 ,1, 贺霞2, 张前2
,1, 贺霞2, 张前2Anthocyanins and Flavonoids Accumulation Forms of Five Different Color Tree Peony Cultivars at Blooming Stages
CUI HuLiang ,1, HE Xia2, ZHANG Qian2
,1, HE Xia2, ZHANG Qian2通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-08-28修回日期:2020-11-8网络出版日期:2021-07-01
| 基金资助: |
Received:2020-08-28Revised:2020-11-8Online:2021-07-01
作者简介 About authors
崔虎亮,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1420KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
崔虎亮, 贺霞, 张前. 不同牡丹品种开花期间花瓣花青素和类黄酮组成的动态变化[J]. 中国农业科学, 2021, 54(13): 2858-2869 doi:10.3864/j.issn.0578-1752.2021.13.014
CUI HuLiang, HE Xia, ZHANG Qian.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】牡丹(Paeonia suffruticosa Andr.)是我国十大名花之一,具有极高的观赏价值,且根可入药,种子可榨油,是一种多功能用途植物[1,2,3]。牡丹目前有1 000多个品种[2],花色较为丰富,通常分为9大色系,即白色系、粉色系、红色系、紫色系、蓝色系、黑色系、绿色系、黄色系和复色系[4]。研究牡丹不同花色品种的花青素苷和类黄酮苷的差异及其开花过程中的动态变化,对明确观赏植物花色呈色机理和花色育种具有重要意义。【前人研究进展】经过多年的研究,初步明确了牡丹花瓣中花青素苷和类黄酮苷的组成。WANG等[5]在中原牡丹品种和日本牡丹品种花瓣中鉴定出了6种花青素物质,分别为芍药素-3-葡萄糖苷(peonidin-3-glucoside)、芍药素-3,5-葡萄糖苷(peonidin-3,5-glucoside)、矢车菊素-3-葡萄糖苷(cyaniding-3-glucoside)、矢车菊素-3,5-葡萄糖苷(cyaniding-3,5-glucoside)、天竺葵素-3-葡萄糖苷(pelargonidin-3-glucoside)和天竺葵素-3,5-葡萄糖苷(pelargonidin-3,5-glucoside)。FAN等[6]通过液相色谱(HPLC)对48个中原牡丹品种进行研究,最终鉴定出5种花青素、3种黄酮醇和6种黄酮类化合物,并且首次分离鉴定出芹菜素戊己糖苷(apigenin pento-hexoside)和芹菜素葡萄糖醛苷(apigenin hexo-glucuronide)。BAO等[7]对紫斑牡丹(P. rockii)花瓣进行UHPLC-ESI-HRMSn分析,并分离鉴定了11种类黄酮物质。LI等[8]对黄色牡丹品种花瓣呈色物质进行HPLC分析,鉴定出26种类黄酮物质,其中山奈酚、芦丁、芹菜素等衍生物是主要成分;而YANG等[9]进一步对牡丹、芍药和伊藤杂种中的黄色品种进行HPLC-DAD和HPLC-Q-TOF-MS/MS鉴定,最终分离鉴定出29种类黄酮物质,并且发现不同品种间类黄酮物质含量差异显著。ZHAO等[10]对白色品种‘雪塔’和红色品种‘彩绘’的花色素主要成分差异进行比较,发现两者均可检测到芹菜素-7-葡萄糖苷(apigenin- 7-glucoside)和芹菜素脱氧壳聚糖(apigenin deoxyheso- hexoside)2种物质,但是芍药素-3,5-葡萄糖苷(peonidin-3,5-glucoside)仅在红色品种‘彩绘’中检出。JIA等[11]对紫色系、粉色系、白色系和黄色系共41个芍药品种花瓣进行HPLC-DAD分析,最终鉴定出的主要花青素苷与WANG等[5]的结论相似,但是,紫色芍药品种含有4—5种花青素苷,而粉色品种仅含有矢车菊素-3,5-葡萄糖苷和芍药素-3,5-葡萄糖苷,且含量较低。可见,不同花色牡丹品种中花青素物质种类及含量均存在较大差异。【本研究切入点】花青素是广泛分布于植物中的一种水溶性色素,隶属于苯丙氨酸代谢途径,也是目前研究得较为清晰的次生代谢物途径之一[12,13]。目前来看,花青素苷元主要有6种,分别为天竺葵素、矢车菊素、芍药素、飞燕草素、矮牵牛素和锦葵素。不同色素苷元经过羟基化、甲基化和糖基化反应产生较为稳定的结构存在于花瓣中[14]。在牡丹中,花青素苷合成途径中关键节点酶基因的功能分析已有较多研究,如查尔酮合成酶(chalcone synthase,CHS)、二氢黄酮醇4-还原酶(dihydroflavonol 4-reductase,DFR),以及花青素苷合成酶(anthocyanidin synthase,ANS)等[10,15-16]。转录因子对关键基因的转录调控同样受到广泛关注,如PsbHLH1可激活牡丹PsDFR和PsANS等基因的表达[17],紫斑牡丹中R2R3-MYB转录因子调控关键节点酶基因的互作也得到验证[18]。然而,不同花色牡丹花青素含量差异及其开花过程中的差异化积累有待深入研究。【拟解决的关键问题】本研究对5个不同花色的牡丹品种花瓣中花青素苷和类黄酮苷进行HPLC和LC-MS的定性定量检测,分析不同花色牡丹品种中花青素苷和类黄酮苷的动态变化规律,为丰富牡丹花青素苷代谢途径的相关理论提供参考,同时为花色育种提供理论基础。1 材料与方法
1.1 试验材料
本研究选择5种不同花色的牡丹品种为试验材料,分别为白色品种‘白雪塔’、粉色品种‘赵粉’、红色品种‘迎日红’、蓝色品种‘粉荷’和紫色品种‘洛阳红’,均种植于太原迎泽公园牡丹园,株龄均在10年以上。参考李嘉珏等[2]和王莲英等[4]的方法,将牡丹开花时期分为:蕾期(S1)、露色期(S2)、盛开期(S3)和衰败期(S4),并于2020年4—5月采集不同开花时期的花冠下层花瓣(图1),每个品种选择长势一致的5个单株,每个单株分散选取花瓣,随机混合后液氮冷冻带回实验室-80℃保存备用。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同花色牡丹品种开花期间的形态变化
S1:蕾期;S2:露色期;S3:盛开期;S4:衰败期。BXT:白雪塔;ZF:赵粉;YRH:迎日红;FH:粉荷;LYH:洛阳红
Fig. 1Flower phenotypes of different color cultivars of tree peony at different blooming stages
S1, Bud stage; S2, initial blooming stage; S3, blooming stage; S4, wither stage. BXT: Baixueta; ZF: Zhaofen; YRH: Yingrihong; FH: Fenhe; LYH: Luoyanghong
1.2 试剂
色谱级甲醇、甲酸、三氟乙酸(TFA)和乙腈等化学药品购自Fisher Scientific(Fair Lawn,NJ)公司。本研究使用的标准品槲皮素-3-葡萄糖苷(quercetin- 3-glucoside,Qc3g)、杨梅素(myricetin)、山奈酚-3-鼠李糖苷(kaempferol-3-glucorhamnoside,Km3gr)、芹菜素、矢车菊素-3-葡萄糖苷(cyanindin-3-glucoside,Cy3g)和矢车菊素-3-芸香糖苷(cyanindin 3-rutinoside,Cy3r)购自Sigma-Aldrich(St. Louis, MO)公司;山奈酚和芦丁购自Solarbio(Solarbio,China)公司。试验用超纯水由PureLab Ultra(ELGA LabWater,UK)超纯水系统制备,本试验所用其他试剂均为色谱纯。1.3 样品提取及HPLC分析方法
取0.1 g新鲜样品于液氮中充分研磨,利用2 mL甲醇/水/甲酸/TFA(70﹕27﹕2﹕1,v/v/v/v)提取液避光静置提取24 h,然后12 000 r/min离心20 min,取上清液0.22 μm滤膜过滤用于液相检测。HPLC分析使用Thermo Fisher高效液相色谱系统连接996二极管阵列检测器(UltiMate 3000,ThermoFisher,US)。检测波长190—600 nm,色谱柱为Venusil ASB C18(4.6 mm×250 mm,5 μm)。流动相为2%甲酸水(A)和乙腈(B)。梯度条件如下:0 min,8% B;3 min,8% B;23 min,20% B;33 min,40% B; 43 min,40% B;45 min,8% B。柱温35℃,进样量10 μL,流速0.8 mL∙min -1。二级阵列管检测器进行全波长扫描,在350 nm检测类黄酮物质,在520 nm检测花青素。所有样品设定3次生物学重复。1.4 LC-MS分析方法
采用HPLC -microOTOF Q(ThermoFisher,US)飞行时间系统获得质谱数据。全扫描电喷雾电离(either an electrospray ionization,ESI),正负离子模式,HPLC分析条件同1.3,分子量扫描范围m/z 50—1 100,毛细管电压3 500 V,毛细管出口电压500 V,干燥气体(nitrogen)流速为8.0 L∙min-1,干燥气体温度180℃,碰撞频率200 Vpp; 喷雾器压力0.8 bar,预脉冲时间8.0 μs,转移时间80.0 μs,碰撞能量10.0 eV。1.5 定性定量分析方法
运用LC-MS方法,在350 nm和520 nm下分别检测类黄酮和花青素苷,根据质谱色谱信息、分子量、分子式和二级质谱碎片,以及相关文献等信息综合推定待测化合物。有标准品的化合物,采用外标法[19]分别计算各化合物含量;没有标准品对照的化合物,采用相似结构化合物的外标法确定其相似化合物的含量。本研究构建的标准曲线有:矢车菊素-3-葡萄糖苷(y=274.1046x+0.1328,R2=0.99987);槲皮素(y=392.9441x+0.5815,R2=0.99865);槲皮素-3-葡萄糖苷(y=339.3973x-0.3364,R2=0.99997);芦丁(y=214.2924x-0.1913,R2=0.98327);芹菜素(y=516.2105x+ 0.0001,R2=0.99666);杨梅素(y=1040.74x-2.1097,R2=0.99798)。2 结果
2.1 花青素化合物定性分析
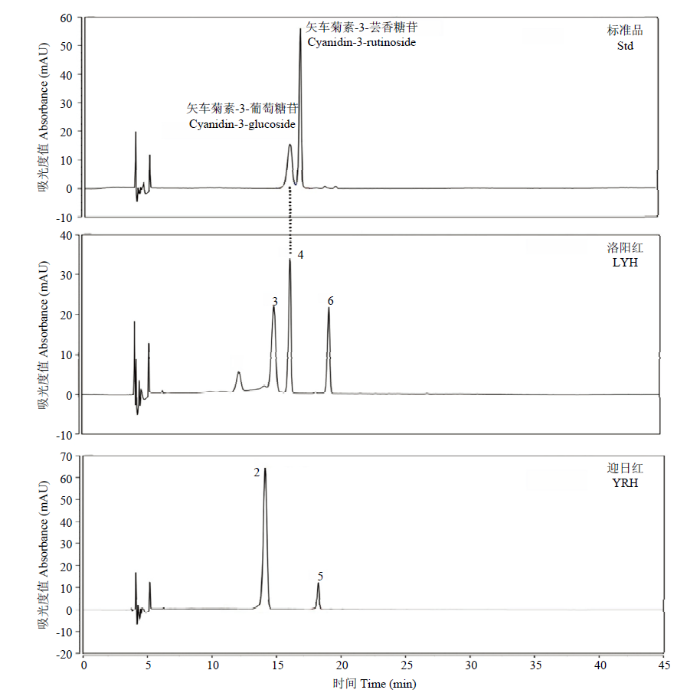
根据出峰时间,最大吸收波长(λmax)、MS质谱数据等综合信息进物质推定,最终共推定出6种花青素苷(图2),相应的出峰时间、最大波长和HPLC-ESI(±)-MS2等数据详见表1。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2牡丹品种‘洛阳红’和‘迎日红’S4时期520 nm下HPLC分离图
峰序号代表的样品信息详见
Fig. 2HPLC chromatograms at 520 nm of standards and anthocyanins extracted from cultivars LYH and YRH at stage 4
Peak numbers were as shown in
Table 1
表1
表1不同牡丹品种花青素结构推定的色谱、波长和质谱信息
Table 1
| 峰序号 Peak number | 推定物质 Putative identification matter | 保留时间 Rt (min) | 吸收波长 λmax (nm) | 母离子+ [M+H]+ | 二级离子 MS2-PI | 参考依据 Reference |
|---|---|---|---|---|---|---|
| 1 | Cy3g5g | 12.22 | 278.22, 512.18 | 611.16 | 287.3 | [6] |
| 2 | Pg3g5g | 14.13 | 274.69, 496.69 | 595.17 | 271.06 | [6, 20] |
| 3 | Pn3g5g | 14.93 | 279.12, 513.17 | 625.15 | 301.1 | [21] |
| 4 | Cy3g | 16.18 | 280.32, 514.05 | 449.1 | 287.3 | 标准品 Std |
| 5 | Pg3g | 18.22 | 266.70, 499.91 | 433.01 | 271.06 | [20, 21] |
| 6 | Pn3g | 19.21 | 279.22, 516.05 | 463.12 | 301.1 | [21] |
新窗口打开|下载CSV
前人研究表明,花青素通常在紫外光区(260—280 nm)和可见光区(500—520 nm)有最大吸收值(λmax),不过天竺葵素衍生物的λmax通常在495—505 nm[20,22]。本研究中,峰2和峰5的λmax均小于500 nm(分别为496.69和499.91 nm),而峰2的质谱数据母离子为m/z 595.17([M-H]+),二级离子m/z 271.06([Y0]+),表明丢失2个葡萄糖苷(162 Da),因此可推断该物质为天竺葵素-3,5-葡萄糖苷(pelargonidin-3,5- glucoside,Pg3g5g);同理,峰5的母离子为m/z 433.01([M-H]+),二级离子m/z 271.06([Y0]+),表明丢失了1个葡萄糖苷(162 Da),推定为天竺葵素-3-葡萄糖苷(pelargonidin-3-glucoside,Pg3g),该物质在日本牡丹品种中已有报道[5],不过FAN等[6]在中原牡丹品种中未检测到Pg3g。本研究供试的5个牡丹品种中,仅‘迎日红’中检测到Pg3g,这与前人结论相似。但是,ZHANG等[21]发现‘霓虹幻彩’和‘桔园少女’2个牡丹品种富含天竺葵衍生物。
峰1和峰4有相同的二级离子m/z 287.3([Y0]+),但是一级离子存在差异,峰1为m/z 611.16([M-H]+),峰4为m/z 449.1([M-H]+)。而峰4与标准品Cy3g的保留时间一致(图2),因此,推断峰1为矢车菊-3,5-葡萄糖苷(cyanidin-3,5-glucoside,Cy3g5g),峰4为矢车菊-3-葡萄糖苷(cyanidin-3-glucoside,Cy3g)。研究表明,花青素衍生物中,双糖苷化合物极性通常大于单糖苷化合物,极性较强的物质在HPLC检测系统中通常分离洗脱顺序早于极性较弱的物质[14,23]。FAN等[6]发现Cy3g5g在大多数中原牡丹品种中极性较强,这一现象同样在本研究中得到验证。此外,矢车菊素极性大于天竺葵素和芍药素的现象在其他花卉作物中也得到证明,如月季[24]、荷花[25]、三色堇[26]等。
同理,峰3和峰6分别推定为芍药素-3,5-葡萄糖苷(peonidin-3,5-glucoside,Pn3g5g)和芍药素-3-葡萄糖苷(peonidin-3-glucoside,Pn3g)。显然,芍药素类衍生物在牡丹不同品种中分布较为广泛[21,27]。
2.2 黄酮类化合物定性分析
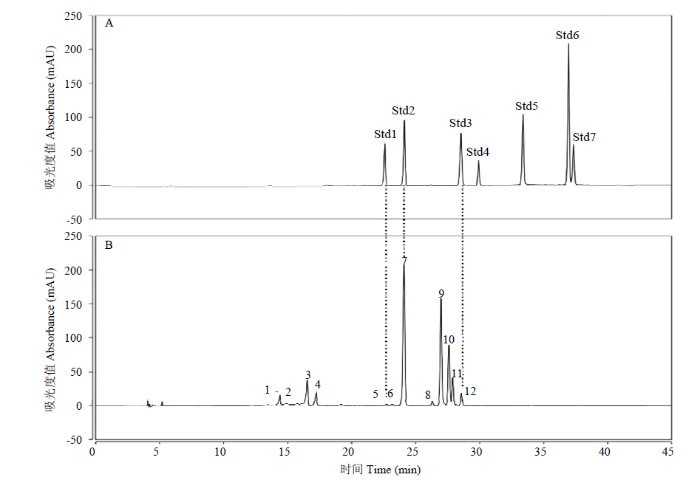
在350 nm波长下,本研究共鉴定出12种黄酮类物质(图3),主要成分为槲皮素、芹菜素和山奈酚的衍生物(表2),这几种物质在植物界分布广泛[13,28]。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3牡丹‘洛阳红’S4时期350 nm下HPLC分离图
A:标准品分离图。Std 1:芦丁;Std 2:槲皮素-3-葡萄糖苷;Std 3:杨梅素;Std 4:山奈酚-3-鼠李糖苷;Std 5:槲皮素;Std 6:芹菜素;Std 7:山奈酚。B:‘洛阳红’分离图。峰序号代表的详细信息见
Fig. 3HPLC chromatograms at 350 nm of ‘LYH’ at stage 4
A: Mix of standards. Std 1: rutin; Std 2: quercetin-3-glucoside; Std 3: myricetin; Std 4: kaempferol-3-glucorhamnoside; Std 5: quercetin; Std 6: apigenin; Std 7: kaempferol. B: ‘LYH’. Peak numbers were as shown in
Table 2
表2
表2牡丹品种花瓣中黄酮类化合物的色谱、光谱和质谱特征数据
Table 2
| 峰序号 Peak number | 推定物质 Putative identification matter | 保留时间 Rt (min) | 最大波长 λmax (nm) | 质谱离子ESIMS (m/z) | 参考依据 Reference | |||
|---|---|---|---|---|---|---|---|---|
| 母离子+ [M+H]+ | 二级离子+ MS2-PI | 母离子- [M-H]- | 二级离子- MS2-NI | |||||
| 1 | 未知 Unknown | 14.41 | 352.7 | - | - | - | - | |
| 2 | 未知 Unknown | 15.78 | 331.9 | 465.08 | 303.02/229.05 | 463.09 | 271.00 | |
| 3 | Km3g7g | 16.54 | 265.11, 345.81 | 633 | 449 | 609 | 447/285.04 | [6,8] |
| 4 | Km3g | 17.24 | 266.22, 352.43 | 471.24 | 287.05 | 447.34 | 284.16/249.04 | [8] |
| 5 | Rutin | 22.76 | 326.9 | 610.53 | 609.1 | 301.04 | std | |
| 6 | Km7g | 23.2 | 266.03, 362.07 | 449.11 | 287.05 | 447.09 | 285.04 | [8] |
| 7 | Qu3g | 24.08 | 254.08, 347.39 | 487.12 | 303.01 | 463.09 | 301.03 | std |
| 8 | 未知Unknown | 26.27 | 269.81, 336.57 | 625.17 | 479.12 | - | - | |
| 9 | Ap5g | 26.97 | 268.33, 336.57 | 433.11 | 256.96 | 431.36 | 269.22 | [6,8] |
| 10 | Aphg | 27.59 | 265.21, 336.51 | 601.28 | 579.26/433.2/271.07 | 577.44 | 431.36/269.17 | [6,8] |
| 11 | Lt7g | 27.88 | 266.01, 344.77 | 449.1 | 287.05/135.05 | 447.21 | 285.01 | [6,8] |
| 12 | My | 28.56 | 269.11, 345.29 | 319.04 | 217.05 | 463.1 | 179 | std |
新窗口打开|下载CSV
峰5、7和12分别与标准品芦丁、槲皮素-3-葡萄糖苷(quercetin-3-glucoside,Qu3g)及杨梅素共洗脱保留时间一致(图3),而质谱数据进一步证明这3个峰可推定为这3个物质(表2)。峰3、4和6的二级离子为m/z 285,表明这3个物质应为山奈酚衍生物;前人报道,山奈酚溶于甲醇之后的λmax位于266 nm(带Ⅱ)和367 nm(带Ⅰ),而槲皮素的λmax位于255 nm(带Ⅱ)和370 nm(带Ⅰ),但是山奈酚和槲皮素3-羟基的糖苷化通常会导致带Ⅰ的λmax蓝移12—17 nm[29];根据这一规律,峰6的λmax为362.07 nm,可推定为山奈酚-7-葡萄糖苷(kaempferol-7-glucoside,Km7g);峰4的λmax为352.43 nm,表明存在蓝移现象,因此该物质为山奈酚-3-葡萄糖苷(kaempferol- 3-glucoside,Km3g);峰3的λmax为265.11 nm和345.81 nm,母离子为m/z 609([M-H]-),二级离子为m/z 447和m/z 285([Y0]-),表明丢失了2个葡萄糖苷(162 Da),因此该物质可推定为山奈酚-3,7-葡萄糖苷(kaempferol- 3,7-glucoside,Km3g7g),这一物质在牡丹黄色品种中大量分布[8,9]。
芹菜素及其衍生物的λmax在267 nm和335 nm[30,31],可暂定峰9和峰10为芹菜素衍生物。根据质谱信息,峰9母离子为m/z 431.36([M-H]-),二级离子为m/z 269.22([Y0]-),推定为芹菜素-5-葡萄糖苷(apigenin-5-glucoside,Ap5g);峰10的母离子为m/z 577.44([M-H]-),二级离子为m/z 431.36/269.17([Y0]-),推定为芹菜素己糖-葡萄糖苷(apigenin hexo -glucoside,Aphg)。
前人报道木犀草素的λmax为340 nm[31],根据峰11的最大吸收波长和质谱数据(表2),暂定其为木犀草素-7-葡萄糖苷(luteolin-7-glucoside,Lt7g)。本研究中峰1、峰2和峰8等3个物质未能推定,主要原因是其质谱数据、最大吸收波长,以及前人报道等综合信息无法准确印证。如峰2的质谱数据为母离子为m/z 463.09([M-H]-),二级离子为m/z 463.09和271([Y0]-),表明该物质与槲皮素衍生物相似,但是λmax为331.9 nm,这与前人报道的352—370 nm差距较大,不能证明这一假设[32]。
2.3 牡丹不同花色品种花青素苷和类黄酮苷含量的差异
如表3所示,5个牡丹品种花青素含量差异较大。Table 3
表3
表3牡丹4个品种花朵开放期间花青素苷含量动态变化
Table 3
| 品种 Cultivar | 化合物 Compound | 蕾期 Bud Stage | 露色期 Initial blooming stage | 盛开期 Blooming stage | 衰败期 Wither stage |
|---|---|---|---|---|---|
| LYH | Cy3g5g | 4.24±0.73d | 8.70±0.76c | 15.04±1.62b | 26.22±0.78a |
| Pn3g5g | 9.80±1.22d | 29.59±3.52c | 48.72±1.39b | 86.50±3.89a | |
| Cy3g | 2.99±0.29d | 11.04±1.33c | 42.38±1.13b | 139.63±7.10a | |
| Pn3g | 3.47±0.22c | 8.66±1.14c | 26.46±3.42b | 87.38±9.64a | |
| 合计 Total | 20.50±1.74d | 57.99±6.05c | 132.59±5.25b | 340.06±9.50a | |
| FH | Pg3g5g | - | - | - | 2.73±0.82 |
| Pn3g5g | 4.94±0.87b | 7.48±0.73a | 8.02±1.66a | 9.62±1.62a | |
| 合计 Total | 4.94±0.87c | 7.48±0.73b | 8.02±1.66b | 12.35±1.41a | |
| YRH | Pg3g5g | 13.95±4.76c | 22.02±5.65b | 35.48±5.28a | 33.17±2.03a |
| Pg3g | 3.48±1.70c | 6.25±1.68ab | 4.69±1.28bc | 7.72±0.67a | |
| 合计 Total | 16.27±6.24bc | 28.27±7.32ab | 40.17±6.41a | 40.89±1.53a | |
| ZF | Pg3g5g | - | 4.43±2.15b | 7.00±0.83a | 5.12±0.96ab |
新窗口打开|下载CSV
其中‘洛阳红’花青素含量最高,在S4时期达到340.06 μg∙g-1 FW,‘白雪塔’未能检出花青素,而前人研究也证实牡丹白色品种中几乎不含有花青素,如‘香玉’[6]和‘冰山雪莲’[21]等。本研究中,不同颜色的品种花青素物质差异也较为明显,‘洛阳红’检出4种花青素(Cy3g5g、Pn3g5g、Cy3g和Pn3g),‘迎日红’检出2种花青素(Pg3g5g和Pg3g),‘粉荷’检出2种花青素(Pg3g5g和Pn3g5g),‘赵粉’仅有1种,即Pg3g5g。由此可见,不同颜色品种花青素含量存在显著差异,这与前人结论相似[6,8,10]。
本研究共鉴定出12种类黄酮苷物质,表4所示为不同品种类黄酮苷含量在花朵开放期间的动态变化结果,可以看出类黄酮苷物质在不同品种中分布差异较为明显。‘洛阳红’和‘粉荷’两个品种中能够检出全部的类黄酮苷物质,而‘白雪塔’和‘赵粉’未检出芦丁和Km7g。‘迎日红’未检测到Km7g、Lt7g和杨梅素。总体来看,Ap5g(7.18%—58.46%)、Aphg(1.44%—43.72%)和Km3g7g(2.83%—43.44%)这3种物质的相对含量高于其他物质。
Table 4
表4
表4牡丹5个品种花朵开放期间类黄酮苷含量动态变化
Table 4
| 品种 Cultivar | 化合物 Compound | 蕾期 Bud Stage | 露色期 Initial blooming stage | 盛开期 Blooming stage | 衰败期 Wither stage |
|---|---|---|---|---|---|
| LYH | 峰1 Peak 1 | 4.98±0.37d | 14.61±3.06c | 22.58±3.69b | 26.08±7.39a |
| 峰2 Peak 2 | 5.07±0.36b | 10.53±2.35a | 13.55±3.00a | 5.38±3.15b | |
| Km3g7g | 13.11±2.08d | 33.93±7.46c | 50.51±8.56b | 72.10±15.70a | |
| Km3g | 12.00±1.42c | 25.48±6.50b | 26.70±3.72b | 66.86±13.43a | |
| Rutin | 4.32±0.17b | 7.39±1.43a | 8.67±1.72a | 6.33±5.03ab | |
| Km7g | 4.42±0.06b | 6.67±1.24a | 8.29±1.94a | 7.37±2.77a | |
| Qu3g | 206.18±34.09b | 254.00±13.21a | 243.70±21.08a | 232.37±15.81a | |
| 峰8 Peak 8 | 5.62±0.24cd | 8.19±1.57b | 11.73±2.53a | 6.29±3.11bc | |
| Ap5g | 84.13±4.83c | 111.64±16.90b | 159.68±11.85a | 147.41±15.63a | |
| Aphg | 61.19±1.64b | 81.58±4.58b | 83.11±6.68b | 123.29±14.70a | |
| Lt7g | 37.83±4.70b | 38.61±6.78b | 45.37±6.37a | 39.15±3.84b | |
| My | 24.71±2.99a | 20.34±4.93b | 22.77±3.35a | 20.29±4.05b | |
| 山奈酚总含量Total km | 29.54±3.55c | 66.08±14.88b | 85.50±14.14b | 146.34±17.60a | |
| 芹菜素总含量Total ap | 145.32±5.17b | 193.22±15.21b | 242.79±17.93a | 270.70±28.52a | |
| 合计 Total | 463.58±44.14c | 612.98±31.23b | 696.67±12.25ab | 752.93±48.10a | |
| FH | 峰1 Peak 1 | 7.85±2.24a | 6.99±3.72a | 4.83±1.27b | 5.03±3.03b |
| 峰2 Peak 2 | 17.34±2.49a | 12.31±5.82b | 8.94±2.06c | 15.62±3.78ab | |
| Km3g7g | 58.04±7.36b | 77.78±9.03a | 75.13±5.25a | 88.24±5.57a | |
| Km3g | 12.33±0.72ab | 11.11±0.58b | 10.56±1.47bc | 12.69±4.41a | |
| Rutin | 3.12±0.03b | — | 4.22±1.94a | 4.28±0.98a | |
| Km7g | 3.73±0.03a | — | 3.77±1.69a | 1.84±0.43b | |
| Qu3g | 27.41±1.28a | 18.12±2.76ab | 13.57±1.01b | 14.01±2.89b | |
| 峰8 Peak 8 | 29.96±1.14a | 19.99±4.73b | 16.69±4.56b | 15.84±1.48b | |
| Ap5g | 432.95±27.17a | 333.13±13.86b | 247.42±19.42d | 251.88±28.77c | |
| Aphg | 132.20±7.25b | 164.13±1.36a | 125.38±11.46c | 28.09±7.47e | |
| Lt7g | 14.05±0.24b | 5.40±3.34c | 5.17±1.19c | 15.39±3.25a | |
| My | 6.15±0.58a | 4.64±0.43b | 3.33±0.03c | 3.78±0.25b | |
| 山奈酚总含量Total km | 71.61±6.87c | 59.26±15.67d | 88.20±7.35b | 102.16±10.46a | |
| 芹菜素总含量Total ap | 565.15±24.11a | 331.51±27.33b | 372.80±10.57b | 279.96±22.45bc | |
| 合计 Total | 740.56±16.08a | 435.72±37.50c | 515.53±19.46b | 456.07±26.12bc | |
| BXT | 峰1 Peak 1 | 6.26±2.04b | 7.71±0.03b | 12.24±1.43a | 6.00±1.82b |
| 峰2 Peak 2 | 6.23±1.93c | 19.61±1.77b | 39.89±7.15a | 32.92±10.35a | |
| Km3g7g | 10.52±2.48c | 67.29±3.54b | 159.68±15.43a | 179.49±17.09a | |
| Km3g | 13.02±3.61a | 4.04±0.54b | 14.24±1.90a | 8.02±1.63b | |
| Qu3g | 183.68±46.64a | 7.37±1.32d | 12.85±2.15c | 6.08±1.96d | |
| 峰8 Peak 8 | 7.32±2.30c | 14.46±2.66b | 19.15±1.94a | 8.05±1.91c | |
| Ap5g | 74.10±18.05d | 259.97±21.93b | 304.54±25.31a | 145.99±19.20c | |
| Aphg | 4.84±1.64d | 294.44±76.50a | 45.72±5.24b | 16.94±6.61c | |
| 品种 Cultivar | 化合物 Compound | 蕾期 Bud Stage | 露色期 Initial blooming stage | 盛开期 Blooming stage | 衰败期 Wither stage |
| Lt7g | na | 6.01±1.73c | 14.25±0.38a | 5.46±1.41c | |
| My | 24.65±5.72a | 3.10±0.03bc | 7.50±0.80b | 2.42±0.71c | |
| 山奈酚总含量Total km | 29.79±8.14c | 69.98±2.00b | 173.92±17.31a | 187.51±18.57a | |
| 芹菜素总含量Total ap | 78.95±9.66d | 554.41±18.93a | 350.26±30.35b | 162.93±14.23c | |
| 合计 Total | 336.87±6.44c | 673.45±9.96a | 625.98±15.87a | 413.18±25.33b | |
| YRH | 峰1 Peak 1 | 3.89±1.60ab | 5.04±0.53a | 4.14±0.80a | 4.75±0.42a |
| 峰2 Peak 2 | 14.68±1.05b | 12.44±2.58b | 5.44±2.48c | 23.68±8.08a | |
| Km3g7g | 76.80±3.73b | 68.99±2.30b | 72.09±3.25b | 117.40±11.45a | |
| Km3g | 5.94±1.23a | 5.97±0.78a | 4.11±0.45a | 5.32±0.26a | |
| Rutin | 6.96±3.96b | 8.14±1.09ab | 10.83±0.66a | 4.75±1.28c | |
| Qu3g | 4.99±0.71a | 3.79±0.67a | 2.76±0.89a | 4.31±0.54a | |
| 峰8 Peak 8 | 11.52±3.26a | 11.91±2.03a | 8.89±0.61b | 12.20±4.26a | |
| Ap5g | 262.87±5.68a | 250.79±28.04a | 173.83±18.97b | 252.57±30.53a | |
| Aphg | 137.57±10.44a | 128.71±34.98a | 58.71±9.04b | 57.09±18.54b | |
| 山奈酚总含量Total km | 82.75±4.89b | 74.97±2.63b | 76.20±3.57b | 122.72±11.20a | |
| 芹菜素总含量Total ap | 400.44±16.11a | 379.50±14.87a | 232.54±7.63c | 309.66±4.77b | |
| 合计 Total | 525.88±22.38a | 495.79±13.54a | 340.80±5.82b | 482.06±24.03a | |
| ZF | 峰1 Peak 1 | 5.10±0.55b | 7.40±0.67b | 13.16±0.03a | 6.60±1.60b |
| 峰2 Peak 2 | 9.96±4.36ab | 9.63±4.32b | 16.28±9.32a | 16.75±1.48a | |
| Km3g7g | 50.88±3.86b | 69.01±15.50b | 77.41±3.70b | 100.30±1.84a | |
| Km3g | 4.55±0.51a | 5.87±1.59a | 6.29±0.70a | 2.54±0.14b | |
| Qu3g | 7.96±1.39ab | 8.66±2.28a | 10.31±1.93a | 4.37±0.59b | |
| 峰8 Peak 8 | 11.29±2.19a | 12.31±4.53a | 15.07±2.19a | 6.60±1.37b | |
| Ap5g | 231.30±52.55b | 250.31±76.68b | 263.98±30.52a | 173.39±25.33c | |
| Aphg | 154.19±59.74c | 161.46±76.31b | 202.70±21.61a | 124.80±29.55d | |
| Lt7g | 4.08±0.03ab | na | 8.99±0.03a | 3.55±1.08b | |
| My | 3.50±0.22b | 3.29±1.55b | 6.48±0.83a | na | |
| 山奈酚总含量Total km | 55.42±4.34d | 74.88±1.99c | 81.60±0.04b | 102.83±1.98a | |
| 芹菜素总含量Total ap | 385.49±3.30c | 411.77±15.86b | 466.68±2.53a | 298.19±4.19d | |
| 合计 Total | 478.39±8.64ab | 524.38±16.89a | 603.81±6.30a | 438.89±6.62b |
新窗口打开|下载CSV
2.4 牡丹花朵开发不同期间花青素苷和类黄酮苷含量的动态变化
本研究中,6种花青素苷在花朵开放期间总体上不断积累,从蕾期(S1)至衰败期(S4),花青素总含量持续增加,在S4时期达到最高值,这种现象在其他研究中同样存在。ZHAO等[10]测定牡丹品种‘彩绘’发现花青素含量在花期不断积累;而ZHANG等[33]发现‘洛阳红’花朵膨大期至半开期花青素含量不断增加;GU等[18]发现紫斑牡丹品种‘青海湖银波’色斑区花青素含量在开花前20 d开始增加,至花期达到最大值。类黄酮物质总含量在花朵开放至衰老期间呈现先增加后降低的趋势,但不同品种的变化趋势差异明显。‘洛阳红’的类黄酮总含量在S4时期达到最大值((752.93±48.10)μg∙g FW),‘赵粉’在S3时期达到最大值((603.81±6.30)μg∙g FW),‘白雪塔’在露色期(S2)时期达到最大值((673.45±9.96)μg∙g FW),‘迎日红’和‘粉荷’均在现蕾期(S1)达到最大值。这表明类黄酮物质在花朵开放之后开始降解,这一现象在其他花卉作物中同样存在,如兰花(Cymbidium cv. Mystique)花瓣的花期黄酮醇含量是蕾期的1.9倍[31];WAN等[24]发现月季(Rosa)品种‘Sun City’花瓣中主要黄酮醇化合物含量在花开放之前不断积累,然后开始逐渐衰减。
3 讨论
3.1 牡丹花青素和类黄酮合成代谢的特点
花青素是广泛分布于植物中的一种天然色素,其合成代谢隶属于类黄酮代谢途径[12-14,34],CHS是花青素苷合成的核心酶,DHK是关键的分支节点,下游酶往往具有底物特异性;本研究中4个品种的花青素苷均以多种糖基衍生物形式存在,表明下游葡萄糖基转移酶(glycosyl transferases,GT)在牡丹花青素合成代谢途径中较为活跃。FAN等[6]认为花青素的羟基化和甲基化是导致花色变紫或变蓝的原因之一。参照类黄酮代谢途径可知[12,13],芍药素是由矢车菊素苷元及其上游化合物经过甲基化衍生合成,本研究中紫色品种‘洛阳红’含有矢车菊素和芍药素等多种花青素苷,蓝色品种‘粉荷’含有Pg3g5g和Pn3g5g两种花青素苷,而红色品种‘迎日红’和粉色品种‘赵粉’仅含有天竺葵素(Pg3g5g和Pn3g),表明牡丹中矢车菊素经甲基化反应产生芍药素,这是形成蓝色和紫色品种的重要因素。虽然本研究检测到了杨梅素,但相对含量不高,表明DHK向二氢杨梅素(dihydromyricetin,DHM)的转化途径并不活跃。此外,未能检出飞燕草素、矮牵牛素和锦葵素,表明无色飞燕草苷元下游合成途径在牡丹中缺失。因此,可推断DHK是牡丹花青素代谢途径重要的分支节点,F3’H和DFR的不同酶促反应对牡丹不同花色的代谢途径分支起决定性作用。3.2 不同牡丹品种花青素苷和类黄酮苷物质组成差异
植物花色的形成受多种因素影响,如色素组成及含量、金属离子含量、细胞pH、花瓣表皮细胞性状等[35]。牡丹花色多样,不同品种间花色差异明显,结合前人研究及本研究结果,发现紫色品种和蓝色花瓣中矢车菊素和芍药素含量较高,红色品种天竺葵素含量较高,白色品种几乎不含有花青素,因此,复杂的花青素苷组成可能导致了牡丹品种花色的差异。一些牡丹品种开花过程中花色变化明显,如‘金衣花脸’和‘霞光’在初开期分别呈现橙色和红紫色,此后逐渐变为黄色和橙黄色,HPLC分析表明‘金衣花脸’仅含Pn3g5g一种花青素,‘霞光’含有3种花青素,在开花各时期花青素含量不断降低[36]。此外,牡丹花器官中富含多种活性成分,如GUO等[37]对四川牡丹(P. decomposita)和凤丹(P. ostii)花瓣及雄蕊等4个部分中的活性物质成分进行分析,通过B16细胞活力测定发现牡丹花瓣提取物可有效降低黑色素生成。而XIE等[38]对35个凤丹和紫斑牡丹品种的雄蕊进行物质鉴定,发现牡丹雄蕊中富含多酚类、总黄酮和花青素物质,其中紫斑牡丹品种‘紫二乔’雄蕊中多酚类物质含量最高。可见,不同花色牡丹品种中呈色物质多样性较高,而花器官中活性物质分布同样存在显著差异,进一步弄清不同花青素苷组分及其分布规律对牡丹花色育种具有重要意义。4 结论
通过HPLC和LC-MS鉴定出6种花青素苷和12种类黄酮苷,不同颜色牡丹品种花瓣中花青素苷差异较大,紫色品种‘洛阳红’花青素苷种类最多、含量最高,白色品种‘白雪塔’几乎不含有花青素苷。类黄酮苷中芹菜素-5-葡萄糖苷、芹菜素己糖-葡萄糖苷和山奈酚-3,7-葡萄糖苷等3种物质含量较高。花青素苷在花朵开放期间不断积累,而类黄酮苷存在先积累后降解的变化趋势。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
DOI:10.1080/14620316.2017.1381045URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1007/PL00013966URL [本文引用: 3]
DOI:10.1016/j.jff.2011.09.006URL [本文引用: 8]
DOI:10.3390/molecules23020392URL [本文引用: 1]
DOI:10.1021/jf902103bURL [本文引用: 3]
DOI:10.1016/j.hpj.2020.04.002URL [本文引用: 2]
DOI:10.1016/j.bbrc.2015.02.126URL [本文引用: 4]
DOI:10.1016/j.scienta.2008.03.016URL [本文引用: 1]
DOI:10.1111/j.1365-313X.2008.03447.xURL [本文引用: 3]
[本文引用: 3]
[本文引用: 3]
DOI:10.1146/annurev.arplant.57.032905.105248URL [本文引用: 3]
DOI:10.3390/molecules22081364URL [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.plaphy.2020.06.015URL [本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1002/jssc.201600661URL [本文引用: 3]
DOI:10.1016/j.scienta.2007.05.009URL [本文引用: 6]
DOI:10.1021/jf904561eURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1021/acs.jafc.8b01509URL [本文引用: 2]
[本文引用: 1]
DOI:10.1016/j.plaphy.2014.09.012URL [本文引用: 1]
DOI:10.1016/j.chroma.2005.04.017URL [本文引用: 1]
DOI:10.1021/jf8018529URL [本文引用: 1]
DOI:10.1016/S0031-9422(01)00204-7URL [本文引用: 1]
DOI:10.1002/pca.v24.5URL [本文引用: 1]
DOI:10.1016/j.plantsci.2019.110173URL [本文引用: 3]
DOI:10.1016/j.jff.2015.11.042URL [本文引用: 1]
DOI:10.1016/j.postharvbio.2014.05.019URL [本文引用: 1]
DOI:10.1016/S0031-9422(97)00595-5URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/jfbc.2019.43.issue-4URL [本文引用: 1]
DOI:10.1016/j.indcrop.2020.112711URL [本文引用: 1]
