknown to date and warrants further investigation.
DOIURL [本文引用: 1]

Background and aims Nitrogen (N2) fixation in feather moss-cyanobacteria associations is a major source of N for boreal ecosystems. However, mosses experience significant shifts in their moisture...
DOIURLPMID [本文引用: 1]

Abstract Nitrogen (N2) fixation is a major source of available N in ecosystems that receive low amounts of atmospheric N deposition. In boreal forest and subarctic tundra, the feather moss Hylocomium splendens is colonized by N2 fixing cyanobacteria that could contribute fundamentally to increase the N pool in these ecosystems. However, N2 fixation in mosses is inhibited by N input. Although this has been shown previously, the ability of N2 fixation to grow less sensitive towards repeated, increased N inputs remains unknown. Here, we tested if N2 fixation in H. splendens can recover from increased N input depending on the N load (0, 5, 20, 80, 320 kg N ha-1 yr-1) after a period of N deprivation, and if sensitivity towards increased N input can decrease after repeated N additions. Nitrogen fixation in the moss was inhibited by the highest N addition, but was promoted by adding 5 kg N ha-1 yr-1, and increased in all treatments during a short period of N deprivation. The sensitivity of N2 fixation towards repeated N additions seem to decrease in the 20 and 80 kg N additions, but increased in the highest N addition (320 kg N ha-1 yr-1). Recovery of N in leachate samples increased with increasing N loads, suggesting low retention capabilities of mosses if N input is above 5 kg N ha-1 yr-1. Our results demonstrate that the sensitivity towards repeated N additions is likely to decrease if N input does not exceed a certain threshold.
DOIURL [本文引用: 1]

Nitrogen (N) fixation in moss-cyanobacteria associations is one of the main sources of ‘new’ N in pristine ecosystems like subarctic and arctic tundra. This fundamental ecosystem process is driven by
DOIURL [本文引用: 2]

Feather mosses utilize various sources of nitrogen (N): they absorb N deposited on leaf tissue, they host N-2 fixing cyanobacteria, and they are able to take up N directly from soil. In addition to their importance as primary producers in boreal ecosystems, feather mosses play a significant role in N cycling. However, estimates of their ability to take up N from soil in situ are scarce. Further, connecting uptake of N from soil with N-2 fixation could significantly improve our understanding of their role in ecosystem N cycling, but to date this issue has not been addressed. We report results from an uptake experiment in which we tracked C-13-carbon (C), N-15-alanine and N-15-ammonium chloride (NH4Cl) into feather moss (Pleurozium schreberi (Brid.) Mitt.)-soil cores taken along natural fertility gradients in Northern Sweden. The varying fertility conditions coincided with a N-2 fixation gradient in the feather moss. We found that P. schreberi takes up C and N directly from soil. However, the moss did not show a preference for inorganic or organic N sources and only 1.4% of the added amino acid appeared to be taken up from soil in an intact form. No differences in uptake of C or N from soil along the fertility gradients were detected. Nitrogen fixation rates in the moss were thus not correlated with C or N-uptake from soil. Nitrogen fixation as well as uptake of C and N from soil seem to be unaffected by C or N availability in the soil, suggesting that the moss can cover its nutrient demand by absorption of throughfall N and via associated N-2-fixing cyanobacteria without soil-N supplementation. We suggest further, that the moss can represent a (temporary) N-sink in the boreal forest, and that the moss' mechanism of uptake and release thereby will characterize the ecosystem N cycle. (C) 2013 Elsevier Ltd. All rights reserved.
DOIURL [本文引用: 2]

Nitrogen (N) fixation is the main source of ‘new’ N for N-limited ecosystems like subarctic and arctic tundra. This crucial ecosystem function is performed by a wide range of N2fixer (diazotroph)...
DOIURL

Background and aims Nitrogen (N2) fixation by moss-associated bacteria is an important N source in boreal peatlands and forests. Here we studied whether moss species, water table fluctuations,...
DOIURLPMID

Acetylene reduction (AR) rates by cyanobacteria epiphytic on a moss at Marion Island (46 degrees 54' S, 37 degrees 45' E) increased from -5 degrees C to a maximum at 25 to 27 degrees C. Q(10) values between 0 and 25 degrees C were between 2.3 and 2.9, depending on photosynthetic photon flux density. AR rates declined sharply at temperatures above the optimum and were lower at 35 degrees C than at 0 degrees C. Photosynthetic photon flux density at low levels markedly influenced AR, and half of the maximum rate occurred at 84 mumol m s, saturation occurring at ca. 1,000 mumol m s. Higher photosynthetic photon flux density levels decreased AR rates. AR increased up to the highest sample moisture content investigated (3,405%), and the pH optimum was between 5.9 and 6.2. The addition of P, Co, and Mo, individually or together, depressed AR.
DOIURL [本文引用: 1]

Abstract Nitrogen (N) availability is the main constraint on primary production in most Arctic ecosystems, with microbial fixation of atmospheric N as the primary source of N input. However, there are only few reports on N fixation rates in relation to climate change in the Arctic. In order to investigate the effects of anticipated global climate change on N fixation rates in a subarctic moist heath, a field experiment was carried out in Northern Sweden. Warming was induced by plastic tents, and in order to simulate the effects of future increased tree cover, birch litter was added each fall for 9 years before the measurements. We analyzed N fixation rates on both whole-ecosystem level and specifically on two moss species: Sphagnum warnstorfii and Hylocomium splendens . The whole-ecosystem N fixation of the warmed plots almost tripled compared with the control plots. However, in the Sphagnum and Hylocomium mosses we observed either no change or occasionally even a decrease in N fixation after warming. Both measured on whole-ecosystem level and on the two moss species separately, litter addition increased N fixation rates. The results suggest that warming will lead to a general increased ecosystem N input, but also that the N fixation associated to some moss species is likely to decrease. Hence, this study shows that the scale of measurements is crucial when investigating on ecosystem responses to manipulations.
DOIURL [本文引用: 1]

Nitrogen inputs via biological N 2 -fixation are important in arctic environments where N often limits plant productivity. An understanding of the direct and indirect theoretical causal relationships between key intercorrelated variables that drive the process of N 2 -fixation is essential to understanding N input. An exploratory multi-group Structural Equation Modeling (SEM) approach was used to examine the direct and indirect effects of soil moisture, plant community functional composition, and bryophyte and lichen abundance on rates of nitrogen fixation at a low arctic ecosystem, two high arctic oases and a high arctic polar desert in the Canadian Arctic. Increasing soil moisture was strongly associated with an increasing presence of bryophytes and increasing bryophyte abundance was a major factor determining higher N 2 -fixation rates at all sites. Shrubs had a negative effect on bryophyte abundance at all sites with the exception of the polar desert site at Alexandra Fjord highland. The importance of competition from vascular plants appears to be greater in more productive sites and may increase at lower latitudes. Moisture availability may have an indirect effect on ecosystem development by affecting N input into the system with bryophyte-cyanobacterial associations playing an important intermediary role in the process.
[本文引用: 1]
DOIURL [本文引用: 3]

Pleurocarpous feather mosses host di-nitrogen (N2) fixing cyanobacteria, and this association serves as an important source of N input to late-successional natural boreal forests. However, little is known about how forest management affects feather mosses and their associated N2-fixation rates, or how these rates change during post-logging stand development. We established a chronosequence of 32 forest stands used for commercial wood production to better understand how stand development after clear-cutting drives changes in biomass and N2-fixation rates of the two dominant feather mosses, Pleurozium schreberi and Hylocomium splendens. These stands included eight replicate stands of each of four stand types: (1) recently clear-cut and newly planted stands (CC, 4years); (2) pre-commercial thinning stands (PCT, 16years); (3) first thinning stands (T1, 34years); and (4) mature uncut forest (MF, 123years), all dominated by Pinus sylvestris. We found that clear-cutting did not reduce moss biomass relative to the uncut forest. Further, biomass of P. schreberi (but not of H. splendens) increased twofold from CC stands to PCT stands, and remained high throughout the T1 stands. Di-nitrogen fixation capacity, determined as the amount of N fixed per unit moss mass, was ca. six and three times larger in PCT stands compared to the other stand types for P. schreberi and H. splendens respectively. Correlation analyses showed that N2-fixation capacity associated with both moss species increased with increasing Empetrum hermaphroditum biomass, and that N2-fixation capacity of P. schreberi declined with increasing NH4+ availability. Further, correlation analysis showed that N2-fixation capacity of H. splendens declined with increasing tree biomass and decreasing light transmission. The total amount of N fixed at the stand level was highest in the PCT stands (1.0kgha/1year/1 of N), and was associated with both high moss biomass and high N2-fixation capacity. The contribution of N2-fixation to total N accrual per hectare during stand development was ca. 9%, and across the chronosequence N2 was fixed on average at rates of 0.4kgha/1year/1. Our results show that N2-fixation rates in feather moss communities were promoted by the conditions at the PCT stands approximately 16years after clear-cutting, while N2-fixation rates were lowest under conditions at the newly clear-cut and mature stands. Further, it suggests that mosses and associated N2-fixation can be important in maintaining a long-term N balance, and that this source of N input should be accounted for when modeling N balance in N-limited managed boreal forests.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]

1. We separated the effects of plant species controls on decomposition rates from environmental controls in northern peatlands using a full factorial, reciprocal transplant experiment of eight dominant bryophytes in four distinct peatland types in boreal Alberta, Canada. Standard fractionation techniques as well as compound-specific pyrolysis molecular beam mass spectrometry were used to identify a biochemical mechanism underlying any interspecific differences in decomposition rates. 2. We found that over a 3-year field incubation, individual moss species and not micro-environmental conditions controlled early stages of decomposition. Across species, Sphagnum mosses exhibited a trade-off in resource partitioning into metabolic and structural carbohydrates, a pattern that served as a strong predictor of litter decomposition. 3. Decomposition rates showed a negative co-variation between species and their microtopographic position, as species that live in hummocks decomposed slowly but hummock microhabitats themselves corresponded to rapid decomposition rates. By forming litter that degrades slowly, hummock mosses appear to promote the maintenance of macropore structure in surface peat hummocks that aid in water retention. 4. Synthesis . Many northern regions are experiencing rapid climate warming that is expected to accelerate the decomposition of large soil carbon pools stored within peatlands. However, our results suggest that some common peatland moss species form tissue that resists decomposition across a range of peatland environments, suggesting that moss resource allocation could stabilize peatland carbon losses under a changing climate.
DOIURL [本文引用: 1]

This synthesis is the final report from the International SCOPE Project on Nitrogen Transport and Transformations: A Regional and Global Analysis. SCOPE (the Scientific Committee on Problems of the Environment) authorized the Nitrogen Project because of the need to better understand how humans have altered nitrogen cycling globally and at the scale of large regions. The project has synthesized information through a series of workshops over the past 8 years, involving over 250 scientists from over 20 different nations. Papers in this volume explore the extent to which human activity has affected the nitrogen cycle in terrestrial regions and in the worlds oceans, and discuss the implications of accelerated nitrogen cycling for nature and society.
DOIURLPMID [本文引用: 3]

PCR amplification techniques were used to compare cyanobacterial symbionts from a cyanobacterium-bryophyte symbiosis and free-living cyanobacteria from the same field site. Thirty-one symbiotic cyanobacteria were isolated from the hornwort Phaeoceros sp. at several closely spaced locations, and 40 free-living cyanobacteria were isolated from the immediate vicinity of the same plants. One of the symbiotic isolates was a species of Calothrix, a genus not previously known to form bryophyte symbioses, and the remainder were Nostoc spp. Of the free-living strains, two were Calothrix spp., three were Chlorogloeopsis spp. and the rest were Nostoc spp. All of the symbiotic and all but one of the free-living strains were able to reconstitute the symbiosis with axenic cultures of both Phaeoceros and the liverwort Blasia sp. Axenic cyanobacterial strains were compared by DNA amplification using PCR with either short arbitrary primers or primers specific for the regions flanking the 16S-23S rRNA internal transcribed spacer. With one exception, the two techniques produced complementary results and confirmed for the first time that a diversity of symbiotic cyanobacteria infect Phaeoceros in the field. Symbionts from adjacent colonies were different as often as they were the same, showing that the same thallus could be infected with many different cyanobacterial strains. Strains found to be identical by the techniques employed here were often found as symbionts in different thalli at the same locale but were never found free-living. Only one of the free-living strains, and none of the symbiotic strains, was found at more than one sample site, implying a highly localized distribution of strains.
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 2]

The pleurocarpus feather moss, Hylocomium splendens, is one of two co-dominant moss species in boreal forest ecosystems and one of the most common mosses on earth, yet little is known regarding its capacity to host cyanobacterial associates and thus contribute total ecosystem N. In these studies, we evaluated the N-fixation potential of the H. splendens-cyanobacteria. association and contrasted the N-fixation activity with that of the putative N-fixing moss-cyanobacteria association of Pleurozium schreberi. Studies were conducted to: quantify N-fixation in H. splendens and P. schreberi in sites ranging from southern to northern Fennoscandia; assess N and P availability as drivers of N-fixation rates; contrast season-long N-fixation rates for both mosses; and characterize the cyanobacteria that colonize shoots of H. splendens. Nitrogenfixation rates were generally low at southern latitudes and higher at northern latitudes (64-69°N) potentially related to anthropogenic N deposition across this gradient. Nitrogen fixation in H. splendens appeared to be less sensitive to N deposition than P. schreberi. The season-long assessment of N-fixation rates at a mixed feather moss site in northern Sweden showed that H. splendens fixed a substantial quantity of N, but about 50% less total N compared to the contribution from P. schreberi. In total, both species provided 1.6 kg fixed N ha6301 year6301 . Interestingly, H. splendens demonstrated some what higher N-fixation rates at high fertility sites compared to P. schreberi. Nostoc spp. and Stigonema spp. were the primary cyanobacteria found to colonize H. splendens and P. schreberi. These results suggest that H. splendens with associated Nostoc or Stigonema communities contributes a significant quantity of N to boreal forest ecosystems, but the contribution is subordinate to that of P. schreberi at northern latitudes. Epiphytic cyanobacteria are likely a key factor determining the codominant presence of these two feather mosses across the boreal biome.
DOIURL [本文引用: 3]

There is little understanding of successional dynamics of N fixation in northern boreal forests. Recent evidence suggests that N fixation by cyanobacteria in association with the common feather moss Pleurozium schreberi contributes to a significant proportion of the total N economy. The purpose of the work herein was to determine how time since last fire influences N fixation rates in boreal forests. We evaluated seasonal N fixation rates on a total of 12 natural forest preserves varying in time since last fire (35-355 years). Each site was monitored for N fixation activity using a calibrated acetylene reduction assay. Nitrogen fixation rates were found to increase linearly with time since fire. This increase in N fixation with succession is likely a function of degree of colonization by cyanobacteria and site factors such as presence of available N. Surface applications of 4.5 kg N ha-1 yr-1as NH4NO3were found to eliminate N fixation while applications of P resulted in only a slight and temporary increase of N fixation rates. In contrast to common observation our findings suggest that N fixation in boreal forests becomes more important in late succession. Limited N availability in late succession is clearly one of the primary drivers of N fixation rates in boreal forest ecosystems. These findings may help to explain the origin of high rates of net N accumulation in soil unaccounted for at northern boreal sites.
DOIURLPMID [本文引用: 1]

Abstract We investigated the colonization and diversity of arbuscular mycorrhizal (AM) fungi associated with 24 moss species belonging to 16 families in China. AM fungal structures, i.e. spores, vesicles, hyphal coils (including intracellular hyphae), or intercellular nonseptate hyphae, were found in 21 moss species. AM fungal structures (vesicles, hyphal coils, and intercellular nonseptate hyphae) were present in tissues of 14 moss species, and spores and nonseptate hyphae on the surface of gametophytes occurred in 15 species. AM fungal structures were present in 11 of the 12 saxicolous moss species and in six of the ten terricolous moss species, but absent in two epixylous moss species. AM fungal structures were only observed in moss stem and leaf tissues, but not in rhizoids. A total of 15 AM fungal taxa were isolated based on trap culture with clover, using 13 moss species as inocula. Of these AM fungi, 11 belonged to Glomus, two to Acaulospora, one to Gigaspora, and one to Paraglomus. Our results suggest that AM fungal structures commonly occur in most mosses and that diverse AM fungi, particularly Glomus species, are associated with mosses.
URL [本文引用: 1]

对实验室分离荒漠生物结皮中的 具鞘微鞘藻〔Microcoleus vaginatus(Vauch.)Gom.〕、眼点伪枝藻(Scy-tonema ocellatum Lyngb)、地木耳(Nostoc commune Vauch.)3种蓝藻分别进行了培养,利用正交设计的方法研究了pH值、K+、Ca2+、Mg2+4种理化因子对其生长的影响,初步探讨了3种蓝藻在实 验室内生长所需的最适生理条件。研究结果表明,具鞘微鞘藻的最适生理条件:pH值为8,Ca2+浓度为3.54×10-5mol/L,Mg2+浓度为 5.1×10-5mol/L,K+浓度为1.345×10-5mol/L;眼点伪枝藻的最适生理条件为:pH值9,Ca2+浓度为 1.77×10-5mol/L,Mg2+浓度为5.1×10-5mol/L,K+浓度为2.69×10-5mol/L;地木耳的最适生理条件为:pH值为 10,K+浓度为5.38×10-5mol/L,Ca2+浓度为1.77×10-5mol/L,Mg2+浓度为1.02×10-4mol/L。
URL [本文引用: 1]

对实验室分离荒漠生物结皮中的 具鞘微鞘藻〔Microcoleus vaginatus(Vauch.)Gom.〕、眼点伪枝藻(Scy-tonema ocellatum Lyngb)、地木耳(Nostoc commune Vauch.)3种蓝藻分别进行了培养,利用正交设计的方法研究了pH值、K+、Ca2+、Mg2+4种理化因子对其生长的影响,初步探讨了3种蓝藻在实 验室内生长所需的最适生理条件。研究结果表明,具鞘微鞘藻的最适生理条件:pH值为8,Ca2+浓度为3.54×10-5mol/L,Mg2+浓度为 5.1×10-5mol/L,K+浓度为1.345×10-5mol/L;眼点伪枝藻的最适生理条件为:pH值9,Ca2+浓度为 1.77×10-5mol/L,Mg2+浓度为5.1×10-5mol/L,K+浓度为2.69×10-5mol/L;地木耳的最适生理条件为:pH值为 10,K+浓度为5.38×10-5mol/L,Ca2+浓度为1.77×10-5mol/L,Mg2+浓度为1.02×10-4mol/L。
DOIURL [本文引用: 1]

Abstract The influence of environmental factors on the nitrogen fixation activity in soil and vegetation samples from different types of plant communities from the Sassen Valley (78°N, 16°E), Svalbard, Norway, was measured under controlled laboratory conditions using the acetylene reduction assay throughout the summers of 1997 and 2000. Samples for study were chosen from six sites along a 2-km-long transect representing different types of arctic vegetation. The influence of temperature, soil water content, and light intensity on acetylene reduction rates was studied. Samples from all sites showed low and almost constant acetylene reduction rates between 0 and 10°C. Above 10°C the activity of all samples increased rapidly and reached its maximum at about 25 and 32°C for the samples with free-living cyanobacteria and moss-associated cyanobacteria, respectively. There was a significant water-dependent increase of acetylene reduction activity for all types of vegetation. The samples showed a clear response to varying light conditions, i.e. a rapid decrease in acetylene reduction rates when light intensity decreased from 140 to 80 μmol m -2 s -1 depending on the type of vegetation.
DOIURL

The course of nitrogen fixation by moss-associated cyanobacteria in Svalbard (78'N, 16'E), Norway, was studied using the acetylene reduction assay. In situ field measurements of nitrogen fixation activity were conducted in six different types of moss-dominated arctic vegetation from the beginning of the snowmelt in early June to the end of July 1998. Concurrently, the water content of the soil/vegetation layer was determined and correlated with the nitrogen fixation rates. At all sites with diminishing water content during the summer season, nitrogen fixation activity was positively correlated with the amount of available water in the vegetation. At two sites, where water content of the vegetation was constantly higher than 80% (w/w) throughout the season, nitrogen fixation activity was correlated with temperature. Depending on the type of vegetation, nitrogen fixation became limited when the water status fell below a minimum threshold level. The most desiccation-tolerant vegetation for nitrogen fixation activity was the cryptobiotic crust, where nitrogen fixation decreased only after the water content of the soil/vegetation was less than 50% of the its fresh weight, while in the other types of vegetation nitrogen fixation stopped when water content was around 60%. The results from the present study confirm that, in arctic regions with low precipitation during the growing season, nitrogen fixation in different types of vegetation is mostly limited either to the period of snowmelt when water is sufficiently available, or to habitats that stay wet during summer.
Cyanobacteria-bryophyte symbioses.
3
2008
... 生态系统净初级生产力常常受到可利用氮的限制(
... ), 其生物固氮作用主要靠固氮酶进行, 且固氮酶集中于异形胞(heterocyst)中, 所以氮固定常发生在蓝藻体内的异形胞中, 而异形胞的数量与蓝藻生境有密切关系(
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
Signalling in Cyanobacteria-?Plant Symbioses. Signaling and Communication in Plant Symbiosis
1
2012
... 有研究发现, 藓-藻共生体所固定的氮只有20%留存于藻类, 其他的转移至苔藓植物内(
Bryophyte-cyanobacteria associations during primary succession in recently deglaciated areas of Tierra del Fuego (Chile).
2
2014
... 角苔类在角苔属(Anthoceros)、树角苔属(Dendroceros)、短角苔属(Notothylas)和黄角苔属(Phaeoceros) 4个属的物种与蓝藻有共生固氮关系(
... 目前已知能与苔藓形成共生关系的固氮蓝藻有8属(
Nitrogen fixation in wet minerotrophic moss communities of a subarctic mire.
1978
Boreal feather mosses secrete chemical signals to gain nitrogen.
2
2013
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
... didymium.在亚高山针叶林和北方针叶林林下常见的藓类塔藓(Hylocomium splendens)和赤茎藓(Pleurozium schreberi)均具有与蓝藻共生固氮的能力, 但在波叶曲尾藓(Dicranum polysetum)和金发藓(Polytrichum commune)中却未发现共生固氮现象(
Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss.
1
2012
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
Dinitrogen fixation (acetylene reduction) in primary succession near Mount Robson, British Columbia, Canada.
1990
Ectomycorrhizas and nutrient transfer in conifer-feather moss ecosystems.
1
1991
... 转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用.(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(
Environment-?regulation of nitrogen-fixation in a high arctic lowland ecosystem.
1991
蓝藻Anabaena 7120固氮的光调节
1
1983
... 蓝藻固氮作用的过程对光具有依赖性.光可以通过光合作用影响其固氮过程中的电子供应链, 或是影响固氮必需的高能量物质的产生.蓝藻固氮酶的活性也受到光的调节, 不同的光照强度下固氮活性呈现相应的高低差异, 且光照可缓解因添加结合态氮对固氮作用的抑制效应(
蓝藻Anabaena 7120固氮的光调节
1
1983
... 蓝藻固氮作用的过程对光具有依赖性.光可以通过光合作用影响其固氮过程中的电子供应链, 或是影响固氮必需的高能量物质的产生.蓝藻固氮酶的活性也受到光的调节, 不同的光照强度下固氮活性呈现相应的高低差异, 且光照可缓解因添加结合态氮对固氮作用的抑制效应(
Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems.
1
1999
... 生态系统净初级生产力常常受到可利用氮的限制(
Comparative cryptogam ecology: A review of bryophyte and lichen traits that drive biogeochemistry.
1
2007
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
Nutrient release from epiphytic bryophytes in tropical montane rain forest (Guadeloupe).
2
1991
... 2011).目前对苔藓植物体内的氮转移方式除菌根真菌方式外, 还有受到干扰(如火烧)后氮释放至土壤中, 但速率很慢(
... 转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用.(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(
Seasonal variation in nitrogen fixation by Nostoc commune Vaucher at the Vestfold Hills, Antarctica.
1
1983
... 目前已知能与苔藓形成共生关系的固氮蓝藻有8属(
Ecosystem feedbacks and nitrogen fixation in boreal forests.
1
2008
... 苔藓-蓝藻共生固氮能力的定量研究到目前为止仅聚焦于藓-藻共生体, 而缺乏角苔-藻与苔-藻共生体的报道.归纳已有的报道发现, 各苔藓-蓝藻共生体的固氮能力介于0.25-1.7 kg·hm-2·a-1, 如
Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden
1
2002a
... 苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(
Ecosystem controls on nitrogen fixation in boreal feather moss communities.
1
2007
... 苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(
Quantifying nitrogen-fixation in feather moss carpets of boreal forest.
4
2002b
... 生态系统净初级生产力常常受到可利用氮的限制(
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
... 附生型的共生关系多见于地钱属(Marchantia)、光萼苔属(Porella)、裂萼苔属(Chiloscyphu)、毛叶苔属(Ptilidium)和挺叶苔属(Anastrophyllum)的苔-藻共生体以及各种藓-藻共生体中(
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
Effects of nitrogen additions on biomass, stoichiometry and nutrient pools of moss Rhytidium rugosum in a boreal forest in northeast China.
1
2014
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
Isotopic analysis of cyanobacterial nitrogen fixation associated with subarctic lichen and bryophyte species.
2010
Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria.
2
2005
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
... 在最适温度下, 提高光照强度会增加共生体固氮能力.Gentili等(2005)发现, 同在13 ℃时, 光照强度160 μmol·m-2·s-1下的共生体固氮能力大于光照强度为80 μmol·m-2·s-1时的固氮能力.目前已发现赤茎藓-蓝藻共生体的最适温度-光照综合值为 23 ℃、130 μmol·m-2·s-1 (
Nitrogen cycling and feedbacks in a global dynamic land model.
1
2010
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
Nitrogen fixation in tundra moss communities. Progress Report, Swedish IBP.
1973
Nitrogen fixation in a subarctic mire.
4
1973
... 目前已知能与苔藓形成共生关系的固氮蓝藻有8属(
... 苔藓和蓝藻的共生关系有2种: 蓝藻内生和附生于苔藓.角苔与蓝藻共生体均为内生型关系.在苔-蓝藻共生关系中, 蓝藻的内生位置至少有2种报道.一种是有关念珠藻属(Nostoc)物种生长于苔类配子体的耳廓(auricles)中(
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
... 已经发现环境pH值能显著影响藓-蓝藻共生关系及其固氮能力.当pH值为3.8时, 3种泥炭藓(Sphagnum balticum、锈色泥炭藓(S. fuscum)和垂枝泥炭藓(S. jensenii))完全没有藻类与之共生; 在pH值为4.2和4.9的环境中, S. lindebergii和岸生泥炭藓有藻类内生; 在pH值更高的环境中, 藻类附生或自由生长(
The impact of simulated chronic nitrogen deposition on the biomass and N2-fixation activity of two boreal feather moss-cyanobacteria associations.
3
2013
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
... ), 共生体的固氮能力相应下降(
... ).长期实验发现, 塔藓-蓝藻共生体的生物量和固氮能力受氮沉降影响的程度高于赤茎藓-蓝藻共生体(
Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests.
4
2011
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
... 之间时, 苔藓-蓝藻共生体的固氮速率出现不同程度的下降, 苔藓植物体表面附生的蓝藻数量也呈现相应的下降趋势(
... ;
The sensitivity of nitrogen fixation by a feathermoss-cyanobacteria association to litter and moisture variability in young and old boreal forests.
2
2009
... 苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制.因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大.水分增加能使苔藓-蓝藻共生体的固氮能力加强(
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
The interactive effects of temperature and light on biological nitrogen fixation in boreal forests.
3
2012a
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
... 在最适温度下, 提高光照强度会增加共生体固氮能力.Gentili等(2005)发现, 同在13 ℃时, 光照强度160 μmol·m-2·s-1下的共生体固氮能力大于光照强度为80 μmol·m-2·s-1时的固氮能力.目前已发现赤茎藓-蓝藻共生体的最适温度-光照综合值为 23 ℃、130 μmol·m-2·s-1 (
... 苔藓-蓝藻共生体关系及其固氮能力对环境(气候)变化十分敏感, 逐渐成为全球气候变化研究的重要对象(
The effect of altered macroclimate on N-fixation by boreal feather mosses.
2
2012b
... 苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制.因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大.水分增加能使苔藓-蓝藻共生体的固氮能力加强(
... ., 2013d), 可见水对蓝藻的固氮能力影响很大, 所以水是苔藓-蓝藻共生体固氮过程中的关键因子(
Nitrogen fixation of epiphytic plants enwrapping trees in Ailao Mountain cloud forests, Yunnan, China.
2
2010
... 苔藓群落组成与生物量在森林的空间差异及其随森林发育演替的变化是明显的, 必然引起苔藓-蓝藻共生体关系及其固氮能力的变化.明确这样的时空变化特点与规律十分必要.Lindo和Whiteley (2011)发现, 在海岸温带雨林中, 地表层为生态系统提供的有效氮较冠层附生苔藓-蓝藻共生体少.从该森林中30、15 m冠层与地表的苔藓-蓝藻共生体固氮量和总的蓝藻(伪枝藻属)细胞数目的比较都显示, 冠层的藻类数目最多, 固氮量最高, 而地表的氮固定量最低(
... 苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(
Identification of two genera of N2-fixing cyanobacteria growing on three feather moss species in boreal forests of Quebec, Canada.
3
2006
... 角苔类在角苔属(Anthoceros)、树角苔属(Dendroceros)、短角苔属(Notothylas)和黄角苔属(Phaeoceros) 4个属的物种与蓝藻有共生固氮关系(
... 目前已知能与苔藓形成共生关系的固氮蓝藻有8属(
... 苔藓和蓝藻的共生关系有2种: 蓝藻内生和附生于苔藓.角苔与蓝藻共生体均为内生型关系.在苔-蓝藻共生关系中, 蓝藻的内生位置至少有2种报道.一种是有关念珠藻属(Nostoc)物种生长于苔类配子体的耳廓(auricles)中(
A unifying framework for dinitrogen fixation in the terrestrial biosphere.
1
2008
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
Response of feather moss associated N2 fixation and litter decomposition to variations in simulated rainfall intensity and frequency.
1
2011
... 苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制.因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大.水分增加能使苔藓-蓝藻共生体的固氮能力加强(
Changes in vegetation and soil characteristics in coastal sand dunes along a gradient of atmospheric nitrogen deposition.
1
2004
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
Ecosystem input of nitrogen through biological ?xation in feather mosses during ecosystem retrogression.
2
2007
... 苔藓-蓝藻共生固氮能力的定量研究到目前为止仅聚焦于藓-藻共生体, 而缺乏角苔-藻与苔-藻共生体的报道.归纳已有的报道发现, 各苔藓-蓝藻共生体的固氮能力介于0.25-1.7 kg·hm-2·a-1, 如
... 苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(
不同含水量下尖叶拟船叶藓光合速率对光温的响应及其模型
1
2004
... 苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制.因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大.水分增加能使苔藓-蓝藻共生体的固氮能力加强(
不同含水量下尖叶拟船叶藓光合速率对光温的响应及其模型
1
2004
... 苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制.因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大.水分增加能使苔藓-蓝藻共生体的固氮能力加强(
Old trees contribute bio-available nitrogen through canopy bryophytes.
1
2011
... 苔藓群落组成与生物量在森林的空间差异及其随森林发育演替的变化是明显的, 必然引起苔藓-蓝藻共生体关系及其固氮能力的变化.明确这样的时空变化特点与规律十分必要.Lindo和Whiteley (2011)发现, 在海岸温带雨林中, 地表层为生态系统提供的有效氮较冠层附生苔藓-蓝藻共生体少.从该森林中30、15 m冠层与地表的苔藓-蓝藻共生体固氮量和总的蓝藻(伪枝藻属)细胞数目的比较都显示, 冠层的藻类数目最多, 固氮量最高, 而地表的氮固定量最低(
青藏高原东部原始林下地表主要苔藓斑块特征及其影响因素
1
2005
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
青藏高原东部原始林下地表主要苔藓斑块特征及其影响因素
1
2005
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
Understory plant assemblages present distinct short-term responses to the clear-cutting of an old-growth spruce forest near an alpine timberline.
1
2014
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
2种蓝藻的生长对不同环境pH的响应
1
2007
... 已经发现环境pH值能显著影响藓-蓝藻共生关系及其固氮能力.当pH值为3.8时, 3种泥炭藓(Sphagnum balticum、锈色泥炭藓(S. fuscum)和垂枝泥炭藓(S. jensenii))完全没有藻类与之共生; 在pH值为4.2和4.9的环境中, S. lindebergii和岸生泥炭藓有藻类内生; 在pH值更高的环境中, 藻类附生或自由生长(
2种蓝藻的生长对不同环境pH的响应
1
2007
... 已经发现环境pH值能显著影响藓-蓝藻共生关系及其固氮能力.当pH值为3.8时, 3种泥炭藓(Sphagnum balticum、锈色泥炭藓(S. fuscum)和垂枝泥炭藓(S. jensenii))完全没有藻类与之共生; 在pH值为4.2和4.9的环境中, S. lindebergii和岸生泥炭藓有藻类内生; 在pH值更高的环境中, 藻类附生或自由生长(
Variation in moss-associated nitrogen fixation in boreal forest stands.
2009
Cyanobacterial-Bryophyte Associations
5
1990
... 生态系统净初级生产力常常受到可利用氮的限制(
... 角苔类在角苔属(Anthoceros)、树角苔属(Dendroceros)、短角苔属(Notothylas)和黄角苔属(Phaeoceros) 4个属的物种与蓝藻有共生固氮关系(
... ;
... 苔藓和蓝藻的共生关系有2种: 蓝藻内生和附生于苔藓.角苔与蓝藻共生体均为内生型关系.在苔-蓝藻共生关系中, 蓝藻的内生位置至少有2种报道.一种是有关念珠藻属(Nostoc)物种生长于苔类配子体的耳廓(auricles)中(
... ), 另一种是生长于配子体腹面的黏液腔(slime cavities)中(
Physiological adaptations in nitrogen-fixing Nostoc-plant symbiotic associations. In: Pawlowski K ed. Prokaryotic symbionts in plants. Microbiology Monographs
2
2007
... 生态系统净初级生产力常常受到可利用氮的限制(
... 有研究发现, 藓-藻共生体所固定的氮只有20%留存于藻类, 其他的转移至苔藓植物内(
Patterns of bryophyte diversity in humid coastal and inland cedar-hemlock forests of British Columbia.
2
2003
... 苔藓群落组成与生物量在森林的空间差异及其随森林发育演替的变化是明显的, 必然引起苔藓-蓝藻共生体关系及其固氮能力的变化.明确这样的时空变化特点与规律十分必要.Lindo和Whiteley (2011)发现, 在海岸温带雨林中, 地表层为生态系统提供的有效氮较冠层附生苔藓-蓝藻共生体少.从该森林中30、15 m冠层与地表的苔藓-蓝藻共生体固氮量和总的蓝藻(伪枝藻属)细胞数目的比较都显示, 冠层的藻类数目最多, 固氮量最高, 而地表的氮固定量最低(
... 苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(
Patterns of desiccation tolerance and recovery in bryophytes.
1
2001
... 苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制.因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大.水分增加能使苔藓-蓝藻共生体的固氮能力加强(
Cyanobacteria in Symbiosis
1
2002
... 2011).目前对苔藓植物体内的氮转移方式除菌根真菌方式外, 还有受到干扰(如火烧)后氮释放至土壤中, 但速率很慢(
Cyanobacterium-plant symbioses.
2
2000
... 生态系统净初级生产力常常受到可利用氮的限制(
... ;
Nitrogen fixation by moss-algal associations in grassland.
2
1981
... 目前已知能与苔藓形成共生关系的固氮蓝藻有8属(
... 附生型的共生关系多见于地钱属(Marchantia)、光萼苔属(Porella)、裂萼苔属(Chiloscyphu)、毛叶苔属(Ptilidium)和挺叶苔属(Anastrophyllum)的苔-藻共生体以及各种藓-藻共生体中(
Functional ecology of free-living nitrogen fixation: A contemporary perspective
1
2011
... 生态系统净初级生产力常常受到可利用氮的限制(
Generic assignments, strain histories and properties of pure cultures of cyanobacteria.
3
1979
... 角苔类在角苔属(Anthoceros)、树角苔属(Dendroceros)、短角苔属(Notothylas)和黄角苔属(Phaeoceros) 4个属的物种与蓝藻有共生固氮关系(
... 目前已知能与苔藓形成共生关系的固氮蓝藻有8属(
... 苔藓和蓝藻的共生关系有2种: 蓝藻内生和附生于苔藓.角苔与蓝藻共生体均为内生型关系.在苔-蓝藻共生关系中, 蓝藻的内生位置至少有2种报道.一种是有关念珠藻属(Nostoc)物种生长于苔类配子体的耳廓(auricles)中(
Biological nitrogen fixation in coniferous forest watershed areas in central Sweden.
1
1980
... 苔藓-蓝藻共生固氮能力的定量研究到目前为止仅聚焦于藓-藻共生体, 而缺乏角苔-藻与苔-藻共生体的报道.归纳已有的报道发现, 各苔藓-蓝藻共生体的固氮能力介于0.25-1.7 kg·hm-2·a-1, 如
Molybdenum and phosphorus limitation of moss- associated nitrogen fixation in boreal ecosystems.
1
2017a
... 苔藓-蓝藻共生体对磷的响应, 或者说磷对苔藓-蓝藻共生体固氮能力的影响, 目前还难有确切定论, 相关结果仍然是矛盾的.Chapin等(1991)发现, 添加磷时北极柳(Salix arctica)环境下的苔藓体固氮能力均增加, 塔藓和赤茎藓-蓝藻固氮能力亦增加(
The cyanobacterial role in the resistance of feather mosses to decomposition—?Toward a new hypothesis.
3
2013a
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
... 时, 赤茎藓共生体的固氮作用并没有受到限制(
... 苔藓-蓝藻共生体关系显然受到形成共生体的苔藓与蓝藻种类的影响, 可以推测也受其他可能影响其微生境与关系的生物类群特性如乔木层类群、灌草类群的影响.已发现, 由于蓝藻自身的固氮能力差异及其对环境适应能力不同, 苔藓-蓝藻共生体的固氮能力有差异(
Exposure to nitrogen does not eliminate N2 fixation in the feather moss Pleurozium schreberi (Brid.) Mitt.
1
2013b
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems.
1
2013c
... 2011).目前对苔藓植物体内的氮转移方式除菌根真菌方式外, 还有受到干扰(如火烧)后氮释放至土壤中, 但速率很慢(
The resilience of nitrogen fixation in feather moss (Pleurozium schreberi)- cyanobacteria associations after a drying and rewetting cycle.
1
2013d
... 苔藓与蓝藻均是变水植物, 对环境中的水分变化十分敏感, 各自均在失水-复水过程中形成了独特的变水适应机制.因此, 水分对苔藓-蓝藻共生体关系及其固氮能力的影响很大.水分增加能使苔藓-蓝藻共生体的固氮能力加强(
The sensitivity of moss- associated nitrogen fixation towards repeated nitrogen input.
1
2016
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
The interactive effects of temperature and moisture on nitrogen fixation in two temperate-arctic mosses.
1
2017b
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
Feather moss nitrogen acquisition across natural fertility gradients in boreal forests.
2
2013e
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
... ,
Nitrogen transfer from four nitrogen-fixer associations to plants and soils.
2
2016f
... 有研究发现, 藓-藻共生体所固定的氮只有20%留存于藻类, 其他的转移至苔藓植物内(
... 2011).目前对苔藓植物体内的氮转移方式除菌根真菌方式外, 还有受到干扰(如火烧)后氮释放至土壤中, 但速率很慢(
Nitrogen fixation in Sphagnum mosses is affected by moss species and water table level.
2015
Effects of abiotic factors on acetylene reduction by cyanobacteria epiphytic on moss at a subantarctic island.
1984
Long-term warming and litter addition affects nitrogen fixation in a subarctic heath.
1
2011
... 在最适温度下, 提高光照强度会增加共生体固氮能力.Gentili等(2005)发现, 同在13 ℃时, 光照强度160 μmol·m-2·s-1下的共生体固氮能力大于光照强度为80 μmol·m-2·s-1时的固氮能力.目前已发现赤茎藓-蓝藻共生体的最适温度-光照综合值为 23 ℃、130 μmol·m-2·s-1 (
Nitrogen inputs by associative cyanobacteria across a low arctic tundra landscape.
2011a
Bryophyte-cyanobacterial associations as a key factor in N2-fixation across the Canadian Arctic.
1
2011b
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
1
1966
... 生态系统净初级生产力常常受到可利用氮的限制(
Nitrogen fixation rates associated with the feather mosses Pleurozium schreberi and Hylocomium splendens during forest stand development following clear-cutting.
3
2015
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
... 苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(
... ); 也有人发现, 林木生物量的增加和林下透光率的减少使苔藓-蓝藻共生体(塔藓-蓝藻)的固氮能力下降(
不同类型生物土壤结皮固氮活性及对环境因子的响应研究
1
2011
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
不同类型生物土壤结皮固氮活性及对环境因子的响应研究
1
2011
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
Nitrogen in Terrestrial Ecosystems. Springer
1
1991
... 生态系统净初级生产力常常受到可利用氮的限制(
Trade-offs in resource allocation among moss species control decomposition in boreal peatlands.
1
2008
... 转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用.(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(
The Nitrogen Cycle at Regional to Global Scales.
1
2002
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
Phenotypic and genotypic comparison of symbiotic and free-living cyanobacteria from a single field site.
3
1997
... 角苔类在角苔属(Anthoceros)、树角苔属(Dendroceros)、短角苔属(Notothylas)和黄角苔属(Phaeoceros) 4个属的物种与蓝藻有共生固氮关系(
... 目前已知能与苔藓形成共生关系的固氮蓝藻有8属(
... 苔藓和蓝藻的共生关系有2种: 蓝藻内生和附生于苔藓.角苔与蓝藻共生体均为内生型关系.在苔-蓝藻共生关系中, 蓝藻的内生位置至少有2种报道.一种是有关念珠藻属(Nostoc)物种生长于苔类配子体的耳廓(auricles)中(
Carbon flux in a subalpine spruce-fir forest: Pulse release from Hylocomium splendens feather-moss mats.
3
1999
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
... 2011).目前对苔藓植物体内的氮转移方式除菌根真菌方式外, 还有受到干扰(如火烧)后氮释放至土壤中, 但速率很慢(
... 转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用.(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(
川西高山林线交错带凋落叶分解速率与初始质量的关系
1
2015
... 转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用.(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(
川西高山林线交错带凋落叶分解速率与初始质量的关系
1
2015
... 转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用.(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(
Nitrogen fixation in mixed Hylocomium splendens moss communities.
2
2009
... 目前有4个实验揭示了大气氮沉降对藓-蓝藻共生体固氮能力的影响.氮沉降量的增加能减少苔藓生物量(
... 苔藓-蓝藻共生体关系显然受到形成共生体的苔藓与蓝藻种类的影响, 可以推测也受其他可能影响其微生境与关系的生物类群特性如乔木层类群、灌草类群的影响.已发现, 由于蓝藻自身的固氮能力差异及其对环境适应能力不同, 苔藓-蓝藻共生体的固氮能力有差异(
Nitrogen fixation increases with successional age in boreal forests.
3
2004
... 苔藓植物是亚高山针叶林和北方针叶林林下的主要层片(
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
... 苔藓-蓝藻共生体的固氮能力在森林演替的后期更高(
Arbuscular mycorrhizal structure and fungi associated with mosses.
1
2007
... 转移至苔藓植物体内的氮以及苔藓自身的氮可能通过以下3种途径进一步为其他植物所利用.(1)苔藓植物体中的养分将逐渐通过凋落物分解过程而释放(
不同理化因子对荒漠生物结皮中三种蓝藻生长的影响
1
2010
... 已经发现环境pH值能显著影响藓-蓝藻共生关系及其固氮能力.当pH值为3.8时, 3种泥炭藓(Sphagnum balticum、锈色泥炭藓(S. fuscum)和垂枝泥炭藓(S. jensenii))完全没有藻类与之共生; 在pH值为4.2和4.9的环境中, S. lindebergii和岸生泥炭藓有藻类内生; 在pH值更高的环境中, 藻类附生或自由生长(
不同理化因子对荒漠生物结皮中三种蓝藻生长的影响
1
2010
... 已经发现环境pH值能显著影响藓-蓝藻共生关系及其固氮能力.当pH值为3.8时, 3种泥炭藓(Sphagnum balticum、锈色泥炭藓(S. fuscum)和垂枝泥炭藓(S. jensenii))完全没有藻类与之共生; 在pH值为4.2和4.9的环境中, S. lindebergii和岸生泥炭藓有藻类内生; 在pH值更高的环境中, 藻类附生或自由生长(
The influence of abiotic factors on biological nitrogen fixation in different types of vegetation in the high Arctic, Svalbard.
1
2002
... 两种大镰刀藓(Drepanocladus exannulatus、D. trichophyllus)和岸生泥炭藓的固氮共生体在11- 16 ℃时具有最大的固氮能力(
Nitrogen fixation in the high arctic: Role of vegetation and environmental conditions.
2005
