0 引言
【研究意义】组蛋白修饰调控是基因转录调控机制中的重要组成部分,其灵活快速的调控特点在细胞对微环境的应答过程中起到了重要作用。在哺乳动物基因组中,组蛋白具有多重修饰形式,包括组蛋白末端的乙酰化[1]、甲基化[2]、磷酸化[3]、泛素化[4]、ADP核糖基化[5]等。这些修饰模式多样,在特定的细胞和状态中对基因转录起到多样化的调控作用。【前人研究进展】 促卵泡素(follicle stimulating hormone,FSH)是垂体前叶嗜酸性细胞分泌的一种糖蛋白激素,能与位于颗粒细胞膜上的特异性受体结合,激活细胞内信号通道,对卵巢卵泡发育,激素分泌过程有重要的调节[6,7]外培养的颗粒细胞中,FSH能促进细胞生长,抑制细胞凋亡[8],影响类固醇的生成,如促进孕酮的合成和分泌以及雌激素合成能力[9]。类固醇合成酶是一类催化性腺中类固醇激素合成的酶类,其催化合成的激素包括睾酮、孕酮和雌二醇等[10]。其中类固醇合成快速调节蛋白(StAR,由STAR基因编码)负责将胆固醇由线粒体外膜或细胞质向线粒体内膜运送[11];P450胆固醇侧链裂解酶(P450scc,由CYP11A1基因编码)催化胆固醇形成孕烯醇酮[12,13];随后在3b-羟甾脱氢酶(3b-HSD,由HSD3B基因编码)的作用下生成孕酮。孕酮是类固醇合成过程中的基础甾体,在卵泡膜细胞中,由C17-20裂解酶(P450c17,由CYP17C编码)催化生成睾酮[14]。最后在颗粒细胞中由芳香化酶(P450arom,由CYP19编码)氧化脱去19-甲基,芳香构化转变成C18雌激素(雌酮和雌二醇)[15] 。FSH对垂体激素受体和凋亡相关基因的诱导具有时间上和物种间的差异,也与体外培养的条件有关。【本研究切入点】一般认为H3K4的2, 3甲基化可能与转录抑制相关,而联合参入的H3K9与 H3K14的乙酰化可能与转录激活相关,单独的H3K14的乙酰化则与转录抑制相关[16,17]。体外培养的颗粒细胞是卵巢研究中常用的实验对象,研究者们对FSH处理引起的基因应答已有一定认识,但组蛋白修饰在这些应答中是否参与,如何参与,是否具有特异性等问题尚不明确。【拟解决的关键问题】本文旨在明确体外培养的猪颗粒细胞中类固醇合成酶基因在FSH处理下的转录变化情况,并探索这些基因转录水平变化与其调控区组蛋白修饰变化的关系。对各组蛋白修饰对FSH的精确应答时间及其在基因调控区的详细分布状况进行深入研究,绘制组蛋白应答的时空图谱,将进一步完善FSH对颗粒细胞生理的调控原理。1 材料与方法
本研究于2016年9月至2017年2月在南京农业大学动物科学类实验教学中心(国家实验教学示范中心)完成。1.1 样品采集
猪卵巢选自淮安苏食肉品屠宰点,屠宰后10min内采集双侧卵巢,置于37℃含双抗(青霉素、链霉素各100U?mL-1)的生理盐水中,3h内带回实验室进行后续试验。1.2 颗粒细胞培养
清洗卵巢,用注射器抽取直径为3—6mm卵泡的卵泡液及颗粒细胞,800×g离心5min去除卵泡液;PBS清洗颗粒细胞两次,800×g离心5min去除PBS后,用完全培养基(含体积分数15%胎牛血清和100 U?mL-1双抗DMEM/F12培养基)重悬细胞,充分吹打至散开并接种于T25培养瓶中;放置于细胞培养箱内,37℃,5%CO2培养。24h后观察颗粒细胞贴壁情况,弃去培养基及未贴壁的颗粒细胞、卵母细胞,清洗贴壁的颗粒细胞,更换培养基继续培养;待细胞长至90%汇合度时传代;传代时用PBS清洗细胞两次,加入1mL胰酶消化细胞,观察到大部分细胞漂起时即加入适量完全培养基终止消化,吹打分散细胞;800×g离心5min,弃去上清,并用完全培养基重悬细胞后接种于6孔板中(约105个/孔)继续后续试验。1.3 FSH激素处理
正常培养颗粒细胞48h后,换无血清DMEM/F12培养基培养16h,试验组加入5IU/mL的FSH(宁波第二激素厂),对照组加入同等体积PBS,继续培养24h。1.4 RNA提取及反转录
用PBS冲洗细胞两次,按照Trizol试剂(Invitrogen公司)说明书提取颗粒细胞RNA(每个孔用量1mL),紫外比色法测定总RNA的浓度和纯度,用1.4%甲醛变性琼脂糖凝胶电泳检测总RNA的质量。取1μg质量合格的总RNA用M-MLV 反转录酶(Promega公司)和oligo(dT)18进行cDNA第一链合成,具体步骤按说明书操作。cDNA于-20℃保存备用。1.5 染色质免疫沉淀(ChIP)
PBS清洗颗粒细胞2次,用终浓度1%的甲醛体外交联颗粒细胞,从培养瓶中收集细胞后,1%SDS裂解液裂解细胞,超声波断裂染色质,加特异性抗体(H3K4me2、H3K4me3、H3K9ac和H3K14ac,CST公司)进行免疫共沉淀,洗脱与逆转交联后,用DNA纯化试剂盒(Qiagen公司)进行DNA纯化,最后以沉淀所得的DNA为模板,进行qPCR。1.6 引物设计与qPCR反应
定量引物设计:根据GenBank数据库中收录的基因mRNA序列和qPCR反应要求,用Primer Premier5.0软件设计引物;ChIP引物设计:根据GenBank数据库中收录的相应基因序列,选取转录起始位点到上游1 000bp以内位置,用Primer Premier5.0软件设计引物。全部引物由上海英骏生物技术有限公司合成,使用时用ddH2O稀释至工作浓度。实时荧光定量PCR反应依试剂盒(Takara公司)说明操作。根据文献[18]的方法进行数据分析,即ΔCT= CT目标基因-CT内参基因,目标基因的CT 相对于内参基因的CT 为2-ΔCT,转基因植株基因表达的变化表示为对照的2-ΔCT记为1时目标基因2-ΔCT的相对值(即2-ΔΔCT),各基因表达量用均值表示。引物序列信息和扩增条件见表1。Table 1
表1
表1PCR引物及反应条件
Table 1Primer and PCR reaction conditions
| 基因Gene | 登陆号Acc. No. | 引物(5'- 3')Primer (5'- 3') | 产物长度Product length(bp) |
|---|---|---|---|
| STAR | XM_005671764.2 | F: GCTGAGCCCTTTCGTGTCTA | 140 |
| R: CATAGGACCTGCCGTGTCTG | |||
| CYP11A1 | NM_214427.1 | F: AGACACTGAGACTCCACCCCA | 110 |
| R: GACGGCCACTTGTACCAATGT | |||
| HSD3B | NM_001004049.1 | F: CCCAGTGTTTTCTGGTTCCT | 131 |
| R: TTCTCCTCCAGCAACAAGTG | |||
| CYP19A | NM_214431.1 | F: TGCTGGACACCTCTAACAA | 264 |
| R: TCAACTCAGTGGCGAAAT | |||
| FSHR | NM_214386.3 | F: GAATTGAAAAGGCCAACAAC | 214 |
| R: CTTTCAAAACTTAGTCCCACG | |||
| LHR | NM_214449.1 | F: ACTCCAATGTGCTCCCGAAC | 222 |
| R: AGTAGCAGGTAGAGCCCCAT | |||
| XIAP | NM_001097436.1 | F: ACTGGCCAGACTATGCTCAC | 107 |
| R: CAGTTTCCCGCCACAACAAA | |||
| FasL-2 | AY033634 | F: CCACCACTCCTGCCATCAA | 135 |
| R: CAGCCCCAATCCAACCA | |||
| GAPDH | AF017079 | F: GGACTCATGACCACGGTCCAT | 220 |
| R: TCAGATCCACAACCGACACGT | |||
| STAR-ChIP | chr15:55501189 -55501271 | F: GAGCAACATTCCTCCCTAGACT | 83 |
| R: CAGCCCCAATCCAACCA | |||
| HSD3B-ChIP | chr4:111565035 -111565175 | F: GCCGGCTGTGACTATTTGGA | 141 |
| R: TGCTTCAAGGACTGTGTCCC | |||
| CYP19A-ChIP | chr1:133903003 -133903130 | F: ACCCTTAACTCAAAAGAACCCA | 128 |
| R: AATTTTGCAGTGGCGGGATT |
新窗口打开
2 结果
2.1 FSH处理对颗粒细胞基因转录水平的影响
2.1.1 类固醇合成酶基因 本试验共检测了4个类固醇合成酶基因的表达水平,分别为STAR、CYP11A1、HSD3B和CYP19A1。在FSH处理24h后的颗粒细胞中,STAR、HSD3B和CYP19A1的表达水平有显著上升,相比对照组而言,处理组的表达水平分别达到了2倍(P<0.01)、2.8倍(P<0.01)和3.6倍(P<0.05)。而CYP11A1表达水平没有显著变化(图1)。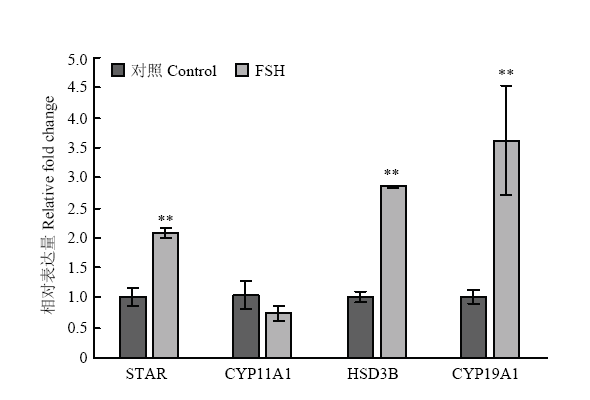 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1FSH处理对颗粒细胞类固醇合成酶基因的表达水平的影响
*. P<0.05,**. P<0.01 下同
-->Fig. 1Steroidogenic enzymes expression in response to FSH treatment in granulosa cells
*. P<0.05,**. P<0.01 The same as below
-->
2.1.2 垂体激素受体基因 颗粒细胞中垂体激素FSH和LH的受体FSHR和LHR的mRNA表达水平在FSH处理24h后相比对照组没有显著差异(图2)。
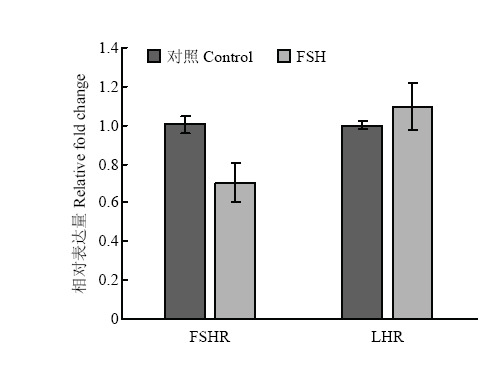 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图2FSH处理对颗粒细胞垂体激素受体基因的表达水平的影响
-->Fig. 2Pituitary hormone receptors expression in response to FSH treatment in granulosa cells
-->
2.1.3 凋亡相关基因 对抗凋亡基因XIAP和凋亡标志基因FasL-2表达水平进行检测,结果证明FSH处理并没有显著改变颗粒细胞中这两个凋亡相关基因的表达水平(图3)。
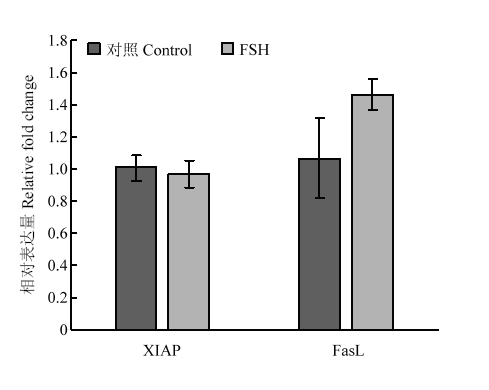 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图3FSH处理对颗粒细胞凋亡相关基因的表达水平的影响
-->Fig. 3Apoptosis related genes expression in response to FSH treatment in granulosa cells
-->
2.2 FSH处理对颗粒细胞类固醇合成酶基因调控区组蛋白修饰的影响
2.2.1 HSD3B基因的组蛋白修饰变化 在FSH处理24 h后,HSD3B基因上游调控区的组蛋白H3修饰发生了显著变化(图4)。其中H3K4me2、H3K4me3、H3K4ac和H3K14ac的结合水平分别上升了14.7倍(P<0.01)、13.6倍(P<0.01)、19.7(P<0.01)倍和2.5倍(P<0.05),差异均达到了显著水平。结果说明HSD3B基因转录调控与所检测的组蛋白修饰关联最为紧密。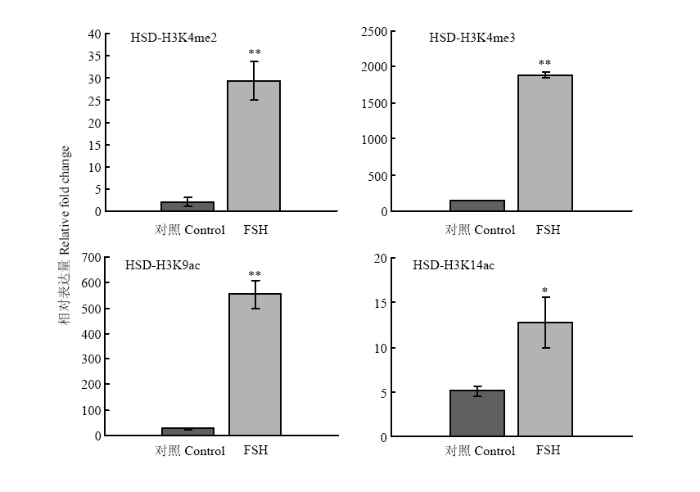 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图4FSH处理对颗粒细胞HSD3B基因上游调控区组蛋白修饰变化的影响
-->Fig. 4Histone modification on HSD3B gene regulatory region in response to FSH treatment in granulosa cells
-->
2.2.2 STAR、CYP19A基因的组蛋白修饰变化 在FSH处理24h后,STAR基因调控区的H3K9ac在处理后有11.1倍的显著下降(P<0.05, 图5);CYP19A 基因调控区的H3K4me3和H3K9ac分别有0.5倍(P<0.01)的上调和10.4倍(P<0.01)的下降,其余组蛋白修饰在处理前后没有显著变化(图6)。结果说明组蛋白修饰具有基因特异性,在不同类固醇合成酶基因中的参与有所不同。
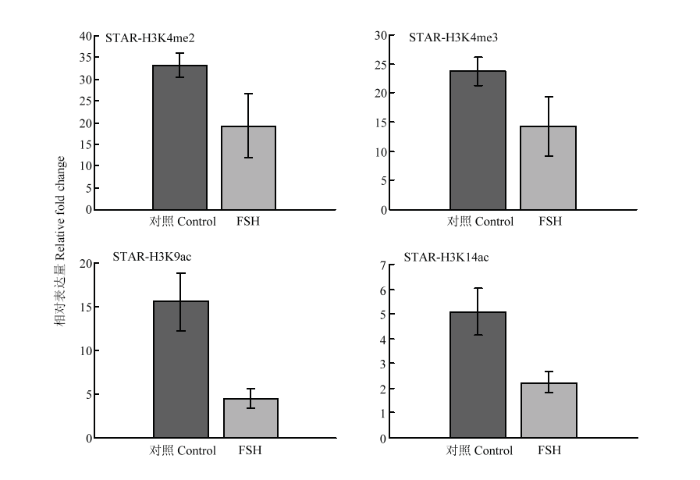 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图5FSH处理对颗粒细胞STAR基因上游调控区组蛋白修饰变化的影响
-->Fig. 5Histone modification on STAR gene regulatory region in response to FSH treatment in granulosa cells
-->
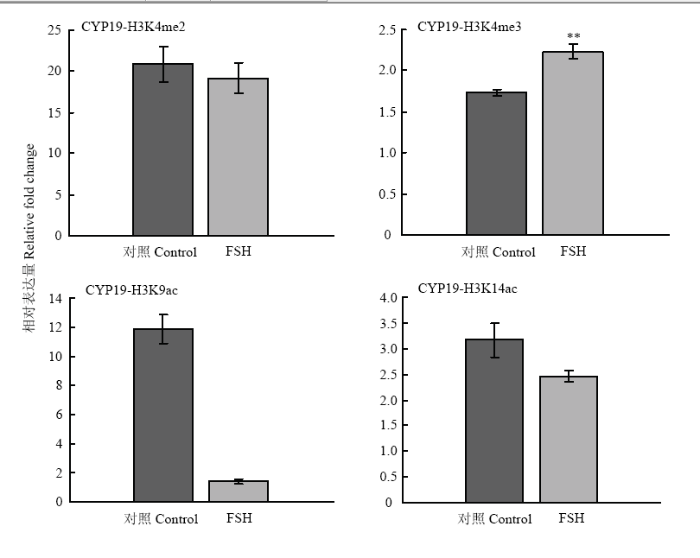 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图6FSH处理对颗粒细胞CYP19A基因上游调控区组蛋白修饰变化的影响
-->Fig. 6Histone modification on CYP19A gene regulatory region in response to FSH treatment in granulosa cells
-->
3 讨论
3.1 FSH提升类固醇合成酶基因表达水平
FSH在雌性动物中能抑制卵泡凋亡、促进卵泡的生长、颗粒细胞增殖以及类固醇激素的合成和分泌[19]。笔者的试验证实,24h的FSH处理让卵泡类固醇合成3个主要合成酶基因STAR、HSD3B和CYP19A1的表达量有了显著上升。在卵巢类固醇激素合成过程中(图7),STAR编码的类固醇合成快速调节蛋白负责类固醇“原料”胆固醇的转运,而HSD3B编码的3b-羟甾脱氢酶是孕酮的主要合成酶,这两种酶表达量的升高理论上为进一步合成雌激素提供了反应底物。而CYP19编码的芳香化酶是雌激素合成的关键酶,其表达水平上升与FSH对颗粒细胞雌激素合成的影响和对卵泡健康发育的维持功能相吻合。同时,另一个合成酶基因CYP11A1的表达量却没有显著变化,一方面可能由于其编码的胆固醇侧链裂解酶及其催化产物孕烯醇酮可能在短期内较为充足,不会限制后续类固醇的合成,另一方面可能由于24h的处理时间不足让此基因在表达水平上产生回应。在小鼠和大鼠体外培养的颗粒细胞模型中有研究报道STAR对FSH的应答极快,3h后既能检测到mRNA的上调,而CYP11A1应答较为缓慢,在24h才检测到mRNA的表达变化[20],这一结果支持了后一种可能。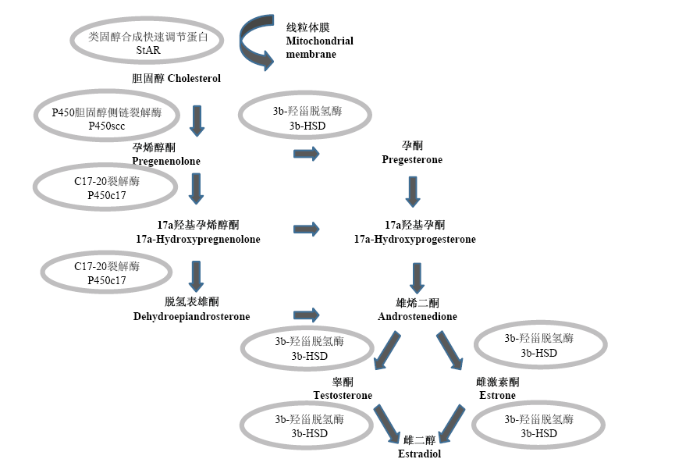 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图7类固醇激素的合成
类固醇用加粗表示,相应合成酶用椭圆表示
-->Fig. 7Biosynthesis of steroid hormones
Steroid hormones are in bold, steroidogenic enzymes are encircled
-->
3.2 FSH处理对垂体激素受体的影响
除了类固醇合成酶,本研究对垂体激素受体基因FSHR和LHR的表达水平进行了检测。在雌性动物卵泡中,FSH和雌激素的协同作用会促进颗粒细胞表达更多FSH及LH受体,增加卵泡对促性腺激素的敏感性,较高的LH受体水平是大卵泡走向成熟和排卵的关键因素之一。但在体外培养的猪颗粒细胞中,24h的FSH处理并没有导致显著的FSHR和LHR水平变化。在其他物种的类似研究中发现,48h的FSH处理能诱导体外培养的小鼠颗粒细胞FSHR水平升高[21], 24h的FSH处理在大鼠颗粒细胞中引起FSHR显著升高[22],在体外培养的羊卵丘卵母细胞复合体(COC)中,10IU?mL-1浓度的FSH能显著上调FSHR和LHR的mRNA水平[23]。然而,在牛颗粒细胞中,单独的FSH处理对FSHR的mRNA水平没有显著影响,仅有在与5-α-二氢睾丸酮(DHT)联合处理细胞时才能引起FSHR水平的升高[24]。在LHR对FSH的应答方面,早年有报道证实LHR对FSH的应答需要颗粒细胞和其他类型细胞的协同配合,因此只有在体内、卵泡培养中才能观测到LHR上调。培养体系中的血清对LHR的应答有较大影响,大鼠无血清培养的颗粒细胞中也有LHR上调的报道[25]。结合的试验结果和他人的研究结论可见,FSH在猪、牛等动物中的作用与在小鼠、大鼠等物种中有所差别,FSH对垂体激素受体的诱导具有时间上和物种间的差异,另外也与体外培养的条件有关。3.3 FSH处理对凋亡相关基因没有显著影响
对抗凋亡基因X连锁凋亡抑制蛋白(XIAP)和凋亡标志基因FasL-2的表达水平的检测证明FSH处理对这2个基因表达没有显著影响。在体内试验和体外培养的卵泡中均有研究证实FSH处理能上调XIAP表达水平[26,27],而下调FasL的表达水平[28]。但由于体外培养的颗粒细胞模型与完整卵泡相比,在FSH应答方面有一定差异,单独的FSH处理不足以引起XIAP和FasL-2的转录变化,但与其他激素,如甲状腺激素三碘甲状腺氨酸(T3)的联合处理则能诱导这两个基因的显著变化[29]。3.4 FSH处理对调控区组蛋白修饰的影响具有基因特异性
目前,对FSH处理的颗粒细胞模型中组蛋白修饰变化的研究鲜有报道,卵泡中的组蛋白修饰研究较多集中在黄体化过程中,在黄体化的大鼠体内试验中,卵巢颗粒细胞对LH的应答使STAR和CYP19A1转录水平发生变化,而这些变化与H4ac和H3K4me3修饰显著相关[30]。DEBORAH等证实了全染色质水平的H3S10磷酸化在FSH处理24h后有显著上升 [31]。本研究首次在基因水平上对体外培养的猪颗粒细胞组蛋白修饰对FSH处理的反应进行了检测,结果证明组蛋白修饰具有基因特异性。在3个受FSH调控上调的类固醇合成酶基因中,HSD3B的调控区组蛋白H3K4me2、H3K4me3、H3K9ac和H3K14ac修饰显著提升,CYP19A1基因调控区的H3K4me3修饰也有显著上升,这些修饰在多数情况下均与转录活化相关[32];相反,STAR和CYP19A1基因调控区的H3K9ac在处理后有所下降,可见FSH处理对调控区组蛋白修饰的影响具有基因特异性。4 结论
FSH处理让卵泡类固醇合成3个主要合成酶基因STAR、HSD3B和CYP19A1的表达量有了显著上升,CYP11A1应答较为缓慢。FSH对垂体激素受体和凋亡相关基因的诱导具有时间上和物种间的差异,也与体外培养的条件有关。对上游调控区组蛋白的检测发现这些基因的表达变化与组蛋白修饰有关,其中HSD3B的表达增高伴随着组蛋白H3K4me2、 H3K4me3、H3K9ac和H3K14ac修饰的显著提升。本研究为进一步完善FSH对卵巢颗粒细胞的影响及调控机理提供了参考。The authors have declared that no competing interests exist.
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | . |
| [2] | . |
| [3] | . |
| [4] | . DNMT1 is recruited by PCNA and UHRF1 to maintain DNA methylation after replication. UHRF1 recognizes hemimethylated DNA substrates via the SRA domain, but also repressive H3K9me3 histone marks with its TTD. With systematic mutagenesis and functional assays, we could show that chromatin binding further involved UHRF1 PHD binding to unmodified H3R2. These complementation assays clearly demonstrated that the ubiquitin ligase activity of the UHRF1 RING domain is required for maintenance DNA methylation. Mass spectrometry of UHRF1-deficient cells revealed H3K18 as a novel ubiquitination target of UHRF1 in mammalian cells. With bioinformatics and mutational analyses, we identified a ubiquitin interacting motif (UIM) in the N-terminal regulatory domain of DNMT1 that binds to ubiquitinated H3 tails and is essential for DNA methylation in vivo. H3 ubiquitination and subsequent DNA methylation required UHRF1 PHD binding to H3R2. These results show the manifold regulatory mechanisms controlling DNMT1 activity that require the reading and writing of epigenetic marks by UHRF1 and illustrate the multifaceted interplay between DNA and histone modifications. The identification and functional characterization of the DNMT1 UIM suggests a novel regulatory principle and we speculate that histone H2AK119 ubiquitination might also lead to UIM-dependent recruitment of DNMT1 and DNA methylation beyond classic maintenance. |
| [5] | . Most published work on post-translational histone modifications focuses on small covalent alterations such as acetylation, methylation and phosphorylation. By contrast, fewer data are available on the modification of histones by ADP-ribose. Discussion of the biological significance of histone ADP-ribosylation has often been restricted to functions of the modifying enzymes, rather than to histones as ADP-ribose acceptors. In particular, the identification of specific lysine residues as ADP-ribose acceptor sites in histones and the identification of ADP-ribose binding modules raise this modification to a par with acetylation, methylation or phosphorylation. We discuss here the functional aspects of histone ADP-ribosylation and its influence on DNA repair, replication and transcription. |
| [6] | . In the human species, follicle growth is a very long process. The ovulatory follicle originates from a cohort of pre-antral follicles that have differentiated their theca interna 85 days earlier under the control of the high peri-ovulatory steroid and gonadotrophin levels. During growth the number of follicles decreases by atresia under the influence of factors relating to the size of the follicles. Their growth rate is regulated by intra- and extra-ovarian hormonal factors. At the end of the luteal phase, 15-20 days before ovulation, the next ovulatory follicle is recruited among a follicle population of 2-5 mm in diameter. At the beginning of the menstrual cycle it can be distinguished only by its size; a few days later it starts its maturation and exhibits its dominance on the other healthy follicles present in the two ovaries. |
| [7] | . Follicle-stimulating hormone (FSH) is necessary and sufficient to induce maturation of ovarian follicles to a mature, preovulatory phenotype in the intact animal, resulting in the generation of mature eggs and production of estrogen. FSH accomplishes these actions by inducing a complex pattern of gene expression in target granulosa cells that is regulated by input from many different signaling cascades, including those for the extracellular regulated kinases (ERKs), p38 mitogen-activated protein kinases (MAPKs), and phosphatidylinositol-3 kinase (PI3K). The upstream kinase that appears to be responsible for initiating all of the signaling that regulates gene expression in these epithelial cells is protein kinase A (PKA). PKA not only signals to directly phosphorylate transcription factors like cAMP response element binding protein and to promote chromatin remodeling by phosphorylating histone H3, this versatile kinase also enhances the activity of the p38 MAPK, ERK, and PI3K pathways. Additionally, accumulating evidence suggests that activation of a single signaling cascade downstream of PKA is not sufficient to activate target gene expression. Rather, cross-talk between and among signaling cascades is required. We will review the signaling cascades activated by FSH in granulosa cells and how these cascades contribute to the regulation of select target gene expression. |
| [8] | . Abstract To investigate the effect of thyroid hormone on the proliferative activity and apoptosis of granulosa cells at the varying stages of follicular growth, porcine granulosa cells obtained from small (1-2 mm), medium (3-5 mm) and large (6-11 mm) follicles were cultured under a serum-free condition in the presence or absence of follicle stimulating hormone (FSH; 20 ng/ml), with or without triiodothyronine (T3; 10-8M). Relative viability, proliferative activity, and apoptosis of cultured granulosa cells were evaluated with 3-(4.5-dimethylahiazol-2-yl)-2,5-diphenyltetrazolium bromide [MTT] assay, Ki67 expression and activated caspase-3 protein expression, respectively. MTT assay showed that T3 had no significant effect on the relative viability of granulosa cells regardless of the follicle size. Ki67-positive rate in small follicle granulosa cells was augmented by treatment with FSH whereas it was not affected by T3. Furthermore, FSH treatment decreased activated caspase-3 protein-positive rate of small follicle granulosa cells. Relative to the treatment with FSH alone, concomitant treatment with FSH and T3 resulted in further decrease in caspase-3 protein-positive rate in small follicle granulosa cells. Treatment with T3 alone did not affect the caspase-3 protein-positive rate. These results suggest that thyroid hormone synergizes with FSH to inhibit apoptosis in small follicle granulosa cells without affecting the proliferative potential of those cells. |
| [9] | . <P>Abstract: Ovarian follicles are composed of granulosa cells (GC), which undergo apoptosis within 24 hours of culture in serum-free medium. The present study was designed to assess the role of progesterone in regulating human GC survival. Human GC were isolated from follicular aspirates of women undergoing in vitro fertilization. GC were then cultured for 24 hours in serum-free media supplemented with progesterone and/or the progesterone antagonist RU486 and dexamethasone. Cells were then fixed and assessed for apoptosis by in situ end labeling of DNA fragments, cell cycle analysis of DNA content, and electron microscopy. When compared with controls, progesterone reduced and RU486 increased the percentage of apoptotic GC (p <0.05), whereas dexamethasone had no effect. In addition, RU486 inhibited the protective effect of progesterone on GC survival (p <0.05). Taken together, these data indicate that progesterone inhibits human GC apoptosis, and this effect is mediated through the progesterone receptor.</P> |
| [10] | . Significant advances have taken place in our knowledge of the enzymes involved in steroid hormone biosynthesis since the last comprehensive review in 1988. Major developments include the cloning, identification, and characterization of multiple isoforms of 3beta-hydroxysteroid dehydrogenase, which play a critical role in the biosynthesis of all steroid hormones and 17beta-hydroxysteroid dehydrogenase where specific isoforms are essential for the final step in active steroid hormone biosynthesis. Advances have taken place in our understanding of the unique manner that determines tissue-specific expression of P450aromatase through the utilization of alternative promoters. In recent years, evidence has been obtained for the expression of steroidogenic enzymes in the nervous system and in cardiac tissue, indicating that these tissues may be involved in the biosynthesis of steroid hormones acting in an autocrine or paracrine manner. This review presents a detailed description of the enzymes involved in the biosynthesis of active steroid hormones, with emphasis on the human and mouse enzymes and their expression in gonads, adrenal glands, and placenta. |
| [11] | . Transcriptional regulation of steroidogenic acute regulatory protein (StAR) determines adrenal and gonadal cell steroidogenesis. Chromatin immunoprecipitation assays were combined with quantitative real-time polymerase chain reaction to assess histone acetylation associated with the StAR promoter. MA-10 cells treated with 8-bromo-cAMP had increased acetylated histone H3 associated with the proximal (but not distal) StAR promoter, nascent StAR transcripts, and progesterone production within 15 min, whereas StAR mRNA increased at 30 min. At 360 min, steroidogenesis remained elevated, but mRNA, nascent RNA, and StAR promoter-associated H3 acetylation all declined. StAR promoter-associated H4 acetylation was unchanged by 8-bromo-cAMP treatment of MA-10 cells. analysis of macaque and human granulosa cells showed that luteinization was associated with increased StAR promoter-associated H3 acetylation. We conclude that acetylation of H3 (but not H4) associated with the proximal promoter is associated with StAR gene transcription, that chromatin modification occurs in discrete regions of the promoter, that the initial steroidogenic response to 8-bromo-cAMP occurs prior to increased StAR mRNA accumulation, and that MA-10 cell StAR gene transcription and promoter-associated H3 acetylation are biphasic during a 6-h treatment period. The union of the chromatin immunoprecipitation assay with quantitative real-time polymerase chain reaction described and validated here should enhance the analysis of gene expression. |
| [12] | . Corpora lutea were collected from cows at four stages of the luteal phase and prepared for immunostaining at the light microscope level. Other corpora lutea, which were fully developed, were dispersed by collagenase treatment and freshly isolated and cultured cells were processed for immunostaining. Electron microscopy was carried out on mature corpora lutea and freshly isolated cells. Positive staining for cholesterol side-chain-cleavage cytochrome P-450 (P-450scc), an inner-mitochondrial membrane enzyme considered to catalyse the rate-limiting step in the conversion of cholesterol to progesterone, was observed in all corpora lutea. The intensity of staining was much greater in mature corpora lutea than in young or regressing corpora lutea. Only small and large luteal cells stained positively and cells of the vasculature and other connective tissue elements did not. When cells were cultured and had become flatter, the intensity of immunostaining was observed to be greater in large luteal cells than in small luteal cells which was interpreted to be due, in part, to the greater volume density of mitochondria in these cells. In some cultured small luteal cells the pattern of immunostaining appeared as whorls of strands encircling the nucleus. This pattern was interpreted as a three-dimensional network of mitochondria organized into 'strands', more than one mitochondrion in cross-section, perhaps formed during the process of attachment and elongation of the cells. Further observations made at the electron microscope level, included the presence of close (5-8 nm) contacts with interconnecting septa between small luteal cells in tissue. |
| [13] | . The objective of the present study was to determine the changes in follicular fluid steroid concentrations and in granulosa cell steroidogenic enzyme expression during the follicular phase, in relation to follicular size and physiological status in the mare. |
| [14] | . The objective of the present study was to characterize the effect of insulin plus hCG on the expression of steroidogenic enzymes (P450scc and CYP17) in polycystic ovaries of rats. Changes in estrous cycle, ovarian morphology, hormonal levels, and protein levels by immunohistochemistry and western-blot were determined. Rats treated with insulin plus hCG displayed abnormal estrous cycles with increasing androgen biosynthesis. Meanwhile, insulin plus hCG resulted in multiple large cysts with diminished granulosa layers and increased thecal layers and stromal-interstitial tissue. Moreover, there was an increase in the expression of P450scc and CYP17 in thecal and stromal cells in our PCOS rat model compared with control rats. These results indicate that administration of insulin with hCG can synergistically result in endogenous hyperandrogenism which may partially upregulate the expression of steroidogenic enzymes in ovarian tissue. |
| [15] | . Although there have been extensive studies on the effects of gonadotrophins and steroids on follicular development, less is known as to the effects these hormones have on the acquisition of oocyte developmental competence. This study investigates the effect of altering the gonadotrophin or steroidal environment on follicular development and on oocyte viability and DNA methylation. Oocytes were obtained from pre-ovulatory follicles after individual follicle culture from the pre-antral stage; gonadotrophin or steroid levels were manipulated during the culture period. Oocytes obtained from follicles grown in gonadotrophin free conditions were able to fertilize and develop to the blastocyst stage despite their impaired follicle development. There was no effect of luteinizing hormone or steroids on follicular growth. Altering the steroidal environment did, however, affect oocyte development. The oocytes of follicles exposed to high estrogen levels had lower fertilization rates, regardless of the presence or absence of high androgen levels. The combined presence of high levels of both steroids altered the level of global methylation. This study demonstrates that gonadotrophins and steroids influence the acquisition of developmental competence of the oocyte and suggests that optimal steroid exposure during follicle development is required for the oocyte to mature correctly. |
| [16] | . |
| [17] | . The monocytic leukemia zinc-finger protein-related factor (MORF) is a transcriptional coactivator and a catalytic subunit of the lysine acetyltransferase complex implicated in cancer and developmental diseases. We have previously shown that the double plant homeodomain finger (DPF) of MORF is capable of binding to acetylated histone H3. Here we demonstrate that the DPF of MORF recognizes many newly identified acylation marks. The mass spectrometry study provides comprehensive analysis of H3K14 acylation states invitro and invivo. The crystal structure of the MORF DPF-H3K14butyryl complex offers insight into the selectivity of this reader toward lipophilic acyllysine substrates. Together, our findings support the mechanism by which the acetyltransferase MORF promotes spreading of histone acylation. |
| [18] | . |
| [19] | . Human gonadotrophin preparations have been used in the treatment of infertility for almost four decades. The earliest preparations were derived from urine from postmenopausal women and contained approximately equal amounts of follicle stimulating hormone (FSH) and luteinizing hormone (LH) activities. However, with the recognition that FSH is the principal regulator of follicular growth and maturation, these have been largely superseded by highly purified urinary FSH preparations and, more recently, recombinant human FSH (r-hFSH). Because of its complexity, r-hFSH is expressed in mammalian cells grown in culture and, from a manufacturing stand point, offers superior purity and batch-to-batch consistency, compared with urinary preparations. A number of clinical trials have compared the efficacy of r-hFSH and urinary FSH in women undergoing assisted reproductive technologies (ART). In general, these have shown that fewer FSH ampoules are required to achieve ovarian stimulation with r-hFSH, while the number of oocytes retrieved and embryos produced are higher than with urinary FSH. Additionally, the results of a recent meta-analysis have also shown that the clinical pregnancy rate per cycle started is significantly higher with r-hFSH, compared with urinary FSH. Furthermore, in poor responder patients, higher implantation rates were seen in patients treated with r-hFSH than in those treated with urinary FSH, suggesting that embryo viability is increased following use of the recombinant preparations. The finding that FSH preparations produce effective ovarian stimulation compared to human menopausal gonadotrophins in women undergoing ART raises the question of whether LH is required for ovarian stimulation. This has been investigated in a number of recent studies. For example, results have suggested that implantation rates may actually be lower in women who received exogenous LH. Such studies suggest, therefore, that in normogonadotrophic women, the addition of LH to an r-hFSH regimen does not add any further clinical benefit and may actually be detrimental. Hence, it appears that LH administration is necessary only in women with hypogonadotrophic hypogonadism. In conclusion, r-hFSH is a consistently pure and high quality gonadotrophin preparation and contributes to increasing the successful outcome of an ART cycle. Together with careful auditing of routine clinical practice, and the application of evidence-based medicine to facilitate clinical decision making, this means that a total quality management approach can be applied to optimize the outcome of assisted reproduction. |
| [20] | . The forkhead box transcription factor FOXO1 is highly expressed in granulosa cells of growing follicles but is down-regulated by FSH in culture or by LH-induced luteinization in vivo. To analyze the function of FOXO1, we infected rat and mouse granulosa cells with adenoviral vectors expressing two FOXO1 mutants: a gain-of-function mutant FOXOA3 that has two serine residues and one threonine residue mutated to alanines rendering this protein constitutively active and nuclear and FOXOA3-mutant DNA-binding domain (mDBD) in which the DBD is mutated. The infected cells were then treated with vehicle or FSH for specific time intervals. Infection of the granulosa cells was highly efficient, caused only minimal apoptosis, and maintained FOXO1 protein at levels of the endogenous protein observed in cells before exposure to FSH. RNA was prepared from control and adenoviral infected cells exposed to vehicle or FSH for 12 and 24 h. Affymetrix microarray and database analyses identified, and real time RT-PCR verified, that genes within the lipid, sterol, and steroidogenic biosynthetic pathways (Hmgcs1, Hmgcr, Mvk, Sqle, Lss, Cyp51, Tm7sf2, Dhcr24 and Star, Cyp11a1, and Cyp19), including two key transcriptional regulators Srebf1 and Srebf2 of cholesterol biosynthesis and steroidogenesis (Nr5a1, Nr5a2), were major targets induced by FSH and suppressed by FOXOA3 and FOXOA3-mDBD in the cultured granulosa cells. By contrast, FOXOA3 and FOXOA3-mDBD induced expression of Cyp27a1 mRNA that encodes an enzyme involved in cholesterol catabolism to oxysterols. The genes up-regulated by FSH in cultured granulosa cells were also induced in granulosa cells of preovulatory follicles and corpora lutea collected from immature mice primed with FSH (equine choriogonadotropin) and LH (human choriogonadotropin), respectively. Conversely, Foxo1 and Cyp27a1 mRNAs were reduced by these same treatments. Collectively, these data provide novel evidence that FOXO1 may play a key role in granulosa cells to modulate lipid and sterol biosynthesis, thereby preventing elevated steroidogenesis during early stages of follicle development. |
| [21] | . Abstract The maturation of ovarian granulosa cells is dependent upon the pituitary gonadotropin FSH, the actions of which are mediated via specific plasma membrane receptors. To study the regulation of ovarian FSH receptor expression at the mRNA level, we used a specific cRNA probe to evaluate changes in FSH receptor transcripts in cultured granulosa cells. Granulosa cells obtained from immature estrogen-treated rats contained two predominant FSH receptor mRNA transcripts (7.0 and 2.5 kilobases), the levels of which declined in a time-related manner during a 2-day culture period. However, inclusion of FSH (30 ng/ml) in the culture medium prevented the decline in FSH receptor mRNA levels. Compared to controls, treatment of granulosa cells for 48 h with FSH (1-100 ng/ml) increased FSH receptor mRNA levels in a dose-dependent manner (ED50, 4.5 ng/ml), with a maximal 5.9 +/- 0.7-fold increase observed in response to 30 ng/ml FSH. The stimulatory actions of FSH were mimicked by the adenyl cyclase activator forskolin (0.1-30 microM), suggesting the involvement of cAMP in FSH receptor gene transcription and/or mRNA stability. Incubation of granulosa cells for 48 h with epidermal growth factor (EGF; 0.3-10 ng/ml), basic fibroblast growth factor (bFGF; 1-30 ng/ml), or insulin-like growth factor-I (IGF-I; 1-30 ng/ml) did not affect basal FSH receptor mRNA levels, whereas the highest doses of EGF and bFGF, but not IGF-I, completely suppressed the stimulatory effects of FSH (30 ng/ml) on its own receptor mRNA levels. Similarly, GnRH (10-1000 nM) attenuated the actions of FSH on its receptor mRNA levels in a dose-dependent manner (ID50, 8 nM). The inhibitory effects of GnRH (100 nM) were reversed by cotreatment with a GnRH antagonist ([Ac-D-Phe1,D-pCl-Phe2,D-Trp3,6]GnRH; 100 nM), indicating that the actions of GnRH are mediated via specific GnRH receptors. These data indicate that treatment of granulosa cells with FSH increases the levels of two FSH receptor mRNA transcripts. However, this positive feedback system, which may lead to an amplification of FSH action, is tightly regulated by the inhibitory actions of EGF, bFGF, and GnRH. Thus, the use of cultured rat granulosa cells provides a model system to analyze the hormonal regulation of FSH receptor gene expression in the ovary. |
| [22] | . Inhibin has long been considered as a suppresser of follicle-stimulating hormone (FSH) secretion from anterior pituitary through pituitary onad negative feedback to regulate follicle development. We demonstrated that addition of inhibin A could significantly suppress FSH-induced FSHR mRNA level in cultured rat granulosa cells (GCs) measured by real-time PCR. The inhibin A exerted its action mainly by inhibiting FSHR promoter activity. Furthermore, exogenous inhibin A could dramatically decrease FSH-induced P450arom and P450scc level and suppress progesterone and estradiol production in the cultured GCs, but it did not decrease forskolin-induced steroidogenesis, indicating that the inhibitory effect of inhibin A on FSH action may be upstream of cAMP signaling. Inhibin A was also capable of suppressing FSH-induced expression of steroidogenic factor 1 (SF-1) and androgen receptor, but stimulating DAX-1 expression in the culture. Our study has provided new evidence to show that inhibin A is capable of feedback antagonizing FSH action on GCs by reducing FSHR expression at ovarian level via a short feedback loop. Transcriptional factor receptors, such as SF-1, AR and DAX-1 were involved in this regulation. |
| [23] | . This study investigated the FSH influence on maturation rates of oocytes in vitro maturation (IVM), and expression levels of the follicle-stimulating hormone receptor ( FSHR ), luteinizing hormone receptor ( LHR ) and gonadotropin releasing hormone receptor ( GnRHR ) of cumulus-oocyte complexes (COCs) response to FSH treatment. 1686 COCs were harvested from 1063 ovaries of sheep. COCs were cultured 2602h at 38.502°C and 5.0% CO 2 in IVM media supplemented with 0, 5, 10, 20 and 30 IU/mL FSH. They were allocated in to FSH-1 (basal line), FSH-2, FSH-3, FSH-4 and FSH-5 groups. The apoptosis of COCs was assessed by Tunel assay. Expression levels of mRNA and protein for FSHR , LHR and GnRHR in sheep COCs were detected using real time RT-PCR and Western blotting respectively. The results showed that the maturation rates of oocytes were improved gradually when FSH supplement increased from 0 to 1002μg/mL. FSH-3 group showed the highest maturation rate. Apoptosis rates of FSH-treated groups were less than that of FSH-1 group with a minimum of FSH-3 group. Expression levels of FSHR and LHR mRNAs in FSH-3 and FSH-4 were significantly higher than in FSH-1. Expression level of GnRHR mRNA in FSH-3 was higher than in FSH-1 ( P02< 020.05). Expression levels of FSHR proteins in FSH-3 and FSH-4 groups were higher than that of FSH-1 group. Expression levels of GnRHR proteins increased gradually with a maximal increment of FSH-5. Maturation rates of COCs had significant positive correlations with mRNA and protein levels of FSHR , LHR and GnRHR . In conclusion, FSH could accelerate the maturation rate of sheep oocytes and reduce their apoptosis rate, also increase the expression levels of FSHR , LHR and GnRHR mRNAs, and strengthen expressions of FSHR and GnRHR proteins. 10 IU/mL FSH additions were the optimal dose for IVM of sheep oocytes. |
| [24] | . Steroidal regulation of gene expression in follicular cells is not completely defined. Granulosa cells from 5 mm bovine follicles were cultured and treated and steady-state mRNA levels determined for FSHR (follicle-stimulating hormone receptor) and CYP19A1 (aromatase). Cells were treated for 5 days with (0.1-300 ng/ml) 17beta-estradiol (E2), testosterone (T), or 5alpha-dihydrotestosterone (DHT). FSHR mRNA was increased by T and DHT but not E2. In contrast, CYP19A1 mRNA was induced by all doses of E2 but only high doses of T and DHT. Similarly, varying treatment duration (1-5 days) showed that FSHR was increased by T and DHT and CYP19A1 mRNA increased by E2 and T at all times. Synergism between steroid hormones and FSH or forskolin was also evaluated. FSH or E2 did not alter FSHR mRNA and did not enhance DHT stimulation of FSHR mRNA. In contrast, DHT alone had no effect on CYP19A1 mRNA but synergized with FSH plus E2 to increase CYP19A1 mRNA, probably due to induction of FSHR by DHT. Effects of E2 and T on CYP19A1 were blocked by ICI 182,780, indicating mediation by estrogen receptors. However, the specific androgen receptor antagonist bicalutamide did not block E2 or T effects on CYP19A1 but did block T and DHT stimulation of FSHR. Thus, FSHR is specifically regulated through androgen receptor, whereas CYP19A1 is regulated by multiple pathways, including estrogen receptors and cAMP/protein kinase A induced by FSHR activation in granulosa cells. These inter- and intracellular regulatory mechanisms may be critical for normal follicle growth and dominant follicle selection. |
| [25] | . |
| [26] | . |
| [27] | . |
| [28] | . <P>The purpose of this study was to establish a culture model for isolated intact porcine antral follicles and investigate the relationship between granulosa cell apoptosis and follicular atresia. Small (<365mm), medium (3–565mm) and large (>565mm) healthy porcine follicles were isolated and cultured in serum-free TCM199 with or without follicular stimulating hormone (FSH). Microscopic identification of healthy follicles was confirmed by histology. A spontaneous onset of apoptotic cell death in granulosa cells was observed from cultured antral follicles. The apoptotic rate of granulosa cells from small follicles cultured for 2465hr was higher than those of large and medium follicles, accompanied with high FasL mRNA abundance in granulosa cells. Supplementation with 3 or 565IU/ml FSH significantly inhibited the percentage of granulosa cells that became apoptotic. FSH did not significantly alter estradiol secretion from cultured follicles. Progesterone secretion significantly decreased after culture for 4865hr, coinciding with the morphological changes observed. FasL and Fas mRNA were expressed in the healthy, early atretic, and progressed atretic porcine follicles regardless of follicular size. However, FasL but not Fas mRNA levels increased during follicular atresia. Addition of FSH significantly decreased FasL rather than Fas mRNA levels in granulosa cells and could attenuate apoptosis. Small follicles seemed to be more susceptible to atresia as compared to medium and large follicles. Mol. Reprod. Dev. 77: 670–678, 2010. 08 2010 Wiley-Liss, Inc.</P> |
| [29] | . Thyroid hormone (TH) is important for normal reproductive function. Our previous studies indicate that FSH increases preantral follicle growth in vitro, a response markedly enhanced by triiodothyronine (T3). However, the nature of this hormonal interaction is poorly understood. The objective of this study was to determine if and how T3 modulate FSH-induced expression and actions of granulosa cell intracellular survival and death intermediates. We investigated the possible involvement of Src and PI3K/Akt pathway in the regulation of granulosa cell survival. We demonstrated that, while ineffective alone (0.1-100 nM), T3 markedly enhanced FSH (100 ng/ml)-induced granulosa cell phospho-Src and phospho-Akt contents and Xiap expression in vitro. The effects of T3 were concentration-dependent, with maximal responses at 1.0 nM. FSH alone decreased Fas Ligand (FasL) content irrespective of the presence of T3. Co-treatment of cell with T3 and FSH decreased Fas content, although neither hormone alone elicited a significant response. Taken together, the present study demonstrates that T3 potentiates the cell survival action of FSH through Src- and PI3K-mediated Xiap up-regulation and decreased Fas and FasL expression. |
| [30] | . The ovulatory LH surge induces rapid up-regulation of steroidogenic acute regulatory (StAR) protein and rapid down-regulation of aromatase (Cyp19a1) in granulosa cells (GCs) undergoing luteinization during ovulation. This study investigated in vivo whether epigenetic mechanisms including histone modifications are involved in the rapid changes of StAR and Cyp19a1 gene expression. GCs were obtained from rats treated with equine chorionic gonadotropin (CG) before (0 h) and after human (h) CG injection. StAR mRNA levels rapidly increased after hCG injection, reached a peak at 4h, and then remained higher compared with 0h until 12 h. Cyp19a1 mRNA levels gradually decreased after hCG injection and reached their lowest level at 12 h. A chromatin immunoprecipitation assay revealed that levels of histone-H4 acetylation (Ac-H4) and trimethylation of histone-H3 lysine-4 (H3K4me3) increased whereas H3K9me3 and H3K27me3 decreased in the StAR promoter after hCG injection. On the other hand, the levels of Ac-H3 and -H4 and H3K4me3 decreased, and H3K27me3 increased in the Cyp19a1 promoter after hCG injection. Chromatin condensation, which was analyzed using deoxyribonuclease I, decreased in the StAR promoter and increased in the Cyp19a1 promoter after hCG injection. A chromatin immunoprecipitation assay also showed that binding activities of CAATT/enhancer-binding protein beta to the StAR promoter increased and binding activities of phosphorylated-cAMP response element binding protein to the Cyp19a1 promoter decreased after hCG injection. These results provide in vivo evidence that histone modifications are involved in the rapid changes of StAR and Cyp19a1 gene expression by altering chromatin structure of the promoters in GCs undergoing luteinization during ovulation. (Endocrinology 154: 458-470, 2013) |
| [31] | . |
| [32] | . |
