
Mesophyll conductance and its limiting factors in plant leaves
HANJi-Mei
通讯作者:
版权声明:2017植物生态学报编辑部本文是遵循CCAL协议的开放存取期刊,引用请务必标明出处。
基金资助:
展开
摘要
关键词:
Abstract
Keywords:
-->0
PDF (1598KB)元数据多维度评价相关文章收藏文章
本文引用格式导出EndNoteRisBibtex收藏本文-->
光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程。CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化。早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素。基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(Farquhar et al., 1980)。随后的一些研究发现, 植物的gm并不是无穷大(Evans et al., 1986; von Caemmerer & Evans, 1991; Harley et al., 1992; Evans & von Caemmerer, 1996)。不同植物的gm存在差异, 而且gm也会随环境的变化而做出适应性的调整(Flexas et al., 2008, 2012)。研究表明, gm对温度(Bernacchi et al., 2002; Flexas & Diaz-Espejo, 2015)、蓝光强度(Loreto et al., 2009)、水分亏缺(Flexas et al., 2002; Miyazawa et al., 2008; Han et al., 2016)等外界环境条件的改变均会做出相应的响应。因此, gm被认为是除气孔限制和羧化限制外的光合效率第三个限制因素, 并认为与气孔导度(gs)同等重要(Flexas et al., 2012)。通常, 依据FvCB光合模型的CO2响应曲线(AN-Ci曲线)拟合方法, 不考虑gm的影响, 往往导致拟合出的光合参数存在偏差。因此, 有研究从考虑gm影响的角度, 对FvCB光合模型AN-Ci曲线拟合方法进行了修正, 例如Ethier和Livingston (2004)对Farquhar等(1980)的光合模型的非直角双曲线模型进行优化, 对AN-Ci曲线进行拟合得到gm, 这种方法降低了光合参数对gm的敏感度。Sharkey等(2007)介绍了一种非线性曲线拟合方法对AN-Ci曲线进行拟合。以上拟合方法均假设gm不受Ci影响且光系统II中光吸收系数为常数。为避免以上问题, Moualeu-Ngangue等(2016)重新提出了一种AN-Ci曲线拟合方法。除AN-Ci曲线拟合gm之外, 气体交换和叶绿素荧光同步测定法与气体交换和同位素同步测定法也可估算gm, 但3种方法均存在一定的缺陷。一般而言, 外界环境通过影响叶片内部物理和(或)生化因素进而影响gm。同时, 由于gm的变化只影响叶片内CO2的运输而不涉及水分散失, 因此有研究提出, gm是实现植物叶片光合速率和水分利用效率同步提高的生理位点。
1 叶片结构和生化因素对叶肉导度的影响
在组织细胞水平, gm的影响因素主要包括结构因素和生化因素。在结构层面, CO2在叶肉细胞中的扩散依次经过细胞间隙、细胞壁、细胞膜、细胞质、叶绿体膜和叶绿体基质等(图1, 图2)。CO2在这些扩散过程中会受到层层阻碍, 从而影响gm。在生化层面, 在叶片传输过程中CO2可以被碳酸酐酶(CA)催化转变成HCO3-进行扩散, 同时水孔蛋白(AQPs)也可以介导CO2的跨膜运输。实际上, 生化层面和结构层面两者密不可分, AQPs存在于生物膜上, CA主要存在于叶绿体基质中。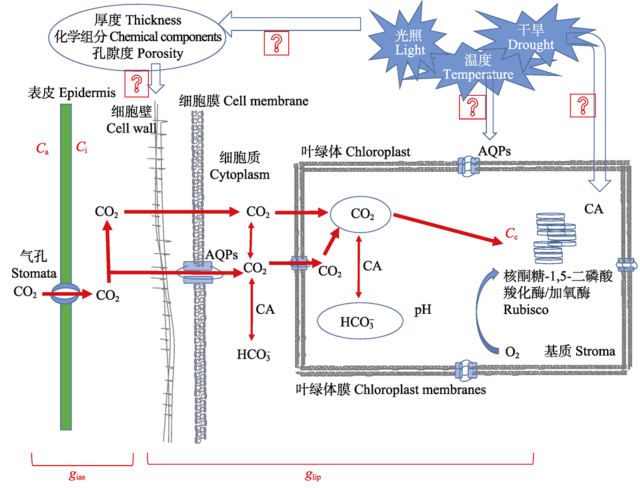 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1CO2运输模式图。AQPs, 水孔蛋白; Ca, 大气CO2浓度; Ci, 胞间CO2浓度; CA, 碳酸酐酶; gias, 气相导度; glip, 液相导度。
-->Fig. 1CO2 transport model. AQPs, aquaporins; Ca, the atmospheric CO2 concentration; Ci, intercellular CO2 concentration; CA, carbonic anhydrase; gias, the gas phase conductance; glip, the liquid phase conductance.
-->
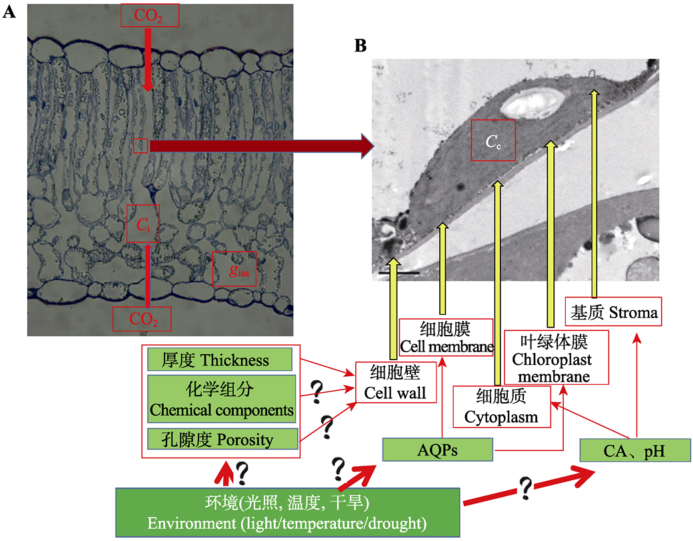 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图2gm反映的CO2扩散路径。A, 光学显微镜拍摄的棉花叶片解剖结构图,代表的是CO2从外界大气进入叶片细胞间隙,完成气相传输。B, 电子显微镜拍摄的棉花叶片超微结构图。代表的是CO2从细胞间隙进入叶绿体羧化位点所经过的部位,完成液相传输。图中简单介绍了影响传输路径的因素及需要进一步深入研究的问题。AQPs, 水孔蛋白; Ci, 胞间CO2浓度; Cc, 叶绿体羧化位点CO2浓度; CA, 碳酸酐酶, gias, 液相导度。
-->Fig. 2The diffusion path of CO2 reflected by gm. A, The leaf anatomical structure in cotton by optical microscope, which represents the CO2 gas phase diffusion from the atmosphere into the leaf intercellular air layer; B, The leaf ultra-micro structure in cotton by electron microscope, which represents the CO2 liquid phase diffusion from intercellular into the chloroplast carboxylation site. AQPs, aquaporins; Ci, intercellular CO2 concentration; Cc, CO2 concentration at chloroplast carboxylation site; CA, carbonic anhydrase; gias, the gas phase conductance.
-->
1.1 结构层面的限制
有研究表明, gm主要由叶片解剖结构决定(Niinemets et al., 2009; Tosens et al., 2012a; Tomás et al., 2013)。大量研究认为面向细胞间隙的叶绿体面积(Sc)与叶片总面积(S)的比值(Sc/S) (von Caemmerer & Evans, 1991; Evans et al., 1994; Pakatas et al., 2003; Flexas et al., 2012)和细胞壁厚度(Hanba et al., 1999, 2002; Terashima et al., 2011)是影响gm的主要结构因素。有研究用一维气体扩散模型定量分析不同组织细胞结构对CO2扩散的重要程度(Niinemets & Reichstein, 2003a; Tosens et al., 2012a; Tomás et al., 2013)。gm被分为气相导度(gias)和液相导度(gliq)两个部分。gias代表CO2从气孔下腔到细胞壁周围扩散阻力的倒数, gliq代表CO2从细胞壁周围到叶绿体内羧化位点扩散阻力的倒数。
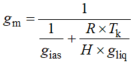
H是亨利定律常数(m3·mol-1·K-1), R为气体常数(Pa·m3·K-1·mol-1), Tk是绝对温度(K)。(R × Tk)/(H × gliq)是亨利定律常数的无因次形式, 可以将气相导度转换为等价的液相导度(Niinemets & Reichstein, 2003b)。
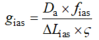
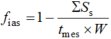
Da (m2·s-1) 是气相中CO2扩散系数(25 ℃下是 1.51 × 10-5), fias (m3·m-3)是细胞间隙体积与叶肉总体积的比值。ΣSs代表叶肉细胞横切面面积; tmes代表上下表皮之间叶肉细胞的厚度; W是切片宽度; ΔLias被近似为叶肉厚度的一半; ζ代表扩散路径弯曲度, 用叶片横切面和纵切面进行估算(Terashima et al., 1995), 一般用常数1.57 mm-1 (Syvertsen et al., 1995; Niinemets & Reichstein, 2003a)。
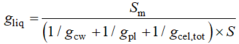
Sm/S代表单位叶片面积内面向细胞间隙的叶肉细胞面积; gcw是细胞壁导度, gpl是质膜导度, gcel,tot是细胞
内部导度。三者通用的表达方式gi计算公式为:
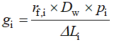
Pi是扩散路径有效孔隙度, 对于胞液和基质用常数1, 细胞壁的孔隙度用常数0.05 (Terashima et al., 2006), Dw是CO2液相扩散系数(1.790 × 10-9 m2·s-1, 25 ℃), rf.i是无量纲因子, 用来表示与CO2在水中的自由扩散相比, 液相扩散导度的下降。针对gcw, rf.i用常数1; ΔLi是指扩散路径长度。其中计算gcel,tot时, 扩散路径较为复杂, 一般将CO2从细胞膜下进入到叶绿体分为两个路径: 一是CO2从细胞质沿垂直细胞膜的方向进入叶绿体; 二是细胞质中的CO2可沿平行细胞膜的方向进入叶绿体侧面(Terashima et al., 2005; Tomás et al., 2013)。gpl和gen (叶绿体膜导度)不适用于此公式, 一般用常数0.003β5 m·s-1 (Evans et al., 1994)表示。
由以上公式可知, 气相扩散路径弯曲度、细胞壁扩散路径有效孔隙度、液相扩散系数等均采用常数。除此之外, 扩散路径的长度, 如细胞壁厚度、叶绿体大小和位置等均对gm的计算有重要影响。尽管气相扩散涉及垂直扩散路径和侧向扩散路径, 但有研究报道气相扩散相对于液相扩散可以忽略不计。同时, 结构量化时假设生物膜限制为常数, 实际上生物膜的限制也是影响gm的不可忽视的因素。Tomás等(2014)认为影响gm的主要因素随物种特性的变化而变化, 例如在肉质植株中影响gm最重要的因素是生物膜通透性、细胞液和叶绿体基质等生化层面的因素; 而在硬叶植株中细胞壁厚度是限制gm的主要因素。
1.1.1 细胞壁
影响gm的细胞壁特性主要为厚度和孔隙度。一般认为细胞壁的厚度与gm呈反比, 扩散路径越大, 厚度越厚, gm越小。研究证实, 细胞壁的厚度与gm确实存在负相关关系(Terashima et al., 2011)。在硬叶植物中gm的限制主要来自于细胞壁厚度的限制(Evans et al., 2009)。同时, 细胞壁越厚的叶片, 单位Sc下的Rubisco含量越少, 叶绿体越小, 光合速率也就越低(Evans et al., 2009)。Tosens等(2012b)认为不仅细胞壁厚度影响着gm, 细胞孔隙度也与gm关系密切。一般认为细胞壁孔隙度越大, gm越大。研究证实, 物种间细胞壁孔隙度随细胞壁厚度的变化而变化(Terashima et al., 2006; Evans et al., 2009; Tosens et al., 2012b)。Tosens等(2012b)利用最小二乘迭代分析法对细胞壁孔隙度进行分析, 结果显示, 细胞壁厚度由0.252 μm到0.420 μm变化的物种, 相对应的细胞孔隙度由0.095变化到0.040。细胞壁的主要成分包括纤维素和半纤维素, 研究细胞壁物理结构和化学组分的相互作用对于理解gm的变化机制至关重要(Flexas & Diaz-Espejo, 2015)。然而, 两者如何受外界因素的影响以及如何相互作用来影响CO2的传输尚未有细致的研究报道。
1.1.2 生物膜
生物膜对脂溶性小分子具有透过性, CO2是亲脂性分子, 能够经过磷脂双分子层进行快速穿膜扩散。早先一直认为CO2在生物膜上的扩散速率非常大, 磷脂双分子层对CO2的阻碍(脂相阻碍)可以忽略不计。然而对水孔蛋白(AQPs)的研究表明, 它不仅介导H2O的跨膜运输, 对CO2的跨膜运输也起着重要作用。Terashima等(2006)认为CO2的穿膜过程主要通过两种途径: 磷脂双分子层和膜上内在蛋白。然而, 不同研究对CO2在生物膜上的通透系数的估测值相差甚大。Missner等(2008)估测膜的通透性是(3.2 ± 1.6) cm·s-1, 认为影响CO2跨膜运输的主要因素是生物膜边界层厚度, 与AQPs关系不大; 而Boron等(2011)在排除AQPs的功能后估测细胞膜通透性为0.015 cm·s-1, 从而认为, AQPs通过影响CO2在生物膜上的运输来调控gm。Uehlein等(2008)证实AQPs基因的沉默降低了叶绿体内CO2的浓度; 而当超表达AQPs时, gm得到了改善, 从而提高了光合速率(Sade et al., 2014)。另外, AQPs也可以通过影响gs从而影响光合作用(Hanba et al., 2004; Flexas et al., 2006b; Heckwolf et al., 2011)。
1.1.3 基质
CO2从细胞膜扩散到叶绿体膜需要经过细胞质, CO2在细胞质中的扩散路径越长, CO2所受到的阻碍越大。通常, 叶绿体会沿细胞膜排列(Sage & Sage, 2009), 这有助于缩短CO2在细胞质中的扩散路径, 从而减少扩散阻碍。同时, CO2进入到叶绿体后还会受到叶绿体基质的阻力, 叶绿体越大, CO2从叶绿体基质扩散到羧化位点的路径越短, 阻力越小, gm越大。面向细胞间隙的叶绿体面积(Sc)是接受CO2的主要部位, 但部分CO2也会通过叶绿体之间的空隙进入到细胞质, 之后在叶绿体侧面扩散进入叶绿体, 但此扩散路径长于垂直进入叶绿体的扩散路径, 不利于CO2的快速扩散和光合速率的改善。
综上所述, 尽管大量证据表明解剖结构对gm的影响极其重要, 但各部分结构因素(例如细胞壁)影响gm的机理并不清楚, 仍需要进一步深入的探讨。
1.2 生化层面的限制
1.2.1 碳酸酐酶CA可以通过催化CO2和HCO3-之间的可逆转换来调节细胞中的pH变化, 促进CO2的传输。近年来, 关于CA对gm产生影响的报道较多, 但结论迥异。Price等(1994)和Williams等(1996)研究发现在CA活性非常低的突变体植株中, 植株光合能力的差异并不大, 认为CA对光合作用无限制作用。但也有作者认为CA的活性具有物种依赖性, 如硬叶植株中CA往往能发挥很大的作用(Gillon & Yakir, 2000)。
Jia和Davies (2007)报道非原质体内pH值为5.5- 6.0, 可能CA不会影响CO2从细胞壁到细胞膜的传输; 细胞膜和叶绿体膜仅对CO2有通透性作用, 对HCO3-具有不透过性; 同时, 细胞质中的pH值小于叶绿体基质中的pH值, Evans等(2009)认为CA不会催化细胞质中CO2和HCO3-的可逆转化。因此, CA可能并未参与CO2在细胞壁、细胞膜、叶绿体膜和细胞质中的扩散过程。研究证实, 植株中CA主要存在于叶绿体基质中(Evans et al., 2009)。CO2不断穿膜进入叶绿体羧化位点, 随着羧化速率的进行, 叶绿体基质内的pH值逐渐升高, HCO3-也逐渐增多, 从而与细胞质中形成了CO2浓度差, 加速了CO2的跨膜扩散; 在此过程中CA通过催化CO2和HCO3-的可逆反应来调节叶绿体内的pH值, 同时加速CO2向叶绿体羧化酶活性部位的扩散, 维持羧化酶周围CO2的浓度以保证其不会随同化过程的进行而降低, 从而保持一定的光合速率(Tholen & Zhu, 2011)。也许这可以证明CA通过加强叶绿体内CO2的扩散来影响gm。然而, 正常生理pH值下, HCO3-的浓度是CO2浓度的85倍左右, HCO3-决定了细胞内无机碳的扩散速率, 但羧化酶的底物是CO2而不是HCO3-。这暗示CO2的扩散量不足以满足CO2固定的需求(Evans et al., 2009)。Tholen和Zhu (2011)通过建立的三维模型估算CA的量, 认为基质中的CA并不够多, 尽管CA调节了CO2和HCO3-的可逆转换, 但是在基质中还是扮演着限制角色。研究CA催化CO2和HCO3-转换的位点和条件对于阐明CA对gm的影响极其重要。
1.2.2 水孔蛋白
Hub和de Groot (2006, 2008)基于分子模型的模拟试验表明, CO2穿过水孔蛋白单体消耗的能量多于CO2直接通过磷脂双分子层的运输能量, 这似乎并不合理。然而, 水孔蛋白家族包括大量点突变的同系物, 不同同系物对CO2的通透性存在差异, 可能存在的点突变可以减少运输CO2所消耗的能量(Hub & de Groot, 2008)。另有研究表明, 水孔蛋白四聚物形成的孔隙介导CO2和H2O的运输, 单体并不会介导CO2的跨膜运输(Otto et al., 2010)。四聚物形成的孔隙运输CO2消耗的能量比单体低。Flexas等(2012)认为, 形成水孔蛋白四聚物的同系物之间存在竞争, 以此来调节水孔蛋白运输H2O和CO2的功能。
水孔蛋白家族根据序列同源性可以分为5类: 质膜内在蛋白(PIPs)、液泡膜内在蛋白(TIPs)、类Nod26膜内在蛋白(NIPs)、小分子碱性膜内在蛋白(SIPs)以及类GlpF膜内在蛋白(GIPs)(Kelly et al., 2014)。研究表明, 大部分PIPs和TIPs均属于选择性通道蛋白, 但TIPs位于液泡膜上, 只有PIPs位于质膜上, 并且既可以运输H2O, 又可以运输CO2。PIPs又分为PIP1和PIP2两个亚类。Otto等(2010)认为PIP1具有转运CO2的功能, PIP2只具有转运H2O的功能; 但是Hanba等(2004)的研究表明PIP2同样介导CO2的转运。AQPs是PIP1家族的成员。研究表明, 在AQPs非特异性抑制剂HgCl2处理的植株中, gm显著下降(Terashima & Ono, 2002)。而且AQPs在细胞水平和整株植株水平均可发挥作用, 提高植株蒸腾速率和净光合速率(Sade et al., 2010)。Perez-Martin等(2014)的研究表明AQPs的表达对干旱条件下油橄榄(Olea europaea)的光合速率具有一定的影响。然而, AQPs对gm的调节机制并不清楚。因此, 研究AQPs如何调控CO2的传输过程对于理解AQPs对gm的调节机理具有重要意义。
2 外界环境对gm的影响
研究表明, 与gs相似, gm对外界环境(水分、温度、光照、氮、CO2浓度等)的响应也具有敏感性。2.1 水分亏缺对gm的影响
水分亏缺是限制植物生长和作物产量的主要环境因素。研究表明, 水分亏缺条件下光合速率下降的根本原因是从外界大气到叶绿体内羧化位点CO2扩散速率的下降(Flexas et al., 2002; Galmés et al., 2007)。水分亏缺条件下gm的降低(Flexas et al., 2004, 2006a; Galmés et al., 2006, 2007)与gs一样是限制光合作用的主要因素。水分亏缺导致gm降低的因素主要包括结构因素(降低Sc、增加细胞壁厚度)和生化因素(AQPs活性的降低、CA活性的改变)。这些因素的变化抑制了水分亏缺条件下叶片组织内部的CO2传输。2.2 温度对gm的影响
Yamori等(2006)的研究表明, 在30和15 ℃下生长的菠菜(Spinacia oleracea)叶片中, gm达到峰值所对应的温度分别是25和20 ℃; 在田间空气温度(波动范围7-32 ℃)下生长的油橄榄叶片gm的最适温度是29 ℃ (Diaz-Espejo et al., 2007); 在5 ℃环境下生长的甘蓝(Brassica oleracea)叶片, 其gm峰值所对应的温度可以达到18 ℃ (Flexas et al., 2008)。研究表明, 纯水中CO2的温度系数Q10 (温度每变化 10 ℃, CO2扩散速率的变化)是1.25, 但在烟草中CO2的Q10达到2.2 (Bernacchi et al., 2002)。以上研究表明, gm对温度的响应可能与酶促反应密切相关。CA和AQPs的化学性质均属于蛋白质, 两者的活性与温度密切相关, 温度可能通过影响CA和AQPs的活性进而对gm产生影响。另外, 温度的提高也会提高蒸气压差, 从而降低细胞的水势和膨压, 引起Sc的下降。2.3 光照强度对gm的影响
研究表明, 光照强度的改变可以调控gm的大小(Flexas et al., 2007)。阴生植物的gm比阳生植物的低(Hanba et al., 2002; Piel et al., 2002; Laisk et al., 2005; Warren et al., 2007)。Hassiotou等(2009)对Banksia的研究发现, 低光强下发育的叶片gm比高光强下平均低20%。光照强度也许通过改变叶绿体的位置来影响Sc的大小, 从而改变gm (Tholen et al., 2008; Boex-Fontvieille et al., 2014)。大量研究还报道了短期改变光照强度和光质对gm的影响。研究发现gm对蓝光反应迅速, 而叶绿体位置不可能瞬间变化, 说明除了光照强度对Sc的影响外, 也许还存在其他的机制影响gm (Loreto et al., 2009)。尽管Tholen等(2012)认为gm对光强的快速响应是由于gm计算过程中忽略了叶绿体中由(光)呼吸作用产生的CO2量所导致的假象。然而, Théroux-Rancourt和Gilbert (2017)通过多层次叶片模型和解剖观察表明, gm对光强存在明显响应。因此, 光照强度影响gm的具体机制还有待深入研究。2.4 氮对gm的影响
植物单位叶面积的氮含量与光合能力具有显著的正相关关系, 这主要是由于氮含量增加会提高核酮糖-1,5-双磷酸羧化/加氧酶(Rubisco)的含量, 以及可以增加CO2扩散导度(包括gs和gm) (Warren, 2004; Li et al., 2009; Yamori et al., 2011)。研究认为, 氮可以通过改变叶片结构来调控gm的大小。高氮条件下Rubisco含量的增加势必会造成叶绿体体积的增大(Li et al., 2009), 从而导致Sc的增加, 并最终影响gm。Xiong等(2015b)对水稻(Oryza sativa)的研究也表明Sc对氮营养的响应较敏感。氮含量也可能通过调控叶片相关的基因表达来影响gm的大小。Clarkson等(2000)认为氮含量的提高会增加PIP2水孔蛋白基因家族的表达量; Hanba等(2004)认为PIP2;1水孔蛋白的超表达可以提高gm。然而, 直至目前, 尚未发现氮含量通过调控AQPs改变gm的直接证据。另外, 虽然有研究发现水稻、小麦(Triticum aestivum)、菠菜等C3植物(Makino et al., 1992)和玉米(Zea mays)等C4植物(Burnell et al., 1990)的CA活性均会受到氮的影响, 但CA对gm的调控还一直存在争议。因此, 基因表达活性蛋白对氮的响应从而对gm的调控机理尚需进一步研究。3 gm的估算方法
目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(Bongi & Loreto, 1989; Harley et al., 1992; Loreto et al., 1992)、气体交换和同位素同步测定法(Evans et al., 1986; von Caemmerer & Evans, 1991)和AN-Ci曲线拟合法(Ethier & Livingston, 2004; Sharkey et al., 2007)。研究表明gm的估算会受到众多因素的影响, 例如细胞间隙CO2浓度(Ci)(Flexas et al., 2007)、光照强度(Flexas et al., 2007; Tholen et al., 2008)等因素。尽管大量研究者致力于gm估算方法的研究与改善, 但现有方法均无法准确估算出gm的绝对数值(Flexas et al., 2008; Warren, 2008)。如Tholen等(2012)认为gm的估算忽略了扩散到叶绿体中由线粒体(光)呼吸作用产生的CO2浓度。而且, 有研究者认为影响gm的因素均是估算方法缺陷导致的假象, 例如Gu和Sun (2013)认为Ci只是gm估算公式中的输入参数, Ci及其他与之协同变化的输入参数的测量误差均会导致gm对Ci响应的假象。近几年, 受限于估算叶肉导度方法的各种缺陷, 叶肉导度研究领域进展相对缓慢。因此, 完善和改良gm的计算和测定方法将是深入推进叶肉导度研究的重要突破口。4 水力导度与gm的关系
CO2是植物进行光合作用的原料, 而H2O是生物体的组成物质, 是进行一切生命活动的必需物质和养分运输的媒介。因此, CO2和H2O在植物中的传输关系备受关注。水力导度(Kleaf)和gm分别是衡量植物叶片内H2O和CO2运输的两个重要变量, 是决定气体交换速率和光合性能的主要指标(Flexas et al., 2013)。虽然Kleaf和gm一直是研究的热点, 但很少有研究探讨两者之间的协同关系(Flexas et al., 2013)。Kleaf可以反应H2O在叶片内传输的效率问题, H2O从叶柄贯穿到叶片的木质部, 然后到达叶脉周围的维管束, 最后到达蒸发位点从而扩散到空气中(Sack & Holbrook, 2006)。通常, Kleaf分为木质部导度(Kxylem)和木质部外导度(Kout-xylem), 在叶脉内的水分传输称为Kxylem, 在叶脉外组织内的传输称为Kout-xylem。Flexas等(2013)研究认为, gm与Kleaf具有相关性, 并且主要与Kout-xylem相关。Kout-xylem对整个Kleaf的影响较大, 但所占比例会随着物种和试验条件的变化而变化(Cochard et al., 2004; Sack et al., 2004)。
叶片组织内H2O和CO2有着共同的传输路径。面向细胞间隙的叶肉细胞面积(Sm)被认为是同时影响gm和Kleaf的因素(Xiong et al., 2016)。尽管相比于Sm, Sc的变化能更好地解释gm的变化(Terashima et al., 2005, 2006; Flexas et al., 2012), 但Sm的大小对gm的影响也是至关重要(Xiong et al., 2016)。同时, Xiong等(2015a)认为面向细胞间隙的叶肉细胞膜也是水分由液相转变成气相从而进行蒸发的位置, 因此Sm可以将两者在一定程度上联系起来。另外, 细胞壁的厚度和孔隙度不仅是影响gm的重要结构因素, 事实上也改变了木质部外水分扩散的路径, 从而影响了Kleaf。
同时, 细胞壁孔隙内存在的结合水也影响着水分的传输。事实上gm与Kleaf的相关性不仅取决于叶片的组织结构, 与生化因素(AQPs)也有着一定的关系(Flexas et al., 2012)。AQPs不仅介导H2O的运输, 也介导着CO2的运输。Otto等(2010)和Flexas等(2012)认为CO2和H2O在膜上的交换运输取决于两种不同的水孔蛋白四聚物(PIP1和PIP2)的比例。有研究证明Kleaf可以对外界环境进行响应, 如温度、光照和水分等(Sack & Holbrook, 2006)。gm和Kleaf均与结构、生化和外部环境有一定的关联, 间接表明两者间存在一定的关系, 但尚需进一步的直接证据。
5 研究展望
CO2在植物叶片细胞内的传输过程极其复杂(图1, 图2; 表1)。细胞水平上每个组分和环节均可能影响CO2的传输。在结构层面, 除了细胞壁厚度外, 开展细胞孔隙度及其化学组分对gm影响机制的研究有助于全面揭示gm与结构之间的关系; 其次叶绿体运动会改变Sc的大小从而影响gm, 那么, CO2传输对叶绿体位置是否具有一定的调节作用也需要进一步开展研究。在生化层面, 尽管大量研究认为水孔蛋白和碳酸酐酶会影响gm的大小, 但水孔蛋白和碳酸酐酶介导CO2传输的规律及其机制仍需深入研究。同时, 叶肉导度估算方法的不确定性, 严重阻碍了对叶肉导度的深入研究。因此, 测定方法的不断优化和改良也将是未来研究的重点。Table 1
表1
表1CO2通过叶肉细胞中各超微组分的扩散方式、运输形态、阻力来源、动力来源和对外界环境的响应时间等的差异
Table 1Diffusion way, transportation form, resistance source, power source when CO2 passes through the ultrastructure components of mesophyll cells and the different response time to the external environment
| CO2扩散方式 CO2 diffusion way | CO2运输形态 CO2 transportation form | 阻力来源 Resistance source | 动力来源 Power source | 对外界环境的响应时间 Response time to the external environment | |
|---|---|---|---|---|---|
| 细胞壁 Cell wall | 物理和生化方式 Physics and biochemical mode | CO2 | 厚度、孔隙度、果胶等组分 Thickness, porosity, pectin etc. | CO2浓度差 Difference of CO2 concentration | 最长 Longest |
| 细胞膜 Cell membrane | 物理和生化方式 Physics and biochemical mode | CO2 | 水孔蛋白、膜两侧pH差值 AQPs, the difference of pH on both sides of the membrane | CO2浓度差、跨膜蛋白主动运输 Difference of CO2 concentration, active transport of transmembrane protein | 较短 Shorter |
| 细胞液 Cytoplasm | 生化和物理方 Biochemical and physical mode | CO2, HCO3- | CA、pH、细胞液组分 CA, pH, cytosol component | pH、CA的催化 pH, catalysis of CA | 较短 Shorter |
| 叶绿体膜 Chloroplast membranes | 生化和物理方 Biochemical and physical mode | CO2 | 水孔蛋白、膜两侧CO2浓度差 AQPs, the difference of CO2 concentration on both sides of the membrane | 跨膜蛋白主动运输 Active transport of transmembrane protein | 较短 Shorter |
| 叶绿体基质 Stroma | 生化和物理方式 Biochemical and physical mode) | CO2, HCO3- | CA, pH | pH、CA的催化 pH, catalysis of CA | 最短 Shortest |
新窗口打开
The authors have declared that no competing interests exist.
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | ABSTRACT CO(2) transfer conductance from the intercellular airspaces of the leaf into the chloroplast, defined as mesophyll conductance (g(m)), is finite. Therefore, it will limit photosynthesis when CO(2) is not saturating, as in C3 leaves in the present atmosphere. Little is known about the processes that determine the magnitude of g(m). The process dominating g(m) is uncertain, though carbonic anhydrase, aquaporins, and the diffusivity of CO(2) in water have all been suggested. The response of g(m) to temperature (10 degrees C-40 degrees C) in mature leaves of tobacco (Nicotiana tabacum L. cv W38) was determined using measurements of leaf carbon dioxide and water vapor exchange, coupled with modulated chlorophyll fluorescence. These measurements revealed a temperature coefficient (Q(10)) of approximately 2.2 for g(m), suggesting control by a protein-facilitated process because the Q(10) for diffusion of CO(2) in water is about 1.25. Further, g(m) values are maximal at 35 degrees C to 37.5 degrees C, again suggesting a protein-facilitated process, but with a lower energy of deactivation than Rubisco. Using the temperature response of g(m) to calculate CO(2) at Rubisco, the kinetic parameters of Rubisco were calculated in vivo from 10 degrees C to 40 degrees C. Using these parameters, we determined the limitation imposed on photosynthesis by g(m). Despite an exponential rise with temperature, g(m) does not keep pace with increased capacity for CO(2) uptake at the site of Rubisco. The fraction of the total limitations to CO(2) uptake within the leaf attributable to g(m) rose from 0.10 at 10 degrees C to 0.22 at 40 degrees C. This shows that transfer of CO(2) from the intercellular air space to Rubisco is a very substantial limitation on photosynthesis, especially at high temperature. |
| [2] | Phototropin-dependent chloroplast movement is essential to the photosynthetic acclimation of mesophyll cells to incident light. Chloroplast movement involves many cellular actors, such as chloroplast- |
| [3] | The effects of two levels of salinity on photosynthetic properties of olive (Olea europea L.) leaves were observed either in low or in high H(2)O vapor pressure deficit (vpd). Under moderate salt stress, stomata were found to be less open and responsive both to light and vpd, but the predominant limitation of photosynthesis was due to the mesophyll capacity of CO(2) fixation. We elaborate a procedure to correlate mesophyll capacity and liquid phase diffusive conductance. The estimated liquid phase diffusive conductance was reduced by salt and especially by high vpd; morphological and physiological changes could be responsible for this reduction. As a result, the chloroplast CO(2) partial pressure was found to decrease both under salt and vpd stress, thus resulting in a ribulose-1,5-bisphosphate carboxylase limitation of assimilation. However, under combined salt and vpd stress, O(2) sensitivity of assimilation increased, as would be expected under conditions of limiting ribulose 1,5-bisphosphate regeneration. Fluorescence induction measurements indicated that, under these conditions, energy supply may become limiting. When Cl(-) concentration exceeded 80 millimolar in tissue water, zero growth and 50% leaf drop was observed. Fluorescence induction showed irreversible damage at Cl(-) levels higher than 200 millimolar and basal leaves reached this concentration earlier than the apical ones. |
| [4] | The past dozen years has seen a series of papers that come to the conclusion that CO2 passes through certain aquaporins and Rhesus proteins. The past three years has seen another series of papers that come to the conclusion that protein channels could not make a meaningful contribution to overall CO2 membrane permeability because of a combination of: 1) a high CO2 permeability of membrane lipids and 2) large unstirred layers, which would render their CO2 resistance much higher than that of a biological membrane. Is this also true for a membrane crowded with proteins? This comment summarizes the current status of the debate. |
| [5] | The regulation of carbonic anhydrase (CA) activity in maize (Zea mays L.) leaves by light and nitrogen nutrition was determined. CA activity increased by more than 100-fold in illuminated leaves and decreased in leaves placed in the dark; low levels of CA activity were observed in leaves illuminated with low light intensities. CA activity was reduced in plants grown under nitrogen deficiency and recovered only slowly when supplemented with nitrate. Parallel studies were conducted to follow the levels of phosphoenolpyruvate carboxylase. Experiments indicate that the level of CA and phosphoenolpyruvate carboxylase present in leaves may be controlled by similar mechanisms. |
| [6] | It has been shown that N-, P- and S-deficiencies result in major reductions of root hydraulic conductivity (Lpr) which may lead to lowered stomatal conductance, but the relationship between the two conductance changes is not understood. In a variety of species, Lpr decreases in the early stages of $\mathrm{N}{\mathrm{O}}_{3}^{-}$, ${\mathrm{H}}_{2}\mathrm{P}{\mathrm{O}}_{4}^{2-}$ and $\mathrm{S}{\mathrm{O}}_{4}^{2-}$ deprivation. These effects can be reversed in 4-24 h after the deficient nutrient is re-supplied. Diurnal fluctuations of root Lpr have also been found in some species, and an example of this is given for Lotus japonicus. In nutrient-sufficient wheat plants, root Lpr is extremely sensitive to brief treatments with HgCl2; these effects are completely reversible when Hg is removed. The low values of Lpr in N- or P-deprived roots of wheat are not affected by Hg treatments. The properties of plasma membrane (PM) vesicles from wheat roots are also affected by $\mathrm{N}{\mathrm{O}}_{3}^{-}$-deprivation of the intact plants. The osmotic permeability of vesicles from N-deprived roots is much lower than that of roots adequately supplied with $\mathrm{N}{\mathrm{O}}_{3}^{-}$, and is insensitive to Hg treatment. In roots of L. japonicus, gene transcripts are found which have a strong homology to those encoding the PIP1 and PIP2 aquaporins of Arabidopsis. There is a very marked diurnal cycle in the abundance of mRNAs of aquaporin gene homologues in roots of L. japonicus. The maxima and minima appear to anticipate the diurnal fluctuations in Lpr by 2-4 h. The temporal similarity between the cycles of the abundance of the mRNAs and root Lpr is most striking. The aquaporin encoded by AtPIP1 is known to have its water permeation blocked by Hg binding. The lack of Hg-sensitivity in roots and PMs from N-deprived roots provides circumstantial evidence that lowered root Lpr may be due to a decrease in either the activity of water channels or their density in the PM. It is concluded that roots are capable, by means completely unknown, of monitoring the nutrient content of the solution in the root apoplasm and of initiating responses that anticipate by hours or days any metabolic disturbances caused by nutrient deficiencies. It is the incoming nutrient supply that is registered as deficient, not the plant's nutrient status. At some point, close to the initiation of these responses, changes in water channel activity may be involved, but the manner in which monitoring of nutrient stress is transduced into an hydraulic response is also unknown. |
| [7] | The hydraulic architecture of Laurus nobilis L. and Juglans regia L. leaves was studied using three different approaches: (1) hydraulic measurements of both intact leaves and of leaves subjected to treatments aimed at removing the extra-vascular resistance; (2) direct measurements of the vascular pressure with a pressure probe; and (3) modelling the hydraulic architecture of leaf venation system on the basis of measurements of vein densities and conductivities. The hydraulic resistance of leaves (R-leaf) either cut, boiled or frozen-thawed was reduced by about 60 and 85% with respect to control leaves for laurel and walnut, respectively. Direct pressure drop measurements suggested that 88% of the resistance resided outside the vascular system in walnut. Model simulations were in agreement with these results provided vein hydraulic conductance was 0.12-0.28 that of the conductance predicted by Poiseuille's law. The results suggest that R-leaf is dominated by substantial extra-vascular resistances and therefore contrast with the conclusions of recent studies dealing with the hydraulic architecture of the leaf. The present study confirms the 'classical' view of the hydraulic architecture of leaves as composed by a low-resistance component (the venation) and a high-resistance component (the mesophyll). [References: 31] |
| [8] | Abstract This study tests the hypothesis that diffusional limitation of photosynthesis, rather than light, determines the distribution of photosynthetic capacity in olive leaves under drought conditions. The crowns of four olive trees growing in an orchard were divided into two sectors: one sector absorbed most of the radiation early in the morning (MS) while the other absorbed most in the afternoon (AS). When the peak of radiation absorption was higher in MS, air vapour pressure deficit (VPD) was not high enough to provoke stomatal closure. In contrast, peak radiation absorption in AS coincided with the daily peak in VPD. In addition, two soil water treatments were evaluated: irrigated trees (I) and non-irrigated trees (nI). The seasonal evolution of leaf water potential, leaf gas exchange and photosynthetic capacity were measured throughout the tree crowns in spring and summer. Results showed that stomatal conductance was reduced in nI trees in summer as a consequence of soil water stress, which limited their net assimilation rate. Olive leaves displayed isohydric behaviour and no important differences in the diurnal course of leaf water potentials among treatments and sectors were found. Seasonal diffusional limitation of photosynthesis was mainly increased in nI trees, especially as a result of stomatal limitation, although mesophyll conductance (g(m)) was found to decrease in summer in both treatments and sectors. A positive relationship between leaf nitrogen content with both leaf photosynthetic capacity and the daily integrated quantum flux density was found in spring, but not in summer. The relationship between photosynthetic capacity and g(m) was curvilinear. Leaf temperature also affected to g(m) with an optimum temperature at 29 degrees C. AS showed larger biochemical limitation than MS in August in both treatments. All these suggest that both diffusional limitation and the effect of leaf temperature could be involved in the seasonal reduction of photosynthetic capacity of olive leaves. This work highlights the need for models of plant growth and ecosystem function to incorporate new parameters affecting the distribution of photosynthetic capacity in canopies. |
| [9] | |
| [10] | . Abstract CO(2) faces a series of resistances while diffusing between the substomatal cavities and the sites of carboxylation within chloroplasts. The absence of techniques to measure the resistance of individual steps makes it difficult to define their relative importance. Resistance to diffusion through intercellular airspace differs between leaves, but is usually of minor importance. Leaves with high photosynthetic capacity per unit leaf area reduce mesophyll resistance by increasing the surface area of chloroplasts exposed to intercellular airspace per unit leaf area, S(c). Cell walls impose a significant resistance. Assuming an effective porosity of the cell wall of 0.1 or 0.05, then cell walls could account for 25% or 50% of the total mesophyll resistance, respectively. Since the fraction of apoplastic water that is unbound and available for unhindered CO(2) diffusion is unknown, it is possible that the effective porosity is 50% of the total resistance and a variable proportion. Most of the remaining resistance is imposed by one or more of the three membranes as mesophyll resistance can be altered by varying the expression of cooporins. The CO(2) permeability of vesicles prepared from chloroplast envelopes has been reduced by RNA interference (RNAi) expression of NtAQP1, but not those prepared from the plasma membrane. Carbonic anhydrase activity also influences mesophyll resistance. Mesophyll resistance is relatively insensitive to the manipulation of any step in the pathway because it represents only part of the total and may also be countered by pleiotropic compensatory changes. The parameters in greatest need of additional measurements are S(c), mesophyll cell wall thickness, and the permeabilities of the plasma membrane and chloroplast envelope. |
| [11] | |
| [12] | ABSTRACT Leaves are beautifully specialized organs that enable plants to intercept light necessary for photosynthesis. The light is dispersed among a large array of chloroplasts that are in close proximity to air and yet not too far from vascular tissue, which supplies water and exports sugars and other metabolites. To control water loss from the leaf, gas exchange occurs through pores in the leaf surface, stomata, which are able to rapidly change their aperture. Once inside the leaf, CO, has to diffuse from the intercel- lular air spaces to the sites of carboxylation in the chloro- plast (for C, species) (Fig. 1) or the cytosol (for C, species). These internal diffusion paths are the topic of this article. There are several reasons why internal diffusion is of interest. First, Rubisco has a poor affinity for CO, and operates at only a fraction of its catalytic capacity in C, leaves. The CO, gradient within the leaf thus affects the efficiency of Rubisco and the overall nitrogen use efficiency of the leaf. Second, prediction of photosynthetic rates of leaves from their biochemical properties requires a good estimate of the partial pressure of CO, at the sites of carboxylation, pc. Third, internal resistance to CO, diffu- sion results in a lower pc and reduces carbon gain relative to water loss during photosynthesis (water-use efficiency). Considerable effort is being invested selecting and identi- fying plants with improved water-use efficiency using the surrogate measure of carbon isotope discrimination, A, of plant dry matter (Ehleringer et al., 1993). The ratio of intercellular to ambient CO, partial pressure, pi/p,, and A are both linearly related to water-use efficiency if pc/pi is constant. To date, we have little knowledge of genetic variation in pc/pi. Until recently, it was not possible to directly measure the gradient in CO, partial pressure to the sites of carboxyla- tion. The gradient could be inferred from a theoretical analysis of the diffusion pathway, but because several steps have unknown permeability constants, the values are un- certain. Opinion has oscillated from the existence of large to small gradients over the last 30 years. There are now two techniques that enable the gradient to be measured in C, leaves. After describing these techniques, we will consider diffusion through intercellular air spaces and diffusion across cell walls and liquid phase to sites of carboxylation. Finally, we will examine CO, diffusion into mesophyll cells and across the bundle sheath in C, leaves. |
| [13] | |
| [14] | |
| [15] | Abstract Mesophyll diffusion conductance to CO(2) is a key photosynthetic trait that has been studied intensively in the past years. The intention of the present review is to update knowledge of g(m), and highlight the important unknown and controversial aspects that require future work. The photosynthetic limitation imposed by mesophyll conductance is large, and under certain conditions can be the most significant photosynthetic limitation. New evidence shows that anatomical traits, such as cell wall thickness and chloroplast distribution are amongst the stronger determinants of mesophyll conductance, although rapid variations in response to environmental changes might be regulated by other factors such as aquaporin conductance. Gaps in knowledge that should be research priorities for the near future include: how different is mesophyll conductance among phylogenetically distant groups and how has it evolved? Can mesophyll conductance be uncoupled from regulation of the water path? What are the main drivers of mesophyll conductance? The need for mechanistic and phenomenological models of mesophyll conductance and its incorporation in process-based photosynthesis models is also highlighted. Copyright 脗漏 2012 Elsevier Ireland Ltd. All rights reserved. |
| [16] | . Abstract Photosynthetic down-regulation and/or inhibition under water stress conditions are determinants for plant growth, survival and yield in drought-prone areas. Current knowledge about the sequence of metabolic events that leads to complete inhibition of photosynthesis under severe water stress is reviewed. An analysis of published data reveals that a key regulatory role for Rubisco in photosynthesis is improbable under water stress conditions. By contrast, the little data available for other Calvin cycle enzymes suggest the possibility of a key regulatory role for some enzymes involved in the regeneration of RuBP. There are insufficient data to determine the role of photophosphorylation. Several important gaps in our knowledge of this field are highlighted. The most important is the remarkable scarcity of data about the regulation/inhibition of photosynthetic enzymes other than Rubisco under water stress. Consequently, new experiments are urgently needed to improve our current understanding of photosynthetic down-regulation under water stress. A second gap is the lack of knowledge of photosynthetic recovery after irrigation of plants which have been subjected to different stages of water stress. This knowledge is necessary in order to match physiological down-regulation by water stress with controlled irrigation programmes. |
| [17] | . |
| [18] | This article comments on:Temperature responses of mesophyll conductance differ greatly between species |
| [19] | The effects of short-term (minutes) variations of CO(2) concentration on mesophyll conductance to CO(2) (g(m)) were evaluated in six different C(3) species by simultaneous measurements of gas exchange, chlorophyll fluorescence, online carbon isotope discrimination and a novel curve-fitting method. Depending on the species, g(m) varied from five- to ninefold, along the range of sub-stomatal CO(2) concentrations typically used in photosynthesis CO(2)-response curves (A(N)-C(i) curves; where A(N) is the net photosynthetic flux and C(i) is the CO(2) concentrations in the sub-stomatal cavity), that is, 50 to 1500 micromol CO(2) mol(-1) air. Although the pattern was species-dependent, g(m) strongly declined at high C(i), where photosynthesis was not limited by CO(2), but by regeneration of ribulose-1,5-bisphosphate or triose phosphate utilization. Moreover, these changes on g(m) were found to be totally independent of the velocity and direction of the C(i) changes. The response of g(m) to C(i) resembled that of stomatal conductance (g(s)), but kinetic experiments suggested that the response of g(m) was actually faster than that of g(s). Transgenic tobacco plants differing in the amounts of aquaporin NtAQP1 showed different slopes of the g(m)-C(i) response, suggesting a possible role for aquaporins in mediating CO(2) responsiveness of g(m). The importance of these findings is discussed in terms of their effects on parameterization of A(N)-C(i) curves. |
| [20] | Abstract Rubisco activity decreases under water stress, for reasons as yet unclear. Here, the covariation of stomatal conductance (gs) and relative water content (RWC), often observed during water stress, was impaired to assess the separate effects of these factors on Rubisco activity. Three different treatments were applied to soybean (Glycine max) and tobacco (Nicotiana tabacum): leaf desiccation (LD), in which stomatal closure was accompanied by large decreases of RWC; water stress (WS), in which minor decreases of RWC were observed along with stomatal closure; and exogenous application of abscisic acid (ABA), which triggered stomatal closure without changing RWC. Decreased RWC did not induce decreased initial Rubisco activity, which was impaired only in soybean by 40% when the gs dropped below 50 mmol m(-2) s(-1), regardless of the treatment. The mechanism for decreased activity differed among treatments, owing to decreased activation in LD and to total activity and protein content in WS and ABA. Despite the occurrence of Rubisco regulation, CO2 availability in the chloroplast, not impairment of Rubisco activity, limits photosynthesis during WS. |
| [21] | |
| [22] | |
| [23] | Two highly contrasting variables summarizing the efficiency of of materials within the leaf are recognized as playing central roles in determining gas exchange and plant performance. This paper summarizes current approaches for the measurement of mesophyll conductance to (g m) and leaf hydraulic conductance (K leaf) and addresses the physiological integration of these parameters. First, the most common methods to determine g m and K leaf are summarized. Next, novel data compilation is analysed, which indicates that, across diverse species, g m is strongly linked with gas exchange parameters such as net assimilation (A area) and stomatal conductance (g s), and with K leaf, independently of leaf vein length per leaf area. Based on their parallel responses to a number of environmental variables, this review proposes that g m is linked to the outside-xylem but not to the xylem component of K leaf. Further, a mechanistic hypothesis is proposed to explain the interactions among all these and other physiological parameters. Finally, the possibility of estimating g m based on this hypothesis was tested using a regression analysis and a neurofuzzy logic approach. These approaches enabled the estimation of g m of given species from K leaf and leaf mass per area, providing a higher predictive power than from either parameter alone. The possibility of estimating g m from measured K leaf or vice-versa would result in a rapid increase in available data. Studies in which g m, K leaf, and leaf mass per area are simultaneously determined are needed in order to confirm and strengthen predictive and explanatory models for these parameters and importantly improve resolution of the integrated hydraulic-stomatal-photosynthetic system. |
| [24] | Abstract In studies about the photosynthesis response to environmental stresses, such as drought, the Rubisco specificity factor (tau) is assumed to be constant or derived indirectly from gas exchange measurements. However, an analysis of the acclimation of tau to drought using in vitro determinations is lacking. The aim of the present work was to analyse the acclimation of tau to different drought intensities in tobacco (Nicotiana tabacum L.). Potted tobacco plants were subjected to three different water regimes (100%, 40%, and 15% of field capacity) and new leaves were allowed to develop. When acclimated leaves were fully developed, they were sampled for gas exchange and chlorophyll fluorescence measurements, as well as for the in vitro analysis of Rubisco kinetic properties. Relative water content and gas exchange decreased with increasing water shortage. The apparent Rubisco specificity factor as estimated in vivo by gas exchange decreased with water stress. However, in vitro estimates of tau were identical among treatments, as were Rubisco specific initial activity and activation state. The reasons for the observed discrepancy between in vitro and in vivo estimates are profusely discussed. It is suggested that the Rubisco specificity factor does not acclimate to water stress in the short term (weeks or months) in tobacco, and the validity of the so-called Laisk gas exchange method to estimate tau under drought is questioned. |
| [25] | . |
| [26] | |
| [27] | AbstractStudies with the variable J method have reported that mesophyll conductance (gm) rapidly decreases with increasing intercellular CO2 partial pressures (Ci) or decreasing irradiance. Similar responses have been suggested with the online isotope discrimination method, although with less consistency. Here we show that even when the true gm is constant, the variable J method can produce an artefactual dependence of gm on Ci or irradiance similar to those reported in previous studies for any of the following factors: day respiration and chloroplastic CO2 photocompensation point are estimated with Laisk method; Ci or electron transport rate is positively biased; net photosynthetic rate is negatively biased; insufficient NADPH is assumed while insufficient ATP limits RuBP regeneration. The isotopic method produces similar artefacts if fractionation of carboxylation or Ci is positively biased or 螖13 negatively biased. A non-zero chloroplastic resistance to CO2 movement results in a qualitatively different dependence of gm on Ci or irradiance and this dependence is only sensitive at low Ci. We thus cannot rule out the possibility that previously reported dependence of gm on Ci or irradiance is a methodological artefact. Recommendations are made to take advantage of sensitivities of the variable J and isotopic methods for estimating gm. |
| [28] | Drought slows net photosynthetic rate ( A N ) but increases water use efficiency (WUE). Farmers give an artificial drought pretreatment to some crops in the early growth stage and find that yield increases accompanying with the improvement of WUE. We conducted well-watered, non-drought, mild drought and moderate drought pretreatments of potted cotton cultivars. The aims of the present study were to analyse the importance of mesophyll conductance ( g m ) as a factor that may simultaneously improve A N and WUE under drought pretreatment conditions, and to analyse the role of anatomical structure and biochemical mechanism in the variability of g m . Our results showed that significant variability of g m estimated by gas exchange and chlorophyll fluorescence was observed between non-drought pretreatment and drought pretreatment associated with change in A N and WUE. There was great difference in anatomical structure and expression of aquaporins ( GhAQP1 ) among all the treatments. In addition, expression of carbonic anhydrase ( CA ) may not be important in the regulation of g m under drought pretreatment conditions. We concluded that the variability of g m offers a potential target for improving leaf A N and WUE simultaneously by the regulation of anatomical structure and GhAQP1 . |
| [29] | |
| [30] | 1. The influence of leaf thickness on internal conductance for CO 2 transfer from substomatal cavity to chloroplast stroma ( g i ) and carbon isotope ratio (未 13 C) of leaf dry matter was investigated for some evergreen tree species from Japanese temperate forests. g i was estimated based on the combined measurements of gas exchange and concurrent carbon isotope discrimination. 2. Leaves with thicker mesophyll tended to have larger leaf dry mass per area (LMA), larger surface area of mesophyll cells exposed to intercellular air spaces per unit leaf area ( S mes ) and smaller volume ratio of intercellular spaces to the whole mesophyll (mesophyll porosity). 3. g i of these leaves was correlated positively to S mes but negatively to mesophyll porosity. The variation in g i among these species would be therefore primarily determined by variation of the conductance in liquid phase rather than that in gas phase. 4. 未 13 C was positively correlated to mesophyll thickness and leaf nitrogen content on an area basis. However, g i values did not correlate to 未 13 C. These results suggest that difference in 未 13 C among the species was not caused by the variation in g i , but mainly by the difference in long-term photosynthetic capacity. 5. Comparison of our results with those of previous studies showed that the correlation between leaf thickness and g i differed depending on leaf functional types (evergreen, deciduous or annual). Differences in leaf properties among these functional types were discussed. |
| [31] | The internal conductance for CO(2) diffusion (g(i)) and CO(2) assimilation rate were measured and the related anatomical characteristics were investigated in transgenic rice leaves that overexpressed barley aquaporin HvPIP2;1. This study was performed to test the hypothesis that aquaporin facilitates CO(2) diffusion within leaves. The g(i) value was estimated for intact leaves by concurrent measurements of gas exchange and carbon isotope ratio. The leaves of the transgenic rice plants that expressed the highest levels of Aq-anti-HvPIP2;1 showed a 40% increase in g(i) as compared to g(i) in the leaves of wild-type rice plants. The increase in g(i) was accompanied by a 14% increase in CO(2) assimilation rate and a 27% increase in stomatal conductance (g(s)). The transgenic plants that had low levels of Aq-anti-HvPIP2;1 showed decreases in g(i) and CO(2) assimilation rate. In the plants with high levels of Aq-anti-HvPIP2;1, mesophyll cell size decreased and the cell walls of the epidermis and mesophyll cells thickened, indicating that the leaves had become xeromorphic. Although such anatomical changes could partially offset the increase in g(i) by the aquaporin, the increase in aquaporin content overcame such adverse effects. |
| [32] | Abstract The conductance for CO(2) diffusion in the mesophyll of leaves can limit photosynthesis. We have studied two methods for determining the mesophyll conductance to CO(2) diffusion in leaves. We generated an ideal set of photosynthesis rates over a range of partial pressures of CO(2) in the stroma and studied the effect of altering the mesophyll diffusion conductance on the measured response of photosynthesis to intercellular CO(2) partial pressure. We used the ideal data set to test the sensitivity of the two methods to small errors in the parameters used to determine mesophyll conductance. The two methods were also used to determine mesophyll conductance of several leaves using measured rather than ideal data sets. It is concluded that both methods can be used to determine mesophyll conductance and each method has particular strengths. We believe both methods will prove useful in the future. |
| [33] | Abstract Leaf photosynthesis (A) is limited by mesophyll conductance (g(m)), which is influenced by both leaf structure and the environment. Previous studies have indicated that the upper bound for g(m) declines as leaf dry mass per area (LMA, an indicator of leaf structure) increases, extrapolating to zero at a LMA of about 240 g m(-2). No data exist on g(m) and its response to the environment for species with LMA values higher than 220 g m(-2). In this study, laboratory measurements of leaf gas exchange and in vivo chlorophyll a fluorescence were used concurrently to derive estimates of g(m) in seven species of the Australian sclerophyllous genus Banksia covering a wide range of LMA (130-480 g m(-2)). Irradiance and CO(2) were varied during those measurements to gauge the extent of environmental effects on g(m). A significant decrease of g(m) with increasing LMA was found. g(m) declined by 35-60% in response to increasing atmospheric CO(2) concentrations at high irradiance, with a more variable response (0-60%) observed at low irradiance, where g(m) was, on average, 22% lower than at high irradiance at ambient CO(2) concentrations. Despite considerable variation in A and LMA between the Banksia species, the CO(2) concentrations in the intercellular air spaces (C(i), 262+/-5 micromol mol(-1)) and in the chloroplasts (C(c), 127+/-4 micromol mol(-1)) were remarkably stable. |
| [34] | Cellular exchange of carbon dioxide (CO60) is of extraordinary importance for life. Despite this significance, its molecular mechanisms are still unclear and a matter of controversy. In contrast to other living organisms, plants are physiologically limited by the availability of CO60. In most plants, net photosynthesis is directly dependent on CO60 diffusion from the atmosphere to the chloroplast. Thus, it is important to analyze CO60 transport with regards to its effect on photosynthesis. A mutation of the Arabidopsis thaliana AtPIP1;2 gene, which was characterized as a non-water transporting but CO60 transport-facilitating aquaporin in heterologous expression systems, correlated with a reduction in photosynthesis under a wide range of atmospheric CO60 concentrations. Here, we could demonstrate that the effect was caused by reduced CO60 conductivity in leaf tissue. It is concluded that the AtPIP1;2 gene product limits CO60 diffusion and photosynthesis in leaves. |
| [35] | Aquaporins facilitate water permeation across biological membranes. Additionally, glycerol and other small neutral solutes are permeated by related aquaglyceroporins. The role of aquaporins in gas permeation has been a long-standing and controversially discussed issue. We present an extensive set of atomistic molecular dynamics simulations that address the question of CO 2 permeation through human aquaporin-1. Free energy profiles derived from the simulations display a barrier of 652302kJ/mol in the aromatic/arginine constriction region of the water pore, whereas a barrier of 65402kJ/mol was observed for a palmitoyloleoylphosphatidylethanolamine lipid bilayer membrane. The results indicate that significant aquaporin-1-mediated CO 2 permeation is to be expected only in membranes with a low intrinsic CO 2 permeability. |
| [36] | Abstract Aquaporins and aquaglyceroporins form a family of pore proteins that facilitate the efficient and selective flux of small solutes across biological membranes. We studied the selectivity of aquaporin-1 (AQP1) and the bacterial glycerol facilitator, GlpF, for O(2), CO(2), NH(3), glycerol, urea, and water. Using molecular dynamics simulations, we calculated potentials of mean force for solute permeation along the aquaporin channels and compared them with the alternative pathway across the lipid bilayer. For small solutes permeating through AQP1, a remarkable anticorrelation between permeability and solute hydrophobicity was observed, whereas the opposite trend was observed for permeation through the membrane. This finding renders AQP1 a selective filter for small polar solutes, whereas GlpF was found to be highly permeable for small solutes and permeable for larger solutes. Surprisingly, not solute-channel but water-channel interactions were found to be the key determinant underlying the selectivity mechanism of aquaporins. Hence, a hydrophobic effect, together with steric restraints, determines the selectivity of aquaporins. |
| [37] | The confocal microscope was used to determine the pH of the leaf apoplast and the pH of microvolumes of xylem sap. We quantified variation in leaf apoplast and sap pH in relation to changes in edaphic and atmospheric conditions that impacted on stomatal sensitivity to a root-sourced abscisic acid signal. Several plant species showed significant changes in the pH of both xylem sap and the apoplast of the shoot in response to environmental perturbation. Xylem sap leaving the root was generally more acidic than sap in the midrib and the apoplast of the leaf. Increasing the transpiration rate of both intact plants and detached plant parts resulted in more acidic leaf apoplast pHs. Experiments with inhibitors suggested that protons are removed from xylem sap as it moves up the plant, thereby alkalinizing the sap. The more rapid the transpiration rate and the shorter the time that the sap resided in the xylem/apoplastic pathway, the smaller the impact of proton removal on sap pH. Sap pH of sunflower (Helianthus annuus) and Commelina communis did not change significantly as soil dried, while pH of tomato (Lycopersicon esculentum) sap increased as water availability in the soil declined. Increasing the availability of nitrate to roots also significantly alkalinized the xylem sap of tomato plants. This nitrogen treatment had the effect of enhancing the sensitivity of the stomatal response to soil drying. These responses were interpreted as an effect of nitrate addition on sap pH and closure of stomata via an abscisic acid-based mechanism. |
| [38] | Abstract Increased expression of the aquaporin NtAQP1, which is known to function as a plasmalemma channel for CO090202 and water, increases the rate of both photosynthesis and transpiration. In contrast, increased expression of Arabidopsis hexokinase1 (AtHXK1), a dual-function enzyme that mediates sugar sensing, decreases the expression of photosynthetic genes and the rate of transpiration and inhibits growth. Here, we show that AtHXK1 also decreases root and stem hydraulic conductivity and leaf mesophyll CO090202 conductance (g(m)). Due to their opposite effects on plant development and physiology, we examined the relationship between NtAQP1 and AtHXK1 at the whole-plant level using transgenic tomato plants expressing both genes simultaneously. NtAQP1 significantly improved growth and increased the transpiration rates of AtHXK1-expressing plants. Reciprocal grafting experiments indicated that this complementation occurs when both genes are expressed simultaneously in the shoot. Yet, NtAQP1 had only a marginal effect on the hydraulic conductivity of the double-transgenic plants, suggesting that the complementary effect of NtAQP1 is unrelated to shoot water transport. Rather, NtAQP1 significantly increased leaf mesophyll CO090202 conductance and enhanced the rate of photosynthesis, suggesting that NtAQP1 facilitated the growth of the double-transgenic plants by enhancing mesophyll conductance of CO090202. |
| [39] | ABSTRACT Top of page ABSTRACT INTRODUCTION MATERIALS AND METHODS RESULTS DISCUSSION ACKNOWLEDGMENTS REFERENCES The present study was performed to investigate the adjustment of the rate parameters of the light and dark reactions of photosynthesis to the natural growth light in leaves of an overstorey species, Betula pendula Roth, a subcanopy species, Tilia cordata P. Mill., and a herb, Solidago virgaurea L., growing in a natural plant community in J盲rvselja, Estonia. Shoots were collected from the site and individual leaves were measured in a laboratory applying a standardized routine of kinetic gas exchange, Chl fluorescence and 820nm transmittance measurements. These measurements enabled the calculations of the quantum yield of photosynthesis and rate constants of excitation capture by photochemical and non-photochemical quenchers, rate constant for P700 + reduction via the cytochrome b 6 f complex with and without photosynthetic control, actual maximum and potential (uncoupled) electron transport rate, stomatal and mesophyll resistances for CO 2 transport, K m (CO 2 ) and V m of ribulose-bisphosphate carboxylase-oxygenase (Rubisco) in vivo . In parallel, N, Chl and Rubisco contents were measured from the same leaves. No adjustment toward higher quantum yield in shade compared with sun leaves was observed, although relatively more N was partitioned to the light-harvesting machinery in shade leaves ( H. Eichelmann etal ., 2004 ). The electron transport rate through the Cyt b 6 f complex was strongly down-regulated under saturating light compared with darkness, and this was observed under atmospheric, as well as saturating CO 2 concentration. In vivo V m measurements of Rubisco were lower than corresponding reported measurements in vitro , and the k cat per reaction site varied widely between leaves and growth sites. The correlation between Rubisco V m and the photosystem I density was stronger than between V m and the density of Rubisco active sites. The results showed that the capacity of the photosynthetic machinery decreases in shade-adjusted leaves, but it still remains in excess of the actual photosynthetic rate. The photosynthetic control systems that are targeted to adjust the photosynthetic rate to meet the plant's needs and to balance the partial reactions of photosynthesis, down-regulate partial processes of photosynthesis: excess harvested light is quenched non-photochemically; excess electron transport capacity of Cyt b 6 f is down-regulated by 螖pH-dependent photosynthetic control; Rubisco is synthesized in excess, and the number of activated Rubisco molecules is controlled by photosystem I-related processes. Consequently, the nitrogen contained in the components of the photosynthetic machinery is not used at full efficiency. The strong correlation between leaf nitrogen and photosynthetic performance is not due to the nitrogen requirements of the photosynthetic apparatus, but because a certain amount of energy must be captured through photosynthesis to maintain this nitrogen within a leaf. |
| [40] | To identify the effect of nitrogen (N) nutrition on photosynthetic efficiency and mesophyll conductance of seedlings (, cv. 'Shanyou 63' hybrid indica China), hydroponic experiments with different concentrations of N were conducted in a greenhouse. Although leaf N concentration on a dry mass basis increased with increasing supply of N, no significant differences in seedling biomass were observed. A higher light-saturated assimilation rate (A) with a high concentration of supplied N was associated with a higher carboxylation efficiency (CE), but not a higher apparent quantum yield (alpha). Based on classic photosynthetic models, both the content and the bisphosphate (RuBP) regeneration rate were sufficient for light-saturated in seedlings; the estimated chloroplastic concentration (C(c)) and mesophyll conductance (g(m)) demonstrated that a low C(c) was the ultimate limiting factor to photosynthetic efficiency with a higher N supply. Due to a greater size (i.e. a shorter distance to the ) with a higher supply of N, the resistance in the liquid phase (g(liq)) in high-N leaves was lower than that in low-N leaves, which resulted in higher g(m) and C(c) in high-N leaves. Although CE(A/Ci) was higher with a high supply of N, there were no differences in CE(A/Cc) between grown with different concentrations of N, indicating that the carboxylation capacity of between grown at different N concentrations was constant. The enhanced photosynthetic rate with supply of a high N concentration was attributed to a higher concentration in the , related to a higher mesophyll conductance due to an increased size. |
| [41] | The resistance to diffusion of CO(2) from the intercellular airspaces within the leaf through the mesophyll to the sites of carboxylation during photosynthesis was measured using three different techniques. The three techniques include a method based on discrimination against the heavy stable isotope of carbon, (13)C, and two modeling methods. The methods rely upon different assumptions, but the estimates of mesophyll conductance were similar with all three methods. The mesophyll conductance of leaves from a number of species was about 1.4 times the stomatal conductance for CO(2) diffusion determined in unstressed plants at high light. The relatively low CO(2) partial pressure inside chloroplasts of plants with a low mesophyll conductance did not lead to enhanced O(2) sensitivity of photosynthesis because the low conductance caused a significant drop in the chloroplast CO(2) partial pressure upon switching to low O(2). We found no correlation between mesophyll conductance and the ratio of internal leaf area to leaf surface area and only a weak correlation between mesophyll conductance and the proportion of leaf volume occupied by air. Mesophyll conductance was independent of CO(2) and O(2) partial pressure during the measurement, indicating that a true physical parameter, independent of biochemical effects, was being measured. No evidence for CO(2)-accumulating mechanisms was found. Some plants, notably Citrus aurantium and Simmondsia chinensis, had very low conductances that limit the rate of photosynthesis these plants can attain at atmospheric CO(2) level. |
| [42] | Abstract Blue light has many direct and indirect effects on photosynthesis. The impact of blue light on mesophyll conductance (g(m)), one of the main diffusive limitation to photosynthesis, was investigated in leaves of Nicotiana tabacum and Platanus orientalis, characterized by high and low g(m), respectively. Leaves were exposed to blue light fractions between 0% and 80% of incident light intensity (300 micromol photons m(-2) s(-1)), the other fraction being supplied as red light. Leaves exposed to blue light showed reduced photosynthesis and unaltered stomatal conductance. The g(m), measured using the chlorophyll fluorescence-based method, was strongly reduced in both plant species. Such a reduction of g(m) may not be real, as several assumptions used for the calculation of g(m) by fluorescence may not hold under blue light. To assess possible artefacts, the electron transport rate measured by fluorescence (J(f)) and by gas-exchange (J(c)) were compared in leaves exposed to different fractions of blue light under non-photorespiratory conditions. The two values were only equal, a prerequisite for correct g(m) measurements, when the illumination was totally provided as red light. Under increasing blue light levels an increasing discrepancy was observed, which suggests that J(f) was not correctly calculated, and that such an error could also upset g(m) measurements. Blue light was not found to change the absorbance of light by leaves, whereas it slightly decreased the distribution of light to PSII. To equate J(f) and J(c) under blue light, a further factor must be added to the J(f) equation, which possibly accounted for the reduced efficiency of energy transfer between the pigments predominantly absorbing blue light (the carotenoids) and the chlorophylls. This correction reduced by about 50% the effect of blue light on g(m). However, the residual reduction of g(m) under blue light was real and significant, although it did not appear to limit the chloroplast CO(2) concentration and, consequently, photosynthesis. Reduction of g(m) might be caused by chloroplast movement to avoid photodamage, in turn affecting the chloroplast surface exposed to intercellular spaces. However, g(m) reduction occurred immediately after exposure to blue light and was complete after less than 3 min, whereas chloroplast relocation was expected to occur more slowly. In addition, fast g(m) reduction was also observed after inhibiting chloroplast movement by cytochalasin. It is therefore concluded that g(m) reduction under blue light is unlikely to be caused by chloroplast movement only, and must be elicited by other, as yet unknown, factors. |
| [43] | The amounts of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), total chlorophyll (Chl), and total leaf nitrogen were measured in fully expanded, young leaves of wheat (Triticum aestivum L.), rice (Oryza sativa L.), spinach (Spinacia oleracea L.), bean (Phaseolus vulgaris L.), and pea (Pisum sativum L.). In addition, the activities of whole-chain electron transport and carbonic anhydrase were measured. All plants were grown hydroponically at different nitrogen concentrations. Although a greater than proportional increase in Rubisco content relative to leaf nitrogen content and Chl was found with increasing nitrogen supply for rice, spinach, bean, and pea, the ratio of Rubisco to total leaf nitrogen or Chl in wheat was essentially independent of nitrogen treatment. In addition, the ratio of Rubisco to electron transport activities remained constant only in wheat. Nevertheless, gas-exchange analysis showed that the in vivo balance between the capacities of Rubisco and electron transport in wheat, rice, and spinach remained almost constant, irrespective of nitrogen treatment. The in vitro carbonic anhydrase activity in wheat was very low and strongly responsive to increasing nitrogen content. Such a response was not found for the other C(3) plants examined, which had 10- to 30-fold higher carbonic anhydrase activity than wheat at any leaf-nitrogen content. These distinctive responses of carbonic anhydrase activity in wheat were discussed in relation to CO(2)-transfer resistance and the in vivo balance between the capacities of Rubisco and electron transport. |
| [44] | Not Available |
| [45] | |
| [46] | Abstract Gas exchange (GE) and chlorophyll fluorescence (CF) measurements are widely used to noninvasively study photosynthetic parameters, for example the rates of maximum Rubisco carboxylation (V cmax ), electron transport rate (J), daytime respiration (R d ) and mesophyll conductance (g m ). Existing methods for fitting GE data (net assimilation rate-intercellular space CO 2 concentration (A-C i ) curve) are based on two assumptions: g m is unvaried with CO 2 concentration in the intercellular space (C i ); and light absorption (伪) and the proportion of quanta absorbed by photosystem II (尾) are constant in the data set. These may result in significant bias in estimating photosynthetic parameters. To avoid the above-mentioned hypotheses, we present a new method for fitting A-C i curves and CF data simultaneously. This method was applied to a data set obtained from cucumber (Cucumis sativus) leaves of various leaf ages and grown under eight different light conditions. The new method had significantly lower root mean square error and a lower rate of failures compared with previously published methods (6.72% versus 24.1%, respectively) and the effect of light conditions on V cmax and J was better observed. Furthermore, the new method allows the estimation of a new parameter, the fraction of incoming irradiance harvested by photosystem II, and the dependence of g m on C i . 漏 2016 The Authors. New Phytologist 漏 2016 New Phytologist Trust. |
| [47] | According to experimental studies, plant emissions of volatile organic compounds (VOC) are controlled by stomata to a varying extent, but the differing responses could not be explained so far. A dynamic emission model developed in a previous study indicated that stomata may limit the emission rate in a nonsteady state conditions, whereas the rate of increase of liquid-phase volatile concentrations controls the degree to which stomata temporarily curtail the emission. Despite its large predictive capability, potentially large number of volatile physico-chemical and leaf structural variables are needed for parameterization of such dynamic models, limiting the usefulness of the approach. We conducted a sensitivity analysis to determine the effect of varying VOC distribution between gas- and liquid-phases (Henry's law constant, H, Pa mmol) and varying internal diffusion conductances in the liquid- and gas-phases. The model was parameterized for three contrasting leaf architectures (conifer, sclerophyll, and mesophytic leaves). The sensitivity analysis indicated that the volatile H value is the key variable affecting the stomatal sensitivity of VOC emissions. Differences in leaf architecture, in particular in leaf liquid volume to area ratio, also modified the emission responses to changes in stomatal aperture, but these structural effects were superimposed by compound gas/liquid phase partitioning. The results of this analysis indicate that major effort in parameterization of dynamic VOC emission models should be directed toward obtaining reliable gas/liquid-phase equilibria for various plant volatiles, and that these models may readily be applied for leaves with contrasting architecture. |
| [48] | [1] Volatile (VOC) flux from leaves may be expressed as G S Δ P , where G S is stomatal conductance to specific compound and Δ P partial pressure gradient between the atmosphere and substomatal cavities. It has been suggested that decreases in G S are balanced by increases in Δ P such that stomata cannot control VOC emission. Yet, responses of emission rates of various volatiles to experimental manipulations of stomatal aperture are contrasting. To explain these controversies, a dynamic emission model was developed considering VOC distribution between gas and liquid phases using Henry's law constant ( H , Pa m 3 mol 611 ). Our analysis demonstrates that highly volatile compounds such as isoprene and monoterpenes with H values on the order of 10 3 have gas and liquid pool half-times of a few seconds, and thus cannot be controlled by stomata. More soluble compounds such as alcohols and carboxylic acids with H values of 10 612 –10 1 are controlled by stomata with the degree of stomatal sensitivity varying with H . Inability of compounds with high solubility to support a high partial pressure, and thus to balance Δ P in response to a decrease in G S is the primary explanation for different stomatal sensitivities. For compounds with low H , the analysis predicts bursts of emission after stomatal opening that accord with experimental observations, but that cannot be currently explained. Large within-leaf VOC pool sizes in compounds with low H also increase the system inertia to environmental fluctuations. In conclusion, dynamic models are necessary to simulate diurnal variability of the emissions of compounds that preferably partition to aqueous phase. |
| [49] | |
| [50] | |
| [51] | The possible implication of leaf anatomical characteristics on the photosynthetic rate was studied in two grapevine cultivars, Ribier (Vitis vinifera L.) and Isabella (Vitis labrusca), grown under field conditions. Ribier exhibited higher photosynthetic rates than Isabella, although there were no significant differences in the Rubisco activity and the stomatal conductance. The fraction of mesophyll volume represented by the intercellular spaces as well as the surface area of mesophyll cells exposed to intercellular air spaces were significantly lower in Isabella. Both gaseous CO$_2$ conductance through intercellular airspaces and liquid phase conductance were significantly higher in Ribier than in Isabella, contributing to a higher photosynthetic rate in this cultivar. |
| [52] | |
| [53] | The acclimation responses of walnut leaf photosynthesis to the irradiance microclimate were investigated by characterizing the photosynthetic properties of the leaves sampled on young trees (Juglans nigraxregia) grown in simulated sun and shade environments, and within a mature walnut tree crown (Juglans regia) in the field. In the young trees, the CO2 compensation point in the absence of mitochondrial respiration (Gamma*), which probes the CO2 versus O-2 specificity of Rubisco, was not significantly different in sun and shade leaves. The maximal net assimilation rates and stomatal and mesophyll conductances to CO2 transfer were markedly lower in shade than in sun leaves. Dark respiration rates were also lower in shade leaves. However, the percentage inhibition of respiration by light during photosynthesis was similar in both sun and shade leaves. The extent of the changes in photosynthetic capacity and mesophyll conductance between sun and shade leaves under simulated conditions was similar to that observed between sun and shade leaves collected within the mature tree crown. Moreover, mesophyll conductance was strongly correlated with maximal net assimilation and the relationships were not significantly different between the two experiments, despite marked differences in leaf anatomy. These results suggest that photosynthetic capacity is a valuable parameter for modelling within-canopies variations of mesophyll conductance due to leaf acclimation to light. |
| [54] | As an approach to understanding the physiological role of chloroplast carbonic anhydrase (CA), this study reports on the production and preliminary physiological characterisation of transgenic tobacco ( Nicotiana tabacum L.) plants where chloroplast CA levels have been specifically suppressed with an antisense construct directed against chloroplast CA mRNA. Primary transformants with CA levels as low as 2% of wild-type levels were recovered, together with intermediate plants with CA activities of about 20–50% of wild-type levels. Plants with even the lowest CA levels were not morphologically distinct from the wild-type plants. Segregation analysis of the low-CA character in plants grown from T 1 selfed seed indicated that at least one of the low-CA plants appears to have two active inserts and that at least two of the intermediate-CA plants have one active insert. Analysis of CO 2 gas exchange of a group of low-CA plants with around 2% levels of CA indicated that this large reduction in chloroplastic CA did not appear to cause a measurable alteration in net CO 2 fixation at 350 μbar CO 2 and an irradiance of 1000 μmol quanta·m 612 ·s 611 . In addition, no significant differences in Rubisco activity, chlorophyll content, dry weight per unit leaf area, stomatal conductance or the ratio of intercellular to ambient CO 2 partial pressure could be detected. However, the carbon isotope compositions of leaf dry matter were significantly lower (0.85%o) for low-CA plants than for wildtype plants. This corresponds to a 15-μbar reduction in the CO 2 partial pressure at the sites of carboxylation. The difference, which was confirmed by concurrent measurement of discrimination with gas exchange, would reduce the CO 2 assimilation rate by 4.4%, a difference that could not be readily determined by gas-exchange techniques given the inherent variability found in tobacco. A 98% reduction in CA activity dramatically reduced the 18 O discrimination in CO 2 passing over the leaf, consistent with a marked reduction in the ratio of hydrations to carboxylations. We conclude that a reduction in chloroplastic CA activity of two orders of magnitude does not produce a major limitation on photosynthesis at atmospheric CO 2 levels, but that normal activities of the enzyme appear to play a role in facilitated transfer of CO 2 within the chloroplast, producing a marginal improvement in the efficiency of photosynthesis in C 3 plants. |
| [55] | |
| [56] | Leaves constitute a substantial fraction of the total resistance to water flow through plants. A key question is how hydraulic resistance within the leaf is distributed among petiole, major veins, minor veins, and the pathways downstream of the veins. We partitioned the leaf hydraulic resistance (R(leaf)) for sugar maple (Acer saccharum) and red oak (Quercus rubra) by measuring the resistance to water flow through leaves before and after cutting specific vein orders. Simulations using an electronic circuit analog with resistors arranged in a hierarchical reticulate network justified the partitioning of total R(leaf) into component additive resistances. On average 64% and 74% of the R(leaf) was situated within the leaf xylem for sugar maple and red oak, respectively. Substantial resistance-32% and 49%- was in the minor venation, 18% and 21% in the major venation, and 14% and 4% in the petiole. The large number of parallel paths (i.e. a large transfer surface) for water leaving the minor veins through the bundle sheath and out of the leaf resulted in the pathways outside the venation comprising only 36% and 26% of R(leaf). Changing leaf temperature during measurement of R(leaf) for intact leaves resulted in a temperature response beyond that expected from changes in viscosity. The extra response was not found for leaves with veins cut, indicating that water crosses cell membranes after it leaves the xylem. The large proportion of resistance in the venation can explain why stomata respond to leaf xylem damage and cavitation. The hydraulic importance of the leaf vein system suggests that the diversity of vein system architectures observed in angiosperms may reflect variation in whole-leaf hydraulic capacity. |
| [57] | |
| [58] | Abstract Tobacco (Nicotiana tabacum; C3) plants increase their water use efficiency (WUE) under abiotic stress and are suggested to show characteristics of C4 photosynthesis in stems, petioles, and transmitting tract cells. The tobacco stress-induced Aquaporin1 (NtAQP1) functions as both water and CO(2) channel. In tobacco plants, overexpression of NtAQP1 increases leaf net photosynthesis (A(N)), mesophyll CO(2) conductance, and stomatal conductance, whereas its silencing reduces root hydraulic conductivity (L(p)). Nevertheless, interaction between NtAQP1 leaf and root activities and its impact on plant WUE and productivity under normal and stress conditions have never been suggested. Thus, the aim of this study was to suggest a role for NtAQP1 in plant WUE, stress resistance, and productivity. Expressing NtAQP1 in tomato (Solanum lycopersicum) plants (TOM-NtAQP1) resulted in higher stomatal conductance, whole-plant transpiration, and A(N) under all conditions tested. In contrast to controls, where, under salt stress, L(p) decreased more than 3-fold, TOM-NtAQP1 plants, similar to maize (Zea mays; C4) plants, did not reduce L(p) dramatically (only by approximately 40%). Reciprocal grafting provided novel evidence for NtAQP1's role in preventing hydraulic failure and maintaining the whole-plant transpiration rate. Our results revealed independent, albeit closely related, NtAQP1 activities in roots and leaves. This dual activity, which increases the plant's water use and A(N) under optimal and stress conditions, resulted in improved WUE. Consequently, it contributed to the plant's stress resistance in terms of yield production under all tested conditions, as demonstrated in both tomato and Arabidopsis (Arabidopsis thaliana) plants constitutively expressing NtAQP1. The putative involvement of NtAQP1 in tobacco's C4-like photosynthesis characteristics is discussed. |
| [59] | Abstract One mechanism to enhance global food stocks radically is to introduce C4 photosynthesis into C3 crops from warm climates, notably rice. To accomplish this, an understanding of leaf structure and function is essential. The chlorenchyma structure of rice and related warm-climate C3 grasses is distinct from that of cool temperate C3 grasses. In temperate C3 grasses, vacuoles occupy the majority of the cell, while chloroplasts, peroxisomes and mitochondria are pressed against the cell periphery. In rice, 66% of protoplast volume is occupied by chloroplasts, and chloroplasts/stromules cover >95% of the cell periphery. Mitochondria and peroxisomes occur in the cell interior and are intimately associated with chloroplasts/stromules. We hypothesize that the chlorenchyma architecture of rice enhances diffusive CO(2) conductance and maximizes scavenging of photorespired CO2. The extensive chloroplast/stromule sheath forces photorespired CO(2) to exit cells via the stroma, where it can be refixed by Rubisco. Deep cell lobing and small cell size, coupled with chloroplast sheaths, creates high surface area exposure of stroma to intercellular spaces, thereby enhancing mesophyll transfer conductance. In support of this, rice exhibits higher mesophyll transfer conductance, greater stromal CO2 content, lower CO2 compensation points at warm temperature and less oxygen sensitivity of photosynthesis than cool temperate grasses. Rice vein length per leaf, mesophyll thickness and intercellular space volume are intermediate between those of most C3 and C4 grasses, indicating that the introduction of Kranz anatomy into rice may not require radical changes in leaf anatomy; however, deep lobing of chlorenchyma cells may constrain efforts to engineer C4 photosynthesis into rice. |
| [60] | ABSTRACT Photosynthetic responses to carbon dioxide concentration can provide data on a number of important parameters related to leaf physiology. Methods for fitting a model to such data are briefly described. The method will fit the following parameters: V cmax , J , TPU , R d and g m [maximum carboxylation rate allowed by ribulose 1路5-bisphosphate carboxylase/oxygenase (Rubisco), rate of photosynthetic electron transport (based on NADPH requirement), triose phosphate use, day respiration and mesophyll conductance, respectively]. The method requires at least five data pairs of net CO 2 assimilation ( A ) and [CO 2 ] in the intercellular airspaces of the leaf ( C i ) and requires users to indicate the presumed limiting factor. The output is (1) calculated CO 2 partial pressure at the sites of carboxylation, C c , (2) values for the five parameters at the measurement temperature and (3) values adjusted to 25掳C to facilitate comparisons. Fitting this model is a way of exploring leaf level photosynthesis. However, interpreting leaf level photosynthesis in terms of underlying biochemistry and biophysics is subject to assumptions that hold to a greater or lesser degree, a major assumption being that all parts of the leaf are behaving in the same way at each instant. |
| [61] | |
| [62] | BACKGROUND AND AIMS: The paper by Monsi and Saeki in 1953 (Japanese Journal of Botany 14: 22-52) was pioneering not only in mathematical modelling of canopy photosynthesis but also in eco-developmental studies of seasonal changes in leaf canopies. SCOPE: Construction and maintenance mechanisms of efficient photosynthetic systems at three different scaling levels--single leaves, herbaceous plants and trees--are reviewed mainly based on the nitrogen optimization theory. First, the nitrogen optimization theory with respect to the canopy and the single leaf is briefly introduced. Secondly, significance of leaf thickness in CO2 diffusion in the leaf and in leaf photosynthesis is discussed. Thirdly, mechanisms of adjustment of photosynthetic properties of the leaf within the herbaceous plant individual throughout its life are discussed. In particular, roles of sugar sensing, redox control and of cytokinin are highlighted. Finally, the development of a tree is considered. CONCLUSIONS: Various mechanisms contribute to construction and maintenance of efficient photosynthetic systems. Molecular backgrounds of these ecologically important mechanisms should be clarified. The construction mechanisms of the tree cannot be explained solely by the nitrogen optimization theory. It is proposed that the pipe model theory in its differential form could be a potential tool in future studies in this research area. |
| [63] | . Abstract The subject of this paper, sun leaves are thicker and show higher photosynthetic rates than the shade leaves, is approached in two ways. The first seeks to answer the question: why are sun leaves thicker than shade leaves? To do this, CO2 diffusion within a leaf is examined first. Because affinity of Rubisco for CO2 is low, the carboxylation of ribulose 1,5-bisphosphate is competitively inhibited by O2, and the oxygenation of ribulose 1,5-bisphosphate leads to energy-consuming photorespiration, it is essential for C3 plants to maintain the CO2 concentration in the chloroplast as high as possible. Since the internal conductance for CO2 diffusion from the intercellular space to the chloroplast stroma is finite and relatively small, C3 leaves should have sufficient mesophyll surfaces occupied by chloroplasts to secure the area for CO2 dissolution and transport. This explains why sun leaves are thicker. The second approach is mechanistic or 'how-oriented'. Mechanisms are discussed as to how sun leaves become thicker than shade leaves, in particular, the long-distance signal transduction from mature leaves to leaf primordia inducing the periclinal division of the palisade tissue cells. To increase the mesophyll surface area, the leaf can either be thicker or have smaller cells. Issues of cell size are discussed to understand plasticity in leaf thickness. |
| [64] | ABSTRACT In this update, we focus on the key structural features that affect CO2 concentration in the chloroplast stroma. First, we analyze the conductance for CO2 diffusion from the substomatal cavity to the chloroplast stroma (mesophyll conductance, gm, also called internal conductance, gi). Because the low gm limits photosynthesis, the mesophyll surface area exposed to the intercellular spaces (Smes, mesophyll surface area exposed to intercellular spaces per unit leaf area) should be maximized to increase the area for CO2 dissolution and the effective pathway for CO2 diffusion, and thereby photosynthesis. Second, we analyze the light environment within a leaf, because, for maximizing photosynthesis, light should be delivered to all the chloroplasts in the leaf, distributing along the cell walls. In relation to the light environment within a leaf, we also point out some technical problems in measuring photosynthetic parameters. Third, the movement and positioning of cellular organelles are discussed. Finally, we discuss the urgent need of ecologically relevant developmental and cell biological studies that clarify the mechanisms that are responsible for structural and cell biological features in nature. |
| [65] | |
| [66] | Abstract Experiments were conducted to examine whether mercury-sensitive aquaporins facilitate photosynthetic CO(2) diffusion across the plasma membrane of leaf mesophyll cells. Discs without abaxial epidermes from Vicia faba leaflets were treated with HgCl(2), an inhibitor of aquaporins. Hydraulic conductivity of the plasma membrane of these discs, measured as the weight loss of the discs in the 1 M sorbitol solution, was inhibited by sub-mM concentrations of HgCl(2) by 70 to 80%. Photosynthetic CO(2) fixation was also inhibited by the HgCl(2) treatment in a similar concentration range. When 0.3 mM HgCl(2) solution was fed to the V. faba leaflets with intact epidermes via the transpiration stream, the rate of photosynthesis on leaf area basis (A) measured at photosynthetically active photon flux density of 700 micromol m(-2) s(-1) and at leaf temperature of 25 degrees C, decreased by about 20 to 30% at any CO(2) concentration in the intercellular spaces (C(i)). However, when CO(2) concentration in the chloroplast stroma (C(c)) was calculated from fluorescence and gas exchange data and A was plotted against C(c), A at low C(c) concentrations did not differ before and after the treatment. The conductance for CO(2) diffusion from the intercellular spaces to the chloroplast stroma (g(i)) decreased to 40 and 30% of the control value, when the leaflets were fed with 0.3 mM and 1.2 mM HgCl(2), respectively. Similar results were obtained with leaves of Phaseolus vulgaris. Although effects of HgCl(2) were not specific, the present results showed that HgCl(2) consistently lowered g(i). It is, thus, probable that the photosynthetic CO(2) uptake across the plasma membrane of the mesophyll cells is facilitated by mercury-sensitive aquaporins. |
| [67] | 08 2016 John Wiley & Sons LtdMesophyll conductance to CO2 (gm) may respond to light either through regulated dynamic mechanisms or due to anatomical and structural factors. At low light, some layers of cells in the leaf cross-section approach photocompensation and contribute minimally to bulk leaf photosynthesis and little to whole leaf gm (gm,leaf). Thus, the bulk gm,leaf will appear to respond to light despite being based upon cells having an anatomically fixed mesophyll conductance. Such behaviour was observed in species with contrasting leaf structure using the variable J or stable isotope method of measuring gm,leaf. A species with bifacial structure, Arbutus × ‘Marina’, and an isobilateral species, Triticum durum L., had contrasting responses of gm,leaf upon varying adaxial or abaxial illumination. Anatomical observations, when coupled with the proposed model of gm,leaf to photosynthetic photon flux density (PPFD) response, successfully represented the observed gas exchange data. The theoretical and observed evidence that gm,leaf apparently responds to light has large implications for how gm,leaf values are interpreted, particularly limitation analyses, and indicates the importance of measuring gm under full light saturation. Responses of gm,leaf to the environment should be treated as an emergent property of a distributed 3D structure, and not solely a leaf area-based phenomenon. |
| [68] | . The relationship between chloroplast arrangement and diffusion of CO from substomatal cavities to the chloroplast stroma was investigated in Arabidopsis thaliana. Chloroplast position was manipulated by varying the amount of blue light and by cytochalasin D (CytD) treatment. We also investigated two chloroplast positioning mutants. Chloroplast arrangement was assessed by the surface area of chloroplasts adjacent to intercellular airspaces (Sc). Although it has been previously shown that long-term acclimation to high light is linked with a large Sc, we found that the short-term chloroplast avoidance response reduces Sc. This effect was not apparent in the blue-light-insensitive phot2 mutant, which did not show the avoidance response. As expected, the smaller Sc induced by the avoidance response was coupled to a similar decrease in internal conductance. This reduction in internal conductance resulted in an increased limitation of the rate of photosynthesis. The limiting effect of Sc on internal conductance and photosynthesis was also shown in chup1, a mutant with a constant small Sc as the result of an unusual chloroplast arrangement. We conclude that chloroplast movements in A. thaliana can rapidly alter leaf morphological parameters, and this has significant consequences for the diffusion of CO through the mesophyll. |
| [69] | Abstract The CO(2) concentration at the site of carboxylation inside the chloroplast stroma depends not only on the stomatal conductance, but also on the conductance of CO(2) between substomatal cavities and the site of CO(2) fixation. This conductance, commonly termed mesophyll conductance (g(m) ), significantly constrains the rate of photosynthesis. Here we show that estimates of g(m) are influenced by the amount of respiratory and photorespiratory CO(2) from the mitochondria diffusing towards the chloroplasts. This results in an apparent CO(2) and oxygen sensitivity of g(m) that does not imply a change in intrinsic diffusion properties of the mesophyll, but depends on the ratio of mitochondrial CO(2) release to chloroplast CO(2) uptake. We show that this effect (1) can bias the estimation of the CO(2) photocompensation point and non-photorespiratory respiration in the light; (2) can affect the estimates of ribulose 1脗路5-bisphosphate carboxylase/oxygenase (Rubisco) kinetic constants in vivo; and (3) results in an apparent obligatory correlation between stomatal conductance and g(m) . We further show that the amount of photo(respiratory) CO(2) that is refixed by Rubisco can be directly estimated through measurements of g(m) . 脗漏 2012 Blackwell Publishing Ltd. |
| [70] | . |
| [71] | |
| [72] | Background and AimsStomatal (gs) and mesophyll conductance (gm) limit photosynthesis significantly and consequently, apart from gs, gm is also a physiological process that could affect leaf water use efficiency (WUE). The aims of the present study were to analyse the genetic variability of gm in the grapevine, to relate the variability of leaf WUE with relative changes in gs and gm and to analyse the importance of leaf structural parameters in the variability of gm.Methods and ResultsThe gm in seven potted grapevine cultivars (Vitis vinifera鈥匧.) were compared in three experiments under irrigation and moderate water stress treatments. Significant variability of gm was observed among cultivars associated with change in intrinsic WUE. No clear relationship with anatomical traits was found.ConclusionsVariability of gm was associated with leaf WUE, although it could not be explained by anatomical variability. It is likely that biochemical mechanisms play an additional important role in the diffusion of CO2 in grapevines.Significance of the StudyThis study shows that the variability of gm in different grapevine cultivars offers a potential target for improving leaf WUE by increasing net photosynthesis (AN) without increasing plant water losses. |
| [73] | Finite mesophyll diffusion conductance (g(m) ) significantly constrains net assimilation rate (A(n) ), but g(m) variations and variation sources in response to environmental stresses during leaf development are imperfectly known. The combined effects of light and water limitations on g(m) and diffusion limitations of photosynthesis were studied in saplings of Populus tremula L. An one-dimensional diffusion model was used to gain insight into the importance of key anatomical traits in determining g(m) . Leaf development was associated with increases in dry mass per unit area, thickness, density, exposed mesophyll (S(mes) /S) and chloroplast (S(c) /S) to leaf area ratio, internal air space (f(ias) ), cell wall thickness and chloroplast dimensions. Development of S(mes) /S and S(c) /S was delayed under low light. Reduction in light availability was associated with lower S(c) /S, but with larger f(ias) and chloroplast thickness. Water stress reduced S(c) /S and increased cell wall thickness under high light. In all treatments, g(m) and A(n) increased and CO(2) drawdown because of g(m) , C(i) -C(c) , decreased with increasing leaf age. Low light and drought resulted in reduced g(m) and A(n) and increased C(i) -C(c) . These results emphasize the importance of g(m) and its components in determining A(n) variations during leaf development and in response to stress. 漏 2011 Blackwell Publishing Ltd. |
| [74] | In sclerophylls, photosynthesis is particularly strongly limited by mesophyll diffusion resistance from substomatal cavities to chloroplasts (r m), but the controls on diffusion limits by integral leaf variables such as leaf thickness, density, and dry mass per unit area and by the individual steps along the diffusion pathway are imperfectly understood. To gain insight into the determinants of r m in leaves with varying structure, the full CO2 physical diffusion pathway was analysed in 32 Australian species sampled from sites contrasting in soil nutrients and rainfall, and having leaf structures from mesophytic to strongly sclerophyllous. r m was estimated based on combined measurements of gas exchange and chlorophyll fluorescence. In addition, r m was modelled on the basis of detailed anatomical measurements to separate the importance of different serial resistances affecting CO2 diffusion into chloroplasts. The strongest sources of variation in r m were S c/S, the exposed surface area of chloroplasts per unit leaf area, and mesophyll cell wall thickness, t cw. The strong correlation of r m with t cw could not be explained by cell wall thickness alone, and most likely arose from a further effect of cell wall porosity. The CO2 drawdown from intercellular spaces to chloroplasts was positively correlated with t cw, suggesting enhanced diffusional limitations in leaves with thicker cell walls. Leaf thickness and density were poorly correlated with S c/S, indicating that widely varying combinations of leaf anatomical traits occur at given values of leaf integrated traits, and suggesting that detailed anatomical studies are needed to predict r m for any given species. |
| [75] | . Photosynthesis is often limited by the rate of CO(2) diffusion from the atmosphere to the chloroplast. The primary resistances for CO(2) diffusion are thought to be at the stomata and at photosynthesizing cells via a combination resulting from resistances of aqueous solution as well as the plasma membrane and both outer and inner chloroplast membranes. In contrast with stomatal resistance, the resistance of biological membranes to gas transport is not widely recognized as a limiting factor for metabolic function. We show that the tobacco (Nicotiana tabacum) plasma membrane and inner chloroplast membranes contain the aquaporin Nt AQP1. RNA interference-mediated decreases in Nt AQP1 expression lowered the CO(2) permeability of the inner chloroplast membrane. In vivo data show that the reduced amount of Nt AQP1 caused a 20% change in CO(2) conductance within leaves. Our discovery of CO(2) aquaporin function in the chloroplast membrane opens new opportunities for mechanistic examination of leaf internal CO(2) conductance regulation. |
| [76] | ABSTRACT Measurements of CO2 and water vapour exchange by leaves were combined with measurements of carbon isotope composition (13C/12C) of CO2 in the air passing over the leaf. Carbon isotope discrimination during CO2 uptake was determined from the difference in carbon isotope composition of the air leaving the leaf chamber with or without a leaf enclosed. Leaves of wheat plants grown with different nitrogen nutrition and leaves of several other species were examined. The measurements, made at different irradiances for a given leaf, showed that carbon isotope discrimination was strongly correlated with the rate of CO2 assimilation as well as the ratio of intercellular to ambient partial pressure of CO2, pI/pa. A function relating carbon isotope discrimination to the rate of CO2 assimilation was used to estimate the CO2 transfer conductance, gw, from the substomatal cavities to the sites of carboxylation for individual leaves. The photosynthetic capacity correlated with the CO2 transfer conductance, gw, and the average ratio of chloroplastic to intercellular partial pressure of CO2, pI/pa, was 0.7. This means that in general under high irradiance, the ratio of chloroplastic to ambient partial pressure of CO2 is about 0.5. In wheat, variation in gw was correlated with the chloroplast surface area appressing intercellular airspaces. |
| [77] | Abstract The internal conductance to CO(2) supply from substomatal cavities to sites of carboxylation may pose a large limitation to photosynthesis, but little is known of how it is affected by nutrient supply. Knowing how internal conductance responds to nutrient supply is critical for interpreting the biochemical responses from A-C(i) curves. The aim of this paper was to examine the response of g(i) and photosynthetic parameters to nutrient supply in glasshouse-grown seedlings of the evergreen perennial Eucalyptus globulus Labill. Seedlings were grown with five different nutrient treatments and g(i) was estimated from concurrent measurements of gas exchange and fluorescence. Internal conductance varied between 0.12 and 0.19 mol m(-2) s(-1) and the relative limitation of photosynthesis due to internal conductance was greater than the stomatal limitation. In most species these two limitations are rather similar, but in E. globulus stomatal limitations were abnormally low due to high stomatal conductance (0.31 to 0.39 mol m(-2) s(-1)). The large positive response of photosynthesis to nutrient supply was not matched by changes in internal conductance, and thus the relative limitation of photosynthesis due to internal conductance increased with increasing nutrient supply. Failure to account for finite internal conductance led to estimates of V(cmax) that were 60% of the true value, which, in turn, led to an underestimation of in vivo Rubisco specific activity (as V(cmax)/Rubisco content). The specific activity of Rubisco in E. globulus (21 mol mol(-1) s(-1)) was close to the maximum published estimates, and thus, despite these leaves containing a large fraction of N as Rubisco (38-44%) there was no evidence that Rubisco activity was down-regulated or that the enzyme was in excess. |
| [78] | Abstract Internal conductance describes the movement of CO(2) from substomatal cavities to sites of carboxylation. Internal conductance has now been measured in approximately 50 species, and in all of these species it is a large limitation of photosynthesis. It accounts for somewhat less than half of the decrease in CO(2) concentrations from the atmosphere to sites of carboxylation. There have been two major findings in the past decade. First, the limitation due to internal conductance (i.e. C(i)-C(c)) is not fixed but varies among species and functional groups. Second, internal conductance is affected by some environmental variables and can change rapidly, for example, in response to leaf temperature, drought stress or CO(2) concentration. Biochemical factors such as carbonic anhydrase or aquaporins are probably responsible for these rapid changes. The determinants of internal conductance remain elusive, but are probably a combination of leaf anatomy, morphology, and biochemical factors. In most plants, the gas phase component of internal conductance is negligible with the majority of resistance resting in the liquid phase from cell walls to sites of carboxylation. The internal conductance story is far from complete and many exciting challenges remain. Internal conductance ought to be included in models of canopy photosynthesis, but before this is feasible additional data on the variation in internal conductance among and within species are urgently required. Future research should also focus on teasing apart the different steps in the diffusion pathway (intercellular spaces, cell wall, plasmalemma, cytosol, and chloroplast envelope) since it is likely that this will provide clues as to what determines internal conductance. |
| [79] | Two separate objectives were considered in this study. We examined (1) internal conductance to CO 2 ( g i) and photosynthetic limitations in sun and shade leaves of 60-year-old Fagus sylvatica, and (2) whether free-air ozone fumigation affects g i and photosynthetic limitations. g i and photosynthetic limitations were estimated in situ from simultaneous measurements of gas exchange and chlorophyll fluorescence on attached sun and shade leaves of F. sylvatica. Trees were exposed to ambient air (1× O 3) and air with twice the ambient ozone concentration (2× O 3) in a free-air ozone canopy fumigation system in southern Germany (Kranzberg Forest). g i varied between 0.12 and 0.24 mol m 612 s 611 and decreased CO 2 concentrations from intercellular spaces ( C i) to chloroplastic ( C c) by approximately 55 μmol mol 611. The maximum rate of carboxylation ( V cmax) was 22–39% lower when calculated on a C i basis compared with a C c basis. g i was approximately twice as large in sun leaves compared to shade leaves. Relationships among net photosynthesis, stomatal conductance and g i were very similar in sun and shade leaves. This proportional scaling meant that neither C i nor C c varied between sun and shade leaves. Rates of net photosynthesis and stomatal conductance were about 25% lower in the 2× O 3 treatment compared with 1× O 3, while V cmax was unaffected. There was no evidence that g i was affected by ozone. |
| [80] | Abstract The physiological role of chloroplastic carbonic anhydrase (CA) was examined by antisense suppression of chloroplastic CA (on average 8% of wild type) in Nicotiana tabacum. Photosynthetic gas-exchange characteristics of low-CA and wild-type plants were measured concurrently with short-term, on-line stable isotope discrimination at varying vapor pressure deficit (VPD) and light intensity. Low-CA and wild-type plants were indistinguishable in the responses of assimilation, transpiration, stomatal conductance, and intercellular CO2 concentration to changing VPD or light intensity. At saturating light intensity, low-CA plants had lower discrimination against 13CO2 than wild-type plants by 1.2 to 1.8[per mille (thousand) sign]. Consequently, tissue of the low-CA plants was higher in 13C than the control plants. It was calculated that low-CA plants had chloroplast CO2 concentrations 13 to 22 [mu]mol mol-1 lower than wild-type plants. Discrimination against C18O16O in low-CA plants was 20% of that of the wild type, confirming a role of chloroplastic CA in the mechanism of discrimination against C18O16O ([delta]C18O16O). As VPD increased, stomatal closure caused a reduction in chloroplastic C02 concentration, and since VPD and chloroplastic CO2 concentration act in opposing directions on [delta]C18O16O, no effect of VPD was seen on [delta]C18O16O. |
| [81] | Abstract Leaf hydraulic conductance (Kleaf ) and mesophyll conductance (gm ) both represent major constraints to photosynthetic rate (A), and previous studies have suggested that Kleaf and gm is correlated in leaves. However, there is scarce empirical information about their correlation. In this study, Kleaf , leaf hydraulic conductance inside xylem (Kx ), leaf hydraulic conductance outside xylem (Kox ), A, stomatal conductance (gs ), gm , and anatomical and structural leaf traits in 11 Oryza genotypes were investigated to elucidate the correlation of H2 O and CO2 diffusion inside leaves. All of the leaf functional and anatomical traits varied significantly among genotypes. Kleaf was not correlated with the maximum theoretical stomatal conductance calculated from stomatal dimensions (gsmax ), and neither gs nor gsmax were correlated with Kx . Moreover, Kox was linearly correlated with gm and both were closely related to mesophyll structural traits. These results suggest that Kleaf and gm are related to leaf anatomical and structural features, which may explain the mechanism for correlation between gm and Kleaf . |
| [82] | |
| [83] | The range of leaf hydraulic conductance across the genus Oryza is caused by leaf morpho-anatomical traits and leaf N status. Leaf hydraulic conductance (K leaf) is a major determinant of photosynthetic rate in plants. Previous work has assessed the relationships between leaf morpho-anatomical traits and K leaf with woody species, but there has been very little focus on cereal crops. The genus Oryza, which includes rice (Oryza sativa) and wild species (such as O. rufipogon cv. Griff), is ideal material for identifying leaf features associated with K leaf and gas exchange. Leaf morpho-anatomical traits, K leaf, leaf N content per leaf area, and CO2 diffusion efficiency were investigated in 11 Oryza cultivars. K leaf was positively correlated with leaf thickness and related traits, and therefore positively correlated with leaf mass per area and leaf N content per leaf area, and negatively with inter-veinal distance. K leaf was also positively correlated with leaf area and its related traits, and therefore negatively correlated with the proportion of minor vein length per area. In addition, coordination between K leaf and CO2 diffusion conductance in leaves was observed. We conclude that leaf morpho-anatomical traits and N content per leaf area strongly influence K leaf. Our results suggest that more detailed anatomical and structural studies are needed to elucidate the impacts of leaf feature traits on K leaf and gas exchange in grasses. |
| [84] | ABSTRACT Effects of nitrogen (N) supply on the limiting step of CO 2 assimilation rate ( A ) at 380 碌 molmol 鈭1 CO 2 concentration ( A 380 ) at several leaf temperatures were studied in several crops, since N nutrition alters N allocation between photosynthetic components. Contents of leaf N, ribulose 1路5-bisphosphate carboxylase/oxygenase (Rubisco) and cytochrome f (cyt f ) increased with increasing N supply, but the cyt f /Rubisco ratio decreased. Large leaf N content was linked to a high stomatal ( g s ) and mesophyll conductance ( g m ), but resulted in a lower intercellular ( C i ) and chloroplast CO 2 concentration ( C c ) because the increase in g s and g m was insufficient to compensate for change in A 380 . The A - C c response was used to estimate the maximum rate of RuBP carboxylation ( V cmax ) and chloroplast electron transport ( J max ). The J max / V cmax ratio decreased with reductions in leaf N content, which was consistent with the results of the cyt f /Rubisco ratio. Analysis using the C 3 photosynthesis model indicated that A 380 tended to be limited by RuBP carboxylation in plants grown at low N concentration, whereas it was limited by RuBP regeneration in plants grown at high N concentration. We conclude that the limiting step of A 380 depends on leaf N content and is mainly determined by N partitioning between Rubisco and electron transport components. |
| [85] | The photosynthetic rate may be strongly limited by internal conductance from the intercellular airspace to the chloroplast stroma (g(i)). However, the effects of growth and leaf temperature on g(i) are still unclarified. In this work, we determined the temperature dependence of g(i) in spinach leaves grown at 30/25 degrees C (high temperature; HT) and 15/10 degrees C (low temperature; LT), using the concurrent measurements of the gas exchange rate and stable carbon isotope ratio. Moreover, we quantified the effects of g(i) on the temperature dependence of the photosynthetic rate. We measured g(i) and the photosynthetic rate at a CO(2) concentration of 360 microl l(-1) under saturating light (A(360)) at different leaf temperatures. The optimum temperature for A(360) was 28.5 degrees C in HT leaves and 22.9 degrees C in LT leaves. The optimum temperatures for g(i) were almost similar to those of A(360) in both HT and LT leaves. There was a strong linear relationship between A(360) and g(i). The photosynthetic rates predicted from the C(3) photosynthesis model taking account of g(i) agreed well with A(360) in both HT and LT leaves. The temperature coefficients (Q(10)) of g(i) between 10 and 20 degrees C were 2.0 and 1.8 in HT and LT leaves, respectively. This suggests that g(i) was determined not only by physical diffusion but by processes facilitated by protein(s). The limitation of the photosynthetic rate imposed by g(i) increased with leaf temperature and was greater than the limitation of the stomatal conductance at any temperature, in both HT and LT leaves. This study suggests that g(i) substantially limits the photosynthetic rate, especially at higher temperatures. |
2
2002
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... Yamori等(2006)的研究表明, 在30和15 ℃下生长的菠菜(Spinacia oleracea)叶片中, gm达到峰值所对应的温度分别是25和20 ℃; 在田间空气温度(波动范围7-32 ℃)下生长的油橄榄叶片gm的最适温度是29 ℃ (
Differential protein phosphorylation regulates chloroplast movement in response to strong light and darkness in
1
2014
... 研究表明, 光照强度的改变可以调控gm的大小(
Gas-exchange properties of salt stressed olive (Olea europea L.) leaves.
1
1989
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels.
2011
Light induction and the effect of nitrogen status upon the activity of carbonic anhydrase in maize leaves.
1
1990
... 植物单位叶面积的氮含量与光合能力具有显著的正相关关系, 这主要是由于氮含量增加会提高核酮糖-1,5-双磷酸羧化/加氧酶(Rubisco)的含量, 以及可以增加CO2扩散导度(包括gs和gm) (
Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress.
2000
Hydraulic architecture of leafblades: Where is the main resistance?
1
2004
... Kleaf可以反应H2O在叶片内传输的效率问题, H2O从叶柄贯穿到叶片的木质部, 然后到达叶脉周围的维管束, 最后到达蒸发位点从而扩散到空气中(
Seasonal evolution of diffusional limitations and photosynthetic capacity in olive under drought.
1
2007
... Yamori等(2006)的研究表明, 在30和15 ℃下生长的菠菜(Spinacia oleracea)叶片中, gm达到峰值所对应的温度分别是25和20 ℃; 在田间空气温度(波动范围7-32 ℃)下生长的油橄榄叶片gm的最适温度是29 ℃ (
On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-
1
2004
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
Resistances along the CO2 diffusion pathway inside leaves
5
2009
... 影响gm的细胞壁特性主要为厚度和孔隙度.一般认为细胞壁的厚度与gm呈反比, 扩散路径越大, 厚度越厚, gm越小.研究证实, 细胞壁的厚度与gm确实存在负相关关系(
... 下的Rubisco含量越少, 叶绿体越小, 光合速率也就越低(
... ;
... Jia和Davies (2007)报道非原质体内pH值为5.5- 6.0, 可能CA不会影响CO2从细胞壁到细胞膜的传输; 细胞膜和叶绿体膜仅对CO2有通透性作用, 对HCO3-具有不透过性; 同时, 细胞质中的pH值小于叶绿体基质中的pH值, Evans等(2009)认为CA不会催化细胞质中CO2和HCO3-的可逆转化.因此, CA可能并未参与CO2在细胞壁、细胞膜、叶绿体膜和细胞质中的扩散过程.研究证实, 植株中CA主要存在于叶绿体基质中(
... 固定的需求(
Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants.
2
1986
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
Carbon dioxide diffusion inside leaves.
1
1996
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco.
2
1994
... 有研究表明, gm主要由叶片解剖结构决定(
... Pi是扩散路径有效孔隙度, 对于胞液和基质用常数1, 细胞壁的孔隙度用常数0.05 (
A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.
1
1980
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis.
5
2012
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... )同等重要(
... 有研究表明, gm主要由叶片解剖结构决定(
... 叶片组织内H2O和CO2有着共同的传输路径.面向细胞间隙的叶肉细胞面积(Sm)被认为是同时影响gm和Kleaf的因素(
... 同时, 细胞壁孔隙内存在的结合水也影响着水分的传输.事实上gm与Kleaf的相关性不仅取决于叶片的组织结构, 与生化因素(AQPs)也有着一定的关系(
Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management
1
2004
... 水分亏缺是限制植物生长和作物产量的主要环境因素.研究表明, 水分亏缺条件下光合速率下降的根本原因是从外界大气到叶绿体内羧化位点CO2扩散速率的下降(
Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations
2
2002
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... 水分亏缺是限制植物生长和作物产量的主要环境因素.研究表明, 水分亏缺条件下光合速率下降的根本原因是从外界大气到叶绿体内羧化位点CO2扩散速率的下降(
Interspecific differences in temperature response of mesophyll conductance: Food for thought on its origin and regulation.
2
2015
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... 影响gm的细胞壁特性主要为厚度和孔隙度.一般认为细胞壁的厚度与gm呈反比, 扩散路径越大, 厚度越厚, gm越小.研究证实, 细胞壁的厚度与gm确实存在负相关关系(
Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves.
3
2007
... 研究表明, 光照强度的改变可以调控gm的大小(
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
... )、光照强度(
a). Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration.
1
2006
... 水分亏缺是限制植物生长和作物产量的主要环境因素.研究表明, 水分亏缺条件下光合速率下降的根本原因是从外界大气到叶绿体内羧化位点CO2扩散速率的下降(
Mesophyll conductance to CO2: Current knowledge and future prospects.
3
2008
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... Yamori等(2006)的研究表明, 在30和15 ℃下生长的菠菜(Spinacia oleracea)叶片中, gm达到峰值所对应的温度分别是25和20 ℃; 在田间空气温度(波动范围7-32 ℃)下生长的油橄榄叶片gm的最适温度是29 ℃ (
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
b). Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2
1
2006
... 生物膜对脂溶性小分子具有透过性, CO2是亲脂性分子, 能够经过磷脂双分子层进行快速穿膜扩散.早先一直认为CO2在生物膜上的扩散速率非常大, 磷脂双分子层对CO2的阻碍(脂相阻碍)可以忽略不计.然而对水孔蛋白(AQPs)的研究表明, 它不仅介导H2O的跨膜运输, 对CO2的跨膜运输也起着重要作用.Terashima等(2006)认为CO2的穿膜过程主要通过两种途径: 磷脂双分子层和膜上内在蛋白.然而, 不同研究对CO2在生物膜上的通透系数的估测值相差甚大.Missner等(2008)估测膜的通透性是(3.2 ± 1.6) cm·s-1, 认为影响CO2跨膜运输的主要因素是生物膜边界层厚度, 与AQPs关系不大; 而Boron等(2011)在排除AQPs的功能后估测细胞膜通透性为0.015 cm·s-1, 从而认为, AQPs通过影响CO2在生物膜上的运输来调控gm.Uehlein等(2008)证实AQPs基因的沉默降低了叶绿体内CO2的浓度; 而当超表达AQPs时, gm得到了改善, 从而提高了光合速率(
Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination.
2
2013
... CO2是植物进行光合作用的原料, 而H2O是生物体的组成物质, 是进行一切生命活动的必需物质和养分运输的媒介.因此, CO2和H2O在植物中的传输关系备受关注.水力导度(Kleaf)和gm分别是衡量植物叶片内H2O和CO2运输的两个重要变量, 是决定气体交换速率和光合性能的主要指标(
... 一直是研究的热点, 但很少有研究探讨两者之间的协同关系(
Acclimation of Rubisco specificity factor to drought in tobacco: Discrepancies between in vitro and in vivo estimations.
1
2006
... 水分亏缺是限制植物生长和作物产量的主要环境因素.研究表明, 水分亏缺条件下光合速率下降的根本原因是从外界大气到叶绿体内羧化位点CO2扩散速率的下降(
Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms
2
2007
... 水分亏缺是限制植物生长和作物产量的主要环境因素.研究表明, 水分亏缺条件下光合速率下降的根本原因是从外界大气到叶绿体内羧化位点CO2扩散速率的下降(
... ,
Internal conductance to CO2 diffusion and C18OO discrimination in C3 leaves.
1
2000
... CA可以通过催化CO2和HCO3-之间的可逆转换来调节细胞中的pH变化, 促进CO2的传输.近年来, 关于CA对gm产生影响的报道较多, 但结论迥异.Price等(1994)和Williams等(1996)研究发现在CA活性非常低的突变体植株中, 植株光合能力的差异并不大, 认为CA对光合作用无限制作用.但也有作者认为CA的活性具有物种依赖性, 如硬叶植株中CA往往能发挥很大的作用(
Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods.
2013
Variability of mesophyll conductance and its relationship with water use efficiency in cotton leaves under drought pretreatment.
1
2016
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand.
2
2002
... 有研究表明, gm主要由叶片解剖结构决定(
... 研究表明, 光照强度的改变可以调控gm的大小(
The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm temperate forests.
1
1999
... 有研究表明, gm主要由叶片解剖结构决定(
Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants.
1
2004
... 生物膜对脂溶性小分子具有透过性, CO2是亲脂性分子, 能够经过磷脂双分子层进行快速穿膜扩散.早先一直认为CO2在生物膜上的扩散速率非常大, 磷脂双分子层对CO2的阻碍(脂相阻碍)可以忽略不计.然而对水孔蛋白(AQPs)的研究表明, 它不仅介导H2O的跨膜运输, 对CO2的跨膜运输也起着重要作用.Terashima等(2006)认为CO2的穿膜过程主要通过两种途径: 磷脂双分子层和膜上内在蛋白.然而, 不同研究对CO2在生物膜上的通透系数的估测值相差甚大.Missner等(2008)估测膜的通透性是(3.2 ± 1.6) cm·s-1, 认为影响CO2跨膜运输的主要因素是生物膜边界层厚度, 与AQPs关系不大; 而Boron等(2011)在排除AQPs的功能后估测细胞膜通透性为0.015 cm·s-1, 从而认为, AQPs通过影响CO2在生物膜上的运输来调控gm.Uehlein等(2008)证实AQPs基因的沉默降低了叶绿体内CO2的浓度; 而当超表达AQPs时, gm得到了改善, 从而提高了光合速率(
Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2.
2
1992
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls.
2009
The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator.
1
2011
... 生物膜对脂溶性小分子具有透过性, CO2是亲脂性分子, 能够经过磷脂双分子层进行快速穿膜扩散.早先一直认为CO2在生物膜上的扩散速率非常大, 磷脂双分子层对CO2的阻碍(脂相阻碍)可以忽略不计.然而对水孔蛋白(AQPs)的研究表明, 它不仅介导H2O的跨膜运输, 对CO2的跨膜运输也起着重要作用.Terashima等(2006)认为CO2的穿膜过程主要通过两种途径: 磷脂双分子层和膜上内在蛋白.然而, 不同研究对CO2在生物膜上的通透系数的估测值相差甚大.Missner等(2008)估测膜的通透性是(3.2 ± 1.6) cm·s-1, 认为影响CO2跨膜运输的主要因素是生物膜边界层厚度, 与AQPs关系不大; 而Boron等(2011)在排除AQPs的功能后估测细胞膜通透性为0.015 cm·s-1, 从而认为, AQPs通过影响CO2在生物膜上的运输来调控gm.Uehlein等(2008)证实AQPs基因的沉默降低了叶绿体内CO2的浓度; 而当超表达AQPs时, gm得到了改善, 从而提高了光合速率(
Does CO2 permeate through aquaporin-1?
2006
Mechanism of selectivity in aquaporins and aquaglyceroporins.
1
2008
... Hub和de Groot (2006, 2008)基于分子模型的模拟试验表明, CO2穿过水孔蛋白单体消耗的能量多于CO2直接通过磷脂双分子层的运输能量, 这似乎并不合理.然而, 水孔蛋白家族包括大量点突变的同系物, 不同同系物对CO2的通透性存在差异, 可能存在的点突变可以减少运输CO2所消耗的能量(
Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals.
2007
Relationship between hexokinase and the aquaporin PIP1 in the regulation of photosynthesis and plant growth.
1
2014
... 水孔蛋白家族根据序列同源性可以分为5类: 质膜内在蛋白(PIPs)、液泡膜内在蛋白(TIPs)、类Nod26膜内在蛋白(NIPs)、小分子碱性膜内在蛋白(SIPs)以及类GlpF膜内在蛋白(GIPs)(
Adjustment of leaf photosynthesis to shade in a natural canopy: Rate parameters.
1
2005
... 研究表明, 光照强度的改变可以调控gm的大小(
Light- saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration.
2
2009
... 植物单位叶面积的氮含量与光合能力具有显著的正相关关系, 这主要是由于氮含量增加会提高核酮糖-1,5-双磷酸羧化/加氧酶(Rubisco)的含量, 以及可以增加CO2扩散导度(包括gs和gm) (
... 的大小.高氮条件下Rubisco含量的增加势必会造成叶绿体体积的增大(
Estimation of mesophyll conductance to CO2 flux by three different methods.
1
1992
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
The impact of blue light on leaf mesophyll conductance.
2
2009
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... 研究表明, 光照强度的改变可以调控gm的大小(
Distinctive responses of ribulose-1,5-bisphosphate carboxylase and carbonic anhydrase in wheat leaves to nitrogen nutrition and their possible relationships to CO2-transfer resistance.
1
1992
... 植物单位叶面积的氮含量与光合能力具有显著的正相关关系, 这主要是由于氮含量增加会提高核酮糖-1,5-双磷酸羧化/加氧酶(Rubisco)的含量, 以及可以增加CO2扩散导度(包括gs和gm) (
Passive transport across bilayer lipid membranes: Overton continues to rule.
2008
Relationship between mesophyll CO2 gas diffusion conductance and leaf plasma-membrane-type aquaporin contents in tobacco plants grown under drought conditions.
1
2008
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
A new method to estimate photosynthetic parameters through net assimilation reteintercellular space CO2 concentration (A-Ci) curve and chlorophyll fluorescence measurements.
2016
a). Controls on the emission of plant volatiles through stomata: A sensitivity analysis.
2
2003
... 有研究用一维气体扩散模型定量分析不同组织细胞结构对CO2扩散的重要程度(
... Da (m2·s-1) 是气相中CO2扩散系数(25 ℃下是 1.51 × 10-5), fias (m3·m-3)是细胞间隙体积与叶肉总体积的比值.ΣSs代表叶肉细胞横切面面积; tmes代表上下表皮之间叶肉细胞的厚度; W是切片宽度; ΔLias被近似为叶肉厚度的一半; ζ代表扩散路径弯曲度, 用叶片横切面和纵切面进行估算(
b). Controls on the emission of plant volatiles through stomata: Sensitivity or insensitivity of the emission rates to stomatal closure explained.
1
2003
... H是亨利定律常数(m3·mol-1·K-1), R为气体常数(Pa·m3·K-1·mol-1), Tk是绝对温度(K).(R × Tk)/(H × gliq)是亨利定律常数的无因次形式, 可以将气相导度转换为等价的液相导度(
Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field.
1
2009
... 有研究表明, gm主要由叶片解剖结构决定(
Aquaporin tetramer composition modifies the function of tobacco aquaporins.
1
2010
... Hub和de Groot (2006, 2008)基于分子模型的模拟试验表明, CO2穿过水孔蛋白单体消耗的能量多于CO2直接通过磷脂双分子层的运输能量, 这似乎并不合理.然而, 水孔蛋白家族包括大量点突变的同系物, 不同同系物对CO2的通透性存在差异, 可能存在的点突变可以减少运输CO2所消耗的能量(
Relationship between CO2 assimilation and leafanatomical characteristics of two grapevine cultivars.
1
2003
... 有研究表明, gm主要由叶片解剖结构决定(
Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins.
2014
Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut.
1
2002
... 研究表明, 光照强度的改变可以调控gm的大小(
Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation.
1994
Leaf hydraulics.
2
2006
... Kleaf可以反应H2O在叶片内传输的效率问题, H2O从叶柄贯穿到叶片的木质部, 然后到达叶脉周围的维管束, 最后到达蒸发位点从而扩散到空气中(
... 同时, 细胞壁孔隙内存在的结合水也影响着水分的传输.事实上gm与Kleaf的相关性不仅取决于叶片的组织结构, 与生化因素(AQPs)也有着一定的关系(
Hydraulic analysis of water flow through leaves of sugar maple and red oak.
1
2004
... Kleaf可以反应H2O在叶片内传输的效率问题, H2O从叶柄贯穿到叶片的木质部, 然后到达叶脉周围的维管束, 最后到达蒸发位点从而扩散到空气中(
Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions.
1
2014
... 生物膜对脂溶性小分子具有透过性, CO2是亲脂性分子, 能够经过磷脂双分子层进行快速穿膜扩散.早先一直认为CO2在生物膜上的扩散速率非常大, 磷脂双分子层对CO2的阻碍(脂相阻碍)可以忽略不计.然而对水孔蛋白(AQPs)的研究表明, 它不仅介导H2O的跨膜运输, 对CO2的跨膜运输也起着重要作用.Terashima等(2006)认为CO2的穿膜过程主要通过两种途径: 磷脂双分子层和膜上内在蛋白.然而, 不同研究对CO2在生物膜上的通透系数的估测值相差甚大.Missner等(2008)估测膜的通透性是(3.2 ± 1.6) cm·s-1, 认为影响CO2跨膜运输的主要因素是生物膜边界层厚度, 与AQPs关系不大; 而Boron等(2011)在排除AQPs的功能后估测细胞膜通透性为0.015 cm·s-1, 从而认为, AQPs通过影响CO2在生物膜上的运输来调控gm.Uehlein等(2008)证实AQPs基因的沉默降低了叶绿体内CO2的浓度; 而当超表达AQPs时, gm得到了改善, 从而提高了光合速率(
The Role of tobacco aquaporin 1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress.
1
2010
... 水孔蛋白家族根据序列同源性可以分为5类: 质膜内在蛋白(PIPs)、液泡膜内在蛋白(TIPs)、类Nod26膜内在蛋白(NIPs)、小分子碱性膜内在蛋白(SIPs)以及类GlpF膜内在蛋白(GIPs)(
The functional anatomy of rice leaves: Implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice.
1
2009
... CO2从细胞膜扩散到叶绿体膜需要经过细胞质, CO2在细胞质中的扩散路径越长, CO2所受到的阻碍越大.通常, 叶绿体会沿细胞膜排列(
Fitting photosynthetic carbon dioxide response curves for C3 leaves.
1
2007
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves.
1
1995
... Da (m2·s-1) 是气相中CO2扩散系数(25 ℃下是 1.51 × 10-5), fias (m3·m-3)是细胞间隙体积与叶肉总体积的比值.ΣSs代表叶肉细胞横切面面积; tmes代表上下表皮之间叶肉细胞的厚度; W是切片宽度; ΔLias被近似为叶肉厚度的一半; ζ代表扩散路径弯曲度, 用叶片横切面和纵切面进行估算(
Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: An eco-developmental treatise.
2
2005
... Pi是扩散路径有效孔隙度, 对于胞液和基质用常数1, 细胞壁的孔隙度用常数0.05 (
... 叶片组织内H2O和CO2有着共同的传输路径.面向细胞间隙的叶肉细胞面积(Sm)被认为是同时影响gm和Kleaf的因素(
Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion
3
2006
... Pi是扩散路径有效孔隙度, 对于胞液和基质用常数1, 细胞壁的孔隙度用常数0.05 (
... 影响gm的细胞壁特性主要为厚度和孔隙度.一般认为细胞壁的厚度与gm呈反比, 扩散路径越大, 厚度越厚, gm越小.研究证实, 细胞壁的厚度与gm确实存在负相关关系(
... 叶片组织内H2O和CO2有着共同的传输路径.面向细胞间隙的叶肉细胞面积(Sm)被认为是同时影响gm和Kleaf的因素(
Leaf functional anatomy in relation to photosynthesis.
2
2011
... 有研究表明, gm主要由叶片解剖结构决定(
... 影响gm的细胞壁特性主要为厚度和孔隙度.一般认为细胞壁的厚度与gm呈反比, 扩散路径越大, 厚度越厚, gm越小.研究证实, 细胞壁的厚度与gm确实存在负相关关系(
Comparative ecophysiology/ anatomy of leaf and canopy photosynthesis.
1
1995
... Da (m2·s-1) 是气相中CO2扩散系数(25 ℃下是 1.51 × 10-5), fias (m3·m-3)是细胞间隙体积与叶肉总体积的比值.ΣSs代表叶肉细胞横切面面积; tmes代表上下表皮之间叶肉细胞的厚度; W是切片宽度; ΔLias被近似为叶肉厚度的一半; ζ代表扩散路径弯曲度, 用叶片横切面和纵切面进行估算(
Effects of HgCl2 on CO2 dependence of leaf photosynthesis: Evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane.
1
2002
... 水孔蛋白家族根据序列同源性可以分为5类: 质膜内在蛋白(PIPs)、液泡膜内在蛋白(TIPs)、类Nod26膜内在蛋白(NIPs)、小分子碱性膜内在蛋白(SIPs)以及类GlpF膜内在蛋白(GIPs)(
The light response of mesophyll conductance is controlled by structure across leaf profiles.
2017
The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves
2
2008
... 研究表明, 光照强度的改变可以调控gm的大小(
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
Variable mesophyll conductance revisited: Theoretical background and experimental implications.
1
2012
... 有研究用一维气体扩散模型定量分析不同组织细胞结构对CO2扩散的重要程度(
The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion
1
2011
... Jia和Davies (2007)报道非原质体内pH值为5.5- 6.0, 可能CA不会影响CO2从细胞壁到细胞膜的传输; 细胞膜和叶绿体膜仅对CO2有通透性作用, 对HCO3-具有不透过性; 同时, 细胞质中的pH值小于叶绿体基质中的pH值, Evans等(2009)认为CA不会催化细胞质中CO2和HCO3-的可逆转化.因此, CA可能并未参与CO2在细胞壁、细胞膜、叶绿体膜和细胞质中的扩散过程.研究证实, 植株中CA主要存在于叶绿体基质中(
Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models.
3
2013
... 有研究表明, gm主要由叶片解剖结构决定(
... 有研究用一维气体扩散模型定量分析不同组织细胞结构对CO2扩散的重要程度(
... Pi是扩散路径有效孔隙度, 对于胞液和基质用常数1, 细胞壁的孔隙度用常数0.05 (
Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency.
2014
a). Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: How structure constrains function.
1
2012
... 有研究表明, gm主要由叶片解剖结构决定(
b). Anatomicalbasis of variation in mesophyll resistance in eastern Australian sclerophylls: News of a long and winding path.
1
2012
... 影响gm的细胞壁特性主要为厚度和孔隙度.一般认为细胞壁的厚度与gm呈反比, 扩散路径越大, 厚度越厚, gm越小.研究证实, 细胞壁的厚度与gm确实存在负相关关系(
Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability
2008
Determination of the average partial pressure of CO2 in chloroplast from leaves of several C3 plants.
3
1991
... 光合作用是绿色植物利用光能, 把CO2和H2O合成有机物, 同时释放O2的生理过程.CO2首先从外界大气扩散到叶片表层, 然后穿过气孔到达气孔下腔, 最后到达叶绿体羧化位点由羧化酶进行同化.早期一些研究认为CO2从气孔下腔到叶绿体羧化位点的扩散阻力(rm, 其倒数为叶肉导度(gm)趋近于无穷小, 从而将CO2对光合速率的限制简化为气孔和非气孔(羧化)两个因素.基于这种假设, 在Farquhar-von-Caemmerer-Berry (FvCB)光合模型中, 叶绿体羧化位点的CO2浓度(Cc)采用胞间CO2浓度(Ci)替代(
... 有研究表明, gm主要由叶片解剖结构决定(
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply.
1
2004
... 植物单位叶面积的氮含量与光合能力具有显著的正相关关系, 这主要是由于氮含量增加会提高核酮糖-1,5-双磷酸羧化/加氧酶(Rubisco)的含量, 以及可以增加CO2扩散导度(包括gs和gm) (
Stand aside stomata, another actor deserves centre stage: The forgotten role of the internal conductance to CO2 transfer.
1
2008
... 目前, 估算gm最常用的3种方法包括气体交换和叶绿素荧光同步测定法(
Internal conductance to CO2 transfer of adult Fagus sylvatica: Variation between sun and shade leaves and due to free-air ozone fumigation.
1
2007
... 研究表明, 光照强度的改变可以调控gm的大小(
Photosynthetic gas exchange and discrimination against 13CO2, and C18O16O in tobacco plants modified by an antisense construct to have low chloroplastic carbonic anhydrase.
1996
Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in
2
2016
... 叶片组织内H2O和CO2有着共同的传输路径.面向细胞间隙的叶肉细胞面积(Sm)被认为是同时影响gm和Kleaf的因素(
... 的影响也是至关重要(
b). Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice.
2015
a). Leaf hydraulic conductance is coordinated with leaf morpho- anatomical traits and nitrogen status in the genus
2015
The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species.
1
2011
... 植物单位叶面积的氮含量与光合能力具有显著的正相关关系, 这主要是由于氮含量增加会提高核酮糖-1,5-双磷酸羧化/加氧酶(Rubisco)的含量, 以及可以增加CO2扩散导度(包括gs和gm) (
Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures.
2006

