 ,, 姜义平, 赵静, 肖留斌
,, 姜义平, 赵静, 肖留斌 ,江苏省农业科学院植物保护研究所,南京 210014
,江苏省农业科学院植物保护研究所,南京 210014Expression Profile of G Protein-Coupled Receptor Kinase 2 Gene (AlGRK2) and Its Function in the Development of Apolygus lucorum
TAN YongAn ,, JIANG YiPing, ZHAO Jing, XIAO LiuBin
,, JIANG YiPing, ZHAO Jing, XIAO LiuBin ,Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences, Nanjing 210014
,Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences, Nanjing 210014通讯作者:
责任编辑: 岳梅
收稿日期:2021-04-16接受日期:2021-06-10
| 基金资助: |
Received:2021-04-16Accepted:2021-06-10
作者简介 About authors
谭永安,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (735KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
谭永安, 姜义平, 赵静, 肖留斌. 绿盲蝽G蛋白偶联受体激酶2基因(AlGRK2)的表达分析及在绿盲蝽生长发育中的功能. 中国农业科学, 2021, 54(22): 4813-4825 doi:10.3864/j.issn.0578-1752.2021.22.009
TAN YongAn, JIANG YiPing, ZHAO Jing, XIAO LiuBin.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】绿盲蝽(Apolygus lucorum)属半翅目盲蝽科,是果树、蔬菜及棉花等多种经济作物上的重要刺吸类害虫[1]。近年来,随着我国农业产业结构的调整及化学农药使用等因素,绿盲蝽在多种作物上危害趋势加重[2,3],造成巨大的经济和生态效益的损失。因此,有效防控绿盲蝽亟待开拓新型无公害防控手段。蜕皮激素(20-hydroxyecdysone,20E)可调控昆虫多种重要的生理活动,挖掘蜕皮激素信号转导中的关键基因,将有利于开发新型绿色昆虫生长调节剂及有效控制绿盲蝽危害。【前人研究进展】20E是一种类固醇激素,主导调控昆虫发育、变态及繁殖等重要生理活动。蜕皮激素信号转导途径一直是昆虫学研究的重点和热点。研究表明,昆虫蜕皮激素转导分为基因组途径及非基因组途径。目前已经明确了基因组转导的途径:20E依靠自身脂溶性穿过细胞膜进入细胞核,激活核受体基因蜕皮激素受体基因(ecdysone receptor,EcR)和超气门蛋白(ultraspiracle protein,USP)形成异源二聚体,启动下游基因的转录,进而通过核受体途径调控昆虫生长发育及繁殖[4,5,6]。20E转导还存在着多种非基因组途径。其中最重要的一种是20E通过细胞膜上的G蛋白偶联受体(G protein-coupled receptor,GPCR),调控细胞内磷脂酶C(phospholipase C,PLC)、Ca2+、蛋白激酶C(protein kinase C,PKC)等因子的表达,进而触发基因组途径的启动,启动20E下游靶标基因的转录[7,8]。G蛋白偶联受体激酶(G protein-coupled receptor kinase,GRK)是一类重要的膜蛋白,属于丝氨酸/苏氨酸蛋白激酶家族成员,可磷酸化GPCR,磷酸化后的GPCR与β-抑制蛋白(β-arrestin)结合后可发生脱敏反应,导致G蛋白不能与GPCR结合,从而参与20E信号通路中一系列的生理活动[9,10]。GRK通常具有4个典型结构域:G蛋白信号调节域(regulator of G-protein signaling,RGS)、AGC激酶家族的Ser/Thr蛋白激酶结构域(Ser/ Thr protein kinase domain)、氨基端N域(N-terminal domain)和羧基端C域(C-terminal domain)[11,12]。此外,根据蛋白结构差异将GRK分为GRK1、GRK2和GRK4这3个亚家族,其中GRK1亚家族包含GRK1和GRK7,其蛋白C端可以发生法尼基化,可促进其与质膜结合[13];GRK2亚家族包含GRK2和GRK3,其蛋白序列C端存在一个PH结构域,可与酸性磷脂和Gβγ亚基相互作用,因可参与肾上腺素传导,又被称为β-肾上腺素受体激酶家族[14,15,16];GRK4亚家族包含GRK4、GRK5和GRK6,其N-和C-末端存在两个多碱基区域,其中GRK4和GRK6蛋白C端可发生棕榈酰化[17,18],GRK5的C端含有碱性氨基酸富集区[9]。研究表明,昆虫的GRK2基因可参与激素信号通路的转导,进而调控昆虫的多种生理活动。在棉铃虫(Helicoverpa armigera)中,20E通过调控GRK2由细胞质向细胞膜移动,随后磷酸化ErGRCR-2(ecdysone-responsible GPCR-2),并与之结合,诱导ErGRCR-2后发生内吞,最后可终止20E信号的转导[19];此外,在黑腹果蝇(Drosophila melanogaster)中,GRK2表达量的减少会导致果蝇胚胎分化异常及成虫无法飞行等[20]。上述研究结果表明,GRK2可参与昆虫20E信号的转导,并在多种生理活动中发挥着重要功能。此外,在家蚕(Bombyx mori)中的研究也发现,当对幼虫注射20E后,发育受到阻遏,呈现出发育历期的缩短及死亡率增加[21];同样在荨麻蛱蝶(Aglais urticae)和孔雀蛱蝶(Inachis io)中也发现,20E也可显著降低幼虫体重及存活率[22]。【本研究切入点】目前,对GRK2的研究多集中在哺乳动物及某些模式昆虫上,对绿盲蝽 GRK2(AlGRK2)的相关研究尚未见报道。【拟解决的关键问题】获得AlGRK2 全长,明确其时空表达动态,分析外源20E对其表达的影响,阐明AlGRK2在绿盲蝽生长发育中的功能。1 材料与方法
试验于2019年5月至2021年3月在江苏省农业科学院植物保护研究所完成。1.1 供试虫源
初始绿盲蝽采自2018年早春的江苏大丰及东台蚕豆(Vicia faba)田,于室内用四季豆(Phaseolus vulgaris)豆荚继代饲养,饲养条件为温度(25±1)℃,相对湿度70%±5%,光周期12L﹕12D。1.2 主要试剂和仪器
Trizol(Invitrogen,Rockville,USA);M-MLV反转录试剂盒(Promega,Madison,USA);PrimeScriptTM RT Master Mix、SMARTer® RACE 5′/3′ Kit、TB Green®Premix Ex TaqTM、pMD®19-T Vector(TaKaRa,Shiga,Japan);2×EXP Taq(Accurate Biotechnology,中国湖南);AxyPrepTM DNA Gel Extraction Kit、AxyPrepTM Plasmid Miniprep Kit(Axygen,Union City,CA,USA);T7 RiboMAXTM Express RNAi System(Promega,Madison,Wisconsin,USA);20E、U73122(Sigma,Missouri,USA)。Allegra 21R台式高速冷冻离心机(Beckman,California,USA);水平电泳系统(Bio-Rad,California,USA);LightCycler 480 II(Roche,Basel,Switzerland);Eppendorf FemtoJet 4i-TransferMan 4r微量自动注射仪(Eppendorf,Hamburg,Germany)。1.3 AlGRK2克隆
取绿盲蝽3龄若虫20头,Trizol法提取绿盲蝽总RNA,M-MLV反转录合成cDNA。基于NCBI报道的已知昆虫GRK2,设计简并引物(AlGRK2-F和AlGRK2-R)(表1),获得保守序列,进一步根据获得的序列设计特异性引物(表1),进行AlGRK2 5′和3′端序列的克隆。5′端序列克隆反应体系:10×PCR缓冲液5.0 μL,25 mmol·L-1 MgCl2 3.0 μL,10 mmol·L-1 dNTP Mix 1.0 μL,10 μmol·L-1 5′-AlGRK2-F 1.0 μL,10 μmol·L-1 5′-AlGRK2-R 1.0 μL,cDNA 2.5 μL,Taq DNA Polymerase 0.5 μL,超纯水补足至20.0 μL;PCR反应条件:94℃ 2 min,94℃ 30 s,55℃ 30 s,72℃ 1 min,循环35次,94℃延伸7 min。3′端序列克隆反应体系:PCR-Grade Water 34.5 μL,10×Advantage PCR Buffer 5.0 μL,10 mmol·L-1 dNTP Mix 1.0 μL,50×Advantage Polymerase Mix 1.0 μL,cDNA 2.5 μL,10 µmol·L-1 3′-AlGRK2-F 1.0 μL,10 µmol·L-1 3′-AlGRK2-R 1.0 μL,超纯水补足至50.0 μL;PCR反应条件:94℃ 2 min,94℃ 30 s,68℃ 30 s,72℃ 1 min,循环30次,72℃延伸7 min。将获得的5′和3′端及保守区域进行测序和拼接(上海生工生物工程有限公司),最终获得AlGRK2全长,最后对全长基因进行克隆验证。Table 1
表1
表1本研究中所使用的引物
Table 1
| 目的 Purpose | 引物名称 Primer name | 引物序列Primer sequence (5′ to 3′) |
|---|---|---|
| 克隆Cloning | AlGRK-F | AGYGTNMGVAGYGTNATGCA |
| AlGRK-R | TCVGCKGCRTARAAYTTCAT | |
| 5′-AlGRK2-F | TGAAGAGAAGGAATCCCAGA | |
| 5′-AlGRK2-R | ACTTCGTTCTTCTTTTCGAG | |
| 3′-AlGRK2-F | GCTGGCACTCAACGAAAGGATCAT | |
| 3′-AlGRK2-R | GTATGACTTACGCCTTCCACACGC | |
| dsRNA | AlGRK2-F | TTCCCGACTCCTTCTCATC |
| AlGRK2-R | TTTCCGTTTCTGCTCCG | |
| AlGRK2-T7F | TAATACGACTCACTATAGGGTTCCCGACTCCTTCTCATC | |
| AlGRK2-T7R | TAATACGACTCACTATAGGGTTTCCGTTTCTGCTCCG | |
| GFP-F | CACAAGTTCAGCGTGTCCG | |
| GFP-R | CACCTTGATGCCGTTC | |
| GFP-T7F | TAATACGACTCACTATAGGGCACAAGTTCAGCGTGTCCG | |
| GFP-T7R | TAATACGACTCACTATAGGGCACCTTGATGCCGTTC | |
| qRT-PCR | AlGRK2-QF | AGGAGCGTGATGCACAAATA |
| AlGRK2-QR | CGCAGTAGTCCTTGAAGAGAAG | |
| β-Actin-QF | ACCTGTACGCCAACACCGT | |
| β-Actin-QR | TGGAGAGAGAGGCGAGGAT |
新窗口打开|下载CSV
1.4 AlGRK2序列分析与进化树构建
采用ExPASy在线分析程序(1.5 AlGRK2时空表达谱
取自然条件下1—16日龄绿盲蝽虫体及羽化8 d后绿盲蝽雌成虫的7个不同组织(头、胸、翅、足、中肠、卵巢和脂肪体),作为时空表达谱分析的样本。各日龄样本每个处理15头绿盲蝽,各组织样本每个处理10头绿盲蝽,4次生物学重复。液氮处理样品后-80℃保存备用。Trizol法提取总RNA,Prime ScriptTM RT Master Mix反转录合成cDNA,-20℃保存。qRT-PCR试验步骤按照SYBR Premix Ex Taq Kit说明书进行。所用的AlGRK2-QF及AlGRK2-QR引物参见表1,并以绿盲蝽持家基因β-actin为内标基因(GenBank登录号:JN616391)。扩增体系:2×TB Green Premix Ex Taq II 10 μL,上下游引物各0.4 μL,cDNA 2.0 μL,ddH2O 7.2 μL,总体积20.0 μL。反应条件:95℃ 30 s;95℃ 5 s,60℃ 20 s,72℃ 10 s,40个循环。1.6 不同药剂处理后AlGRK2表达量分析
分别用20E(2 μmol·L-1)、U73122(20E信号通路中PLC抑制剂[23],50 μmol·L-1)、20E+U73122处理、乙醇(CK)浸泡切除两端的四季豆1 min,晾干后饲喂初孵1龄若虫。取不同处理的1—6日龄绿盲蝽若虫及上述1.5中不同雌成虫组织样本,作为AlGRK2表达特性分析的样本。各日龄样本每个处理15头绿盲蝽,各组织样本每个处理10头绿盲蝽,4次生物学重复。AlGRK2表达量测定方法同1.5。1.7 绿盲蝽若虫发育历期、5龄若虫体重及成虫羽化率
将上述不同处理后的初孵绿盲蝽若虫置于一次性圆形餐盒(15 cm×6.5 cm)内,用一次性纱布封顶。逐日记录若虫发育进度及成虫羽化率,每处理50头绿盲蝽若虫,4次重复。计重绿盲蝽5龄末期若虫(出现翅芽)体重,每处理10头若虫,4次重复。1.8 dsAlGRK2的合成与注射
以测序验证正确的质粒作为DNA模板,利用带T7启动子的引物(AlGRK2-T7F、AlGRK2-T7R,表1)分别进行AlGRK2 PCR产物正义链与反义链的扩增。用T7 RiboMAXTM Express RNAi System合成试剂盒合成AlGRK2双链RNA(dsAlGRK2)和GFP双链RNA(dsGFP),最后用无RNA酶水配制成1 μg·μL-1终浓度的溶液备用。于绿盲蝽初孵4龄若虫的后足与胸的连接处进行注射,每头若虫dsRNA注射量为1 μg。设未注射及注射dsGFP为对照组。1.9 RNAi处理后AlGRK2的表达及相关生物学参数分析
分别于注射后6、12、24和48 h收集上述不同处理的初孵4龄绿盲蝽若虫,每个处理60头,4次重复。参照1.5中的方法分析AlGRK2表达量。此外,将不同处理后的初孵4龄绿盲蝽若虫置于含有新鲜四季豆的一次性圆形餐盒(15 cm×6.5 cm)中,逐日统计死亡率、羽化率及5龄若虫发育历期,并计重5龄末期若虫(出现翅芽)体重,每个处理10头若虫,4次重复。1.10 数据分析
AlGRK2相对表达量变化均采用2-ΔΔCt计算[24]。生物学相关参数的差异显著性统计分析均采用统计软件SAS 8.0 完成。2 结果
2.1 AlGRK2 cDNA序列克隆
利用RACE法获得了AlGRK2全长cDNA序列(GenBank登录号:MN514868)。序列分析结果表明,AlGRK2 cDNA序列全长2 715 bp,开放阅读框2 106 bp,编码701个氨基酸。ExPASy预测其蛋白分子量为80.2 kD,理论等电点为6.56。SMART在线预测AlGRK2蛋白包含4个结构域:G蛋白信号调节区(RGS,54—175 aa)、丝氨酸/苏氨酸激酶结构域(S-TKc,191—454 aa)、丝氨酸/苏氨酸型蛋白激酶的伸展部分(S-TK-X,455—534 aa)和PH结构域(PH,558—655 aa),其中PH结构域具有与肌醇磷酸酯和蛋白质结合的能力(图1),此为GRK2蛋白的典型特征,表明获得的基因是AlGRK2亚型基因。图1
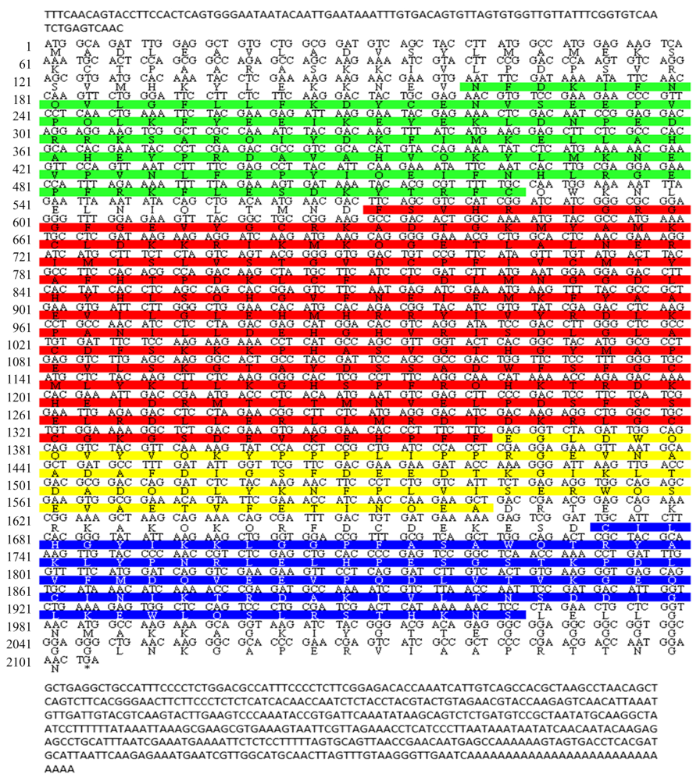 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1AlGRK2核苷酸序列及其推导得出的氨基酸序列
绿色、红色、黄色和蓝色阴影标注的氨基酸分别代表RGS结构域、S-TKc结构域、S-TK-X结构域和PH结构域
Fig. 1Nucleotide and amino acid sequences of AlGRK2
Green, red, yellow and blue shaded amino acids indicate the RGS, S-TKc, S-TK-X and PH domains, respectively
2.2 AlGRK2系统发育
对AlGRK2核苷酸推导出的氨基酸序列进行Blast比对。结果表明,AlGRK2氨基酸序列与半翅目蝽科茶翅蝽(Halyomorpha halys)GRK 2 isoform X2基因序列同源性最高,为92.3%,表明克隆获得的基因为GRK2。利用MEGA6.0软件中的NJ法对包括绿盲蝽在内的18种不同种类昆虫的GRK2氨基酸序列构建系统进化树(图2),结果显示,绿盲蝽GRK2与茶翅蝽GRK2亲缘关系最近。图2
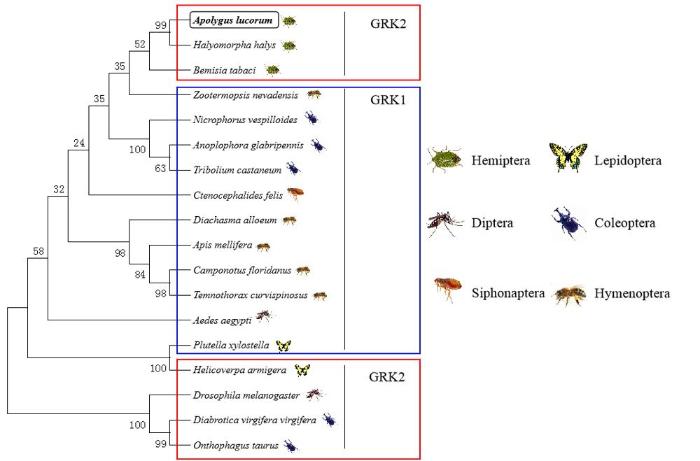 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2AlGRK2与其他已知昆虫GRK氨基酸序列的系统进化树
绿盲蝽Apolygus lucorum GRK2(MN514868)、茶翅蝽Halyomorpha halys GRK2(XP_014284300)、烟粉虱Bemisia tabaci GRK2(XP_018907528)、湿木白蚁Zootermopsis nevadensis GRK1(XP_021942271)、大红葬甲Nicrophorus vespilloides GRK1(XP_017785563)、光肩星天牛Anoplophora glabripennis GRK1(XP_023313123)、赤拟谷盗Tribolium castaneum GRK1(XP_015838145)、猫蚤Ctenocephalides felis GRK1(XP_026471851)、胡锋Diachasma alloeum GRK1(XP_015116743)、西方蜜蜂Apis mellifera GRK1(XP_026297912)、佛罗里达弓背蚁Camponotus floridanus GRK1(XP_011267492)、切胸蚁Temnothorax curvispinosus GRK1(XP_024874981)、埃及伊蚊Aedes aegypti GRK1(XP_021706708)、小菜蛾Plutella xylostella GRK1(XP_011558851)、棉铃虫Helicoverpa armigera GRK2(ANZ22924)、黑腹果蝇Drosophila melanogaster GRK2(NP_476867)、玉米根萤叶甲Diabrotica virgifera virgifera GRK2(XP_028135185)、食粪金龟Onthophagus taurus GRK2(XP_022917771)
Fig. 2Phylogenetic tree based on amino acid sequences of AlGRK and other known insects
2.3 AlGRK2时空表达特性
AlGRK2在1—16日龄绿盲蝽中均有表达,mRNA表达量呈现出波动式下降的模式,以1日龄绿盲蝽表达量最高(图3-A,P<0.05)。综合来看,AlGRK2在绿盲蝽初始龄期的表达量较高,而在末龄期的表达量显著下降。AlGRK2在绿盲蝽8日龄雌成虫卵巢和脂肪体中高表达,而在胸和足中微弱表达(图3-B,P<0.05),显示AlGRK2具有典型的龄期和组织表达特异性。图3
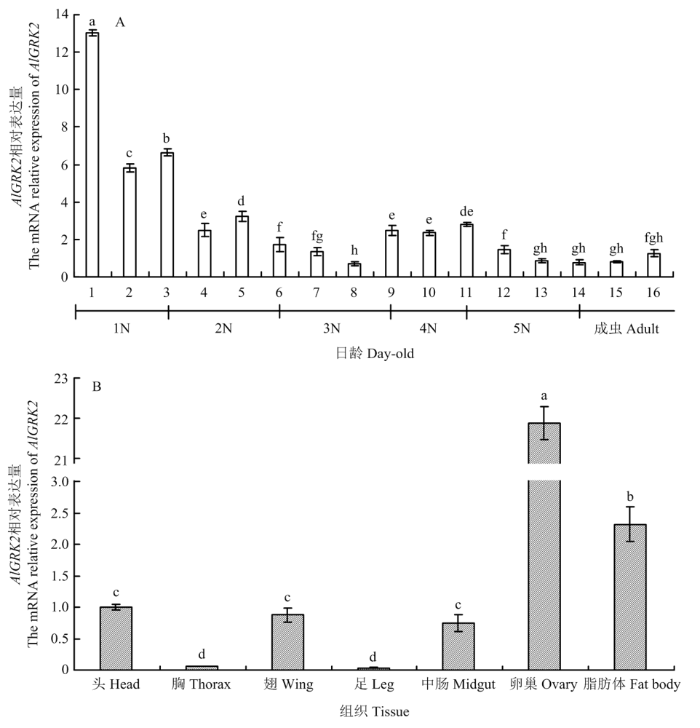 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3绿盲蝽不同日龄(A)和组织(B)中AlGRK2相对表达量
图中数据为平均数±标准误,柱上不同字母表示差异达显著水平(P<0.05)。下同
Fig. 3Relative expression levels of AlGRK2 in different days (A) and tissues (B) of A. lucorum
Data in the figure are means±standard error, and different lowercases above bars indicate significant difference (P<0.05). The same as below
2.4 不同药剂处理对绿盲蝽若虫发育历期的影响
与对照相比,20E处理后绿盲蝽若虫期的发育历期显著缩短(14.94 d vs 17.04 d,P<0.05);相反,20E信号通路中PLC抑制剂U73122处理组若虫期的发育历期显著延长(18.09 d vs 17.04 d,P<0.05)。绿盲蝽若虫各龄期发育历期与整个若虫期发育趋势一致,基本为U73122>20E+U73122>乙醇(CK)>20E。显示出20E可加快绿盲蝽若虫的发育(表2)。Table 2
表2
表2不同药剂处理下绿盲蝽若虫发育历期
Table 2
| 处理 Treatment | 若虫历期Developmental period of nymphs (d) | |||||
|---|---|---|---|---|---|---|
| 1龄1st instar | 2龄2nd instar | 3龄3rd instar | 4龄4th instar | 5龄5th instar | 若虫期Nymph | |
| 乙醇Ethanol (CK) | 2.62±0.07bc | 3.51±0.07b | 3.60±0.07b | 3.55±0.07a | 3.77±0.09a | 17.04±0.18b |
| 20E | 2.42±0.07c | 3.17±0.05c | 3.02±0.09c | 2.96±0.04b | 3.38±0.08b | 14.94±0.19c |
| U73122 | 2.93±0.09a | 3.85±0.05a | 3.80±0.06a | 3.67±0.07a | 3.83±0.09a | 18.09±0.18a |
| 20E+U73122 | 2.79±0.07ab | 3.66±0.07b | 3.79±0.06ab | 3.62±0.07a | 3.89±0.11a | 17.77±0.21a |
新窗口打开|下载CSV
2.5 不同药剂处理对绿盲蝽若虫体重及羽化率的影响
20E处理组5龄若虫体重显著低于对照(P<0.05),降低了9.81%(4-A);成虫羽化率结果表明(图4-B),与对照相比,20E处理组成虫羽化率最低,其次是U73122处理组,分别下降了15.36%和12.19%。此外,20E与其抑制剂U73122处理后的成虫羽化率差异不显著。图4
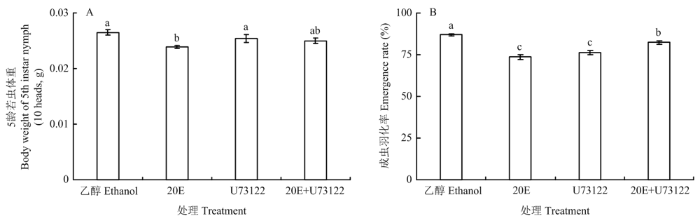 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4不同处理对绿盲蝽5龄若虫体重(A)和成虫羽化率(B)的影响
Fig. 4The effect of different treatments on 5th instar nymph body weight (A) and adult emergence rate (B) of A. lucorum
2.6 不同药剂处理对AlGRK2表达量的影响
与对照相比,20E处理后AlGRK2的表达显著下降(1、3日龄)(P<0.05);而20E抑制剂U73122处理后,供试的1—6日龄绿盲蝽若虫AlGRK2的表达均下调,其中1、3和5日龄下调表达显著(P<0.05);总体来看,不同处理的AlGRK2表达量大致趋势为乙醇(CK)>20E>U73122>20E+U73122(图5-A),显示20E有抑制绿盲蝽若虫AlGRK2表达的效应。此外,与对照相比,20E处理后绿盲蝽雌成虫各组织中AlGRK2表达均上调,其中以卵巢和脂肪体中表达量最高(48.57和199.64倍,P<0.05);相反,U73122处理后绿盲蝽雌成虫各组织中AlGRK2的表达均下调,其中以卵巢和脂肪体中表达量下调最为显著(P<0.05)(图5-B),表明20E有提高绿盲蝽雌成虫组织中AlGRK2表达的效应。图5
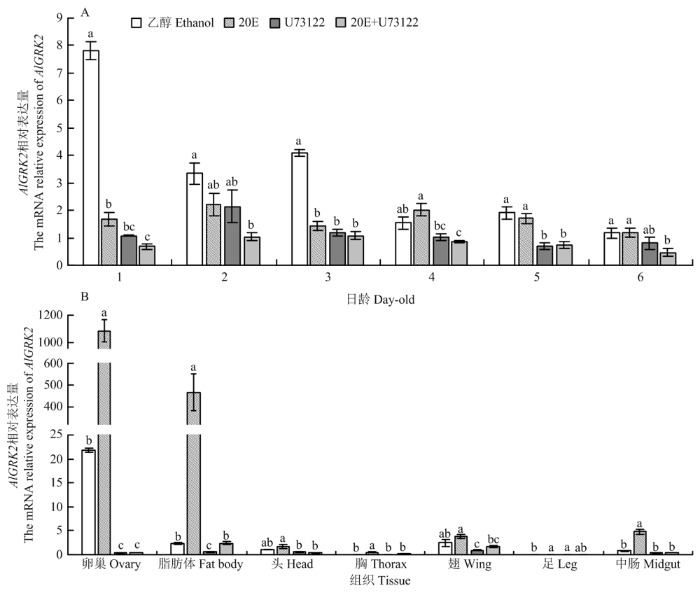 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5不同处理下绿盲蝽不同日龄(A)和组织(B)中AlGRK2相对表达量
Fig. 5Relative expression levels of AlGRK2 in different days (A) and tissues (B) of A. lucorum under different treatments
2.7 dsAlGRK2注射对AlGRK2表达量的影响
qRT-PCR检测结果显示,与CK相比,注射dsAlGRK2后的AlGRK2表达水平均显著下降(P<0.05,图6)。其中以注射后6 h的下降率最高,与对照组相比分别下降了66.77%(dsAlGRK2 vs CK)和65.62%(dsAlGRK2 vs dsGFP);与注射dsGFP对照组相比,注射后12、24和48 h检测发现,绿盲蝽若虫中AlGRK2表达量分别下降了50.31%、38.27%和23.37%。此外,AlGRK2表达量在未注射处理和注射dsGFP处理两个对照组之间无显著差异(P>0.05)。表明注射AlGRK2的dsRNA对该基因具有明显的沉默效应。图6
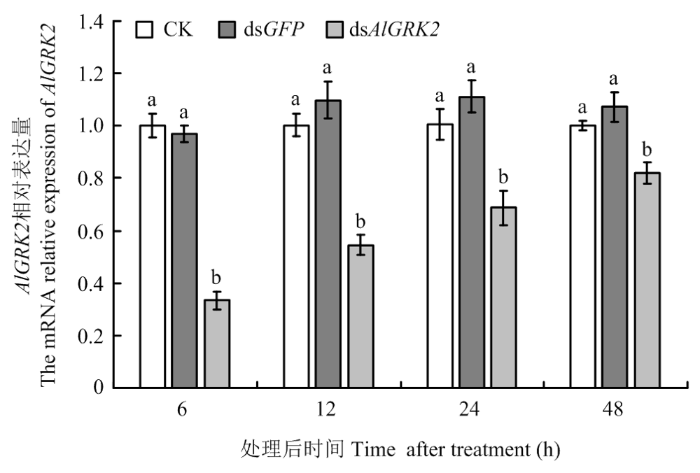 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6dsRNA处理后绿盲蝽若虫AlGRK2的相对表达量
Fig. 6Relative expression levels of AlGRK2 of A. lucorum nymph after dsRNA-injection treatments
2.8 dsAlGRK2注射对绿盲蝽生长发育的影响
注射dsRNA后3 d内死亡率统计结果显示(图7-A),与对照组相比,注射dsAlGRK2后,绿盲蝽若虫死亡率显著增加(P<0.05),分别增加了73.77%(dsAlGRK2 vs CK)和53.63%(dsAlGRK2 vs dsGFP)。若虫死亡率在未注射(CK)和注射dsGFP两个对照组之间无显著差异(P>0.05)。注射dsRNA后绿盲蝽成虫羽化率结果表明,注射dsAlGRK2处理组绿盲蝽羽化率最低(图7-B),与注射dsGFP组相比,下降了33.33%。未注射(CK)和注射dsGFP两个对照组之间羽化率无显著差异(P>0.05)。图7
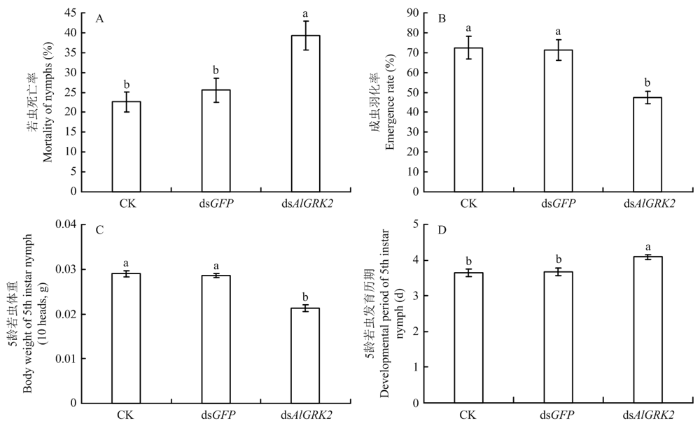 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7dsRNA处理对绿盲蝽若虫死亡率(A)、成虫羽化率(B)、5龄若虫体重(C)和发育历期(D)的影响
Fig. 7The effect of dsRNA-injection treatments on the nymph mortality (A), adult emergence rate (B), 5th instar nymph body weight (C) and developmental period (D) of A. lucorum
注射dsAlGRK2对5龄若虫体重和发育历期均存在显著影响(P<0.05)(图7-C、7-D),其中注射dsAlGRK2后末龄若虫体重显著减少了25.52%(dsAlGRK2 vs dsGFP),而发育历期显著延长,推迟了11.42%(dsAlGRK2 vs dsGFP)。未注射(CK)和注射dsGFP两个对照组之间均无显著差异(P>0.05)。
3 讨论
蜕皮激素又称蜕皮酮,在昆虫幼虫或若虫阶段主要由前胸腺分泌,并在P450酶催化下转化为有活性的20-羟基蜕皮酮,进而与保幼激素协同合作以调控昆虫的生长发育与变态。此外,成虫阶段的性腺器官也是主要分泌器官之一,进一步调控昆虫的生殖和胚胎发育[25,26,27,28]。蜕皮激素作为昆虫生长发育的关键激素,其滴度含量对昆虫生长发育具有核心的调控作用,一旦昆虫体内激素含量出现异常,可能会对昆虫产生不可逆的损伤。MALAUSA等[29]对桃蚜(Myzus persicae)的研究发现,高浓度20E显著降低其若虫数量;SUN等[30]对小菜蛾(Plutella xylostella)的研究发现,外源20E显著降低其幼虫体重、发育历期、繁殖和存活率。本研究结果显示,外源20E显著缩短了绿盲蝽若虫发育历期,且末龄若虫体重和羽化率均显著降低。另外,20E抑制剂U73122处理下可显著延长绿盲蝽若虫发育历期,并有降低羽化率的效应。表明20E可促进绿盲蝽的生长发育。迄今为止,科学家研究证实蜕皮激素的转导途径存在基因组和非基因组两种途径。其中,20E通过细胞膜上的GPCR调控PLC、Ca2+、PKC等效应因子的表达,进而诱导基因组途径的启动,是目前已明确的非基因组途径[7,8]。GPCR是目前发现的最大跨膜受体家族,能传递多种细胞外信号,而GRK作为GPCR负调控因子,是GPCR信号转导过程中的重要调控开关[31,32]。根据蛋白结构差异,GRK一般包含3个亚族的7个成员。目前该激酶已在哺乳动物体内广泛研究,并发现多与疾病相关[33]。但是,昆虫GRK的功能目前仅在一些模式昆虫中有相关报道。果蝇中报道有3类GRK蛋白,而在一些鳞翅目、半翅目和鞘翅目昆虫中发现1—2类亚型[19,20],且其具体的功能尚未清楚。因此,本文以绿盲蝽为研究对象,首先通过RACE方法,克隆获得了一种绿盲蝽GRK,进一步通过昆虫同源性分析及蛋白结构推导,发现该基因具备GRK2亚型典型的PH结构域特征,因此将该基因命名为AlGRK2。
在本研究中,AlGRK2在绿盲蝽前3日龄若虫中表达量显著高于其他日龄,1—16日龄整体表达量呈现波动下降趋势;AlGRK2在绿盲蝽雌成虫卵巢和脂肪体中高表达,而在胸和足中表达量较低。这些结果暗示AlGRK2 在绿盲蝽组织和龄期中具有表达特异性,可能存在着不同的功能。具体来说,AlGRK2在绿盲蝽若虫蜕皮前后高表达,笔者推测GRK2是20E信号通路中的重要分子,参与了20E调控绿盲蝽的蜕皮进程;AlGRK2在卵巢和脂肪体中高表达,表明AlGRK2参与了绿盲蝽的卵巢发育及卵子形成。此外,外源20E处理后,1日龄和3日龄的AlGRK2相对表达量显著下调,说明20E对绿盲蝽初孵若虫AlGRK2的表达存在抑制作用。但ZHAO等[19]通过对棉铃虫6龄幼虫进行注射处理6 h后,发现20E上调表皮中GRK2的表达水平。在本研究中,20E促进了绿盲蝽若虫的发育,降低了AlGRK2的表达、5龄若虫的体重及成虫羽化率;而dsAlGRK2处理后,AlGRK2的表达量、5龄若虫的体重及成虫羽化率均呈现出下降趋势,但绿盲蝽若虫的发育却受到了延缓。实际上,过快发育或发育历期的显著增加,可能是不利于昆虫正常生长发育的两个方面。此外,出现上述结果的原因,还可能与处理方式及处理龄期的差异有关。APPLE等研究发现,一定浓度蜕皮激素处理6 h后,果蝇表皮蛋白相关基因显著上调表达,而当蜕皮激素持续处理果蝇后,表皮蛋白相关基因表达量反而下调[34,35]。上述结果表明,昆虫发育和变态过程中体内蜕皮激素含量是动态的,含量过高会抑制下游基因的表达。此外,在哺乳动物中,β抑制蛋白及Mdm2(一种泛素连接酶E3)等介导的磷酸化作用对GRK2的降解具有调控作用[36,37,38]。β抑制蛋白和GRK共同作用可导致GPCR发生脱敏反应,SALCEDO等[36]研究发现,β抑制蛋白可促进泛素连接酶E3与GRK2结合,促进GRK2蛋白的降解。
GRK2是哺乳动物胚胎发育过程中必不可少的蛋白,GRK2表达量下调会导致胚胎发育迟缓,而该基因的缺失甚至会引发胚胎死亡[39,40]。WANG等[41]研究发现,GRK2功能丧失不利于秀丽隐杆线虫(Caenorhabditis elegans)产卵。本研究发现绿盲蝽雌虫经过20E处理后,各组织中AlGRK2表达量均有所上升,其中卵巢和脂肪体中显著上调表达,而20E抑制剂U73122处理雌虫后,卵巢、脂肪体和翅中AlGRK2 表达量均显著下调,说明20E对绿盲蝽组织中AlGRK2的表达存在诱导作用。昆虫脂肪体和卵巢分别是卵黄及卵子发生场所,以往的研究表明,昆虫卵产生过程中,蜕皮激素能够调控脂肪体中卵黄蛋白的合成、卵壳的发生及卵子的成熟等过程[26,42],而GRK2作为细胞周期调节器在早期胚胎发育过程中担任重要角色[43]。因此笔者推测,AlGRK2在蜕皮激素转导途径中参与调控雌虫生育和繁殖过程,这也是笔者团队后续正在进行的工作。
RNA干扰(RNA interference,RNAi)是由保守的双链RNA(double-stranded RNA,dsRNA)特异性与靶标基因mRNA序列配对,从而高效、特异性降解靶标基因mRNA的表达[44],最终导致靶标基因沉默。该技术作为一种重要的研究手段,在昆虫基因功能的研究中已被广泛应用[45,46]。本研究通过GRK2全长序列设计引物合成dsRNA,dsAlGRK2注射后6、12、24和48 h检测AlGRK2表达量均显著低于对照,其中注射后6 h绿盲蝽体内AlGRK2表达量最低。在家蚕中,GRK2参与脂动激素受体内吞作用,进而调控家蚕体内脂类和糖类代谢[47]。此外,GRK2还可参与棉铃虫20E的内化过程,并且GRK2表达减少会导致果蝇躯体分化异常和无法飞行[19,20]。本研究发现,注射dsAlGRK2后绿盲蝽AlGRK2表达显著下降,且若虫死亡率显著增加,而成虫羽化率和末龄若虫体重均显著降低。这与本研究中20E有抑制AlGRK2表达的效应,并对成虫羽化率和末龄若虫体重存在副效应的结果吻合。说明AlGRK2是20E信号调控绿盲蝽生长发育过程中的重要分子,下一步的研究重点为明确AlGRK2介导20E信号转导的内在分子机制。
4 结论
AlGRK2在绿盲蝽体内的表达谱显示出发育阶段和组织特异性;外源20E抑制剂及RNAi处理后,均可抑制AlGRK2的表达,同时还可对绿盲蝽生长发育产生不利影响,表明AlGRK2参与了20E对绿盲蝽生长发育的调控。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOI:10.1126/science.1187881URL [本文引用: 1]
DOI:10.1371/journal.pone.0117153URL [本文引用: 1]
DOI:10.1371/journal.pgen.1000102URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1210/en.2013-2020URL [本文引用: 2]
[D].
[本文引用: 2]
[D].
[本文引用: 2]
DOI:10.1016/S0898-6568(03)00099-8URL [本文引用: 2]
DOI:10.4062/biomolther.2016.186URL [本文引用: 1]
DOI:10.1016/j.pharmthera.2011.08.001URL [本文引用: 1]
DOI:10.1016/j.cellsig.2017.07.004URL [本文引用: 1]
DOI:10.1038/359147a0URL [本文引用: 1]
DOI:10.1038/321869a0URL [本文引用: 1]
DOI:10.1016/S0021-9258(17)42306-4URL [本文引用: 1]
[本文引用: 1]
DOI:10.1016/S0021-9258(18)46852-4URL [本文引用: 1]
DOI:10.1074/jbc.271.11.6403URL [本文引用: 1]
DOI:10.1038/srep29205URL [本文引用: 4]
DOI:10.1093/jmcb/mju025URL [本文引用: 3]
[本文引用: 1]
PMID:12769893 [本文引用: 1]

A comparative survey was carried out to investigate the effects, distribution and metabolism of ingested 20-hydroxyecdysone in four species of lepidopteran larvae in relation to the phytoecdysteroid content of the insect's host plants. Analysis of the leaves of the host plants of each of the species revealed a strong relationship between the levels of phytoecdysteroids and the relative tolerance of the larvae to ingested 20-hydroxyecdysone. Monophagous or oligophagous species (Aglais urticae, Inachis io) feeding on ecdysteroid-negative host plants were either deterred from feeding or showed marked abnormalities in growth and development after incorporation of 20-hydroxyecdysone in their diets. Oligophagous or polyphagous species (Tyria jacobaeae, Cynthia cardui) which feed on host plants from families which are known to contain phytoecdysteroid-positive species, were able to tolerate low levels of 20-hydroxyecdysone in their diets, but exhibited developmental defects at high concentrations. These species were termed semi-tolerant. In each of the species, ingested [3H]20-hydroxyecdysone appeared to follow the same fate as injected [3H]20-hydroxyecdysone. The data are compared to those obtained in previous studies, where truly polyphagous species were shown to tolerate very high concentrations of 20-hydroxyecdysone in their diets by the production of ecdysteroid 22-fatty acyl esters. Copyright 1997 Elsevier Science Ltd. All rights reserved
DOI:10.1074/jbc.M110.191783URL [本文引用: 1]
DOI:10.1006/meth.2001.1262URL [本文引用: 1]
[本文引用: 1]
DOI:10.1016/0012-1606(88)90255-2URL [本文引用: 2]
DOI:10.1002/(ISSN)1520-6327URL [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.jinsphys.2006.01.007URL [本文引用: 1]
DOI:10.1653/024.098.0233URL [本文引用: 1]
DOI:10.1016/S0165-6147(00)01678-3URL [本文引用: 1]
DOI:10.1161/CIRCRESAHA.110.231233URL [本文引用: 1]
DOI:10.1007/s00726-020-02864-xURL [本文引用: 1]
DOI:10.1016/0012-1606(91)90257-4URL [本文引用: 1]
DOI:10.1083/jcb.101.1.189URL [本文引用: 1]
DOI:10.1038/sj.emboj.7601351URL [本文引用: 2]
DOI:10.1152/ajpgi.00440.2013URL [本文引用: 1]
DOI:10.1021/acs.jmedchem.5b02000URL [本文引用: 1]
[本文引用: 1]
DOI:10.1091/mbc.e08-05-0448URL [本文引用: 1]
DOI:10.1074/jbc.M116.760850URL [本文引用: 1]
DOI:10.1042/bj2210459URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/imb.v30.3URL [本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
