 ,, 廖露露, 齐永霞, 丁克坚, 陈莉
,, 廖露露, 齐永霞, 丁克坚, 陈莉 ,安徽农业大学植物保护学院/作物有害生物综合治理安徽省重点实验室/植物病虫害生物学与绿色防控安徽普通高校重点实验室,合肥 230036
,安徽农业大学植物保护学院/作物有害生物综合治理安徽省重点实验室/植物病虫害生物学与绿色防控安徽普通高校重点实验室,合肥 230036Functional Analysis of the Nucleoporin Gene FgNup42 in Fusarium graminearium
ZHANG ChengQi ,, LIAO LuLu, QI YongXia, DING KeJian, CHEN Li
,, LIAO LuLu, QI YongXia, DING KeJian, CHEN Li ,School of Plant Protection, Anhui Agricultural University/Anhui Province Key Laboratory of Integrated Pest Management on Crops/Key Laboratory of Biology and Sustainable Management of Plant Diseases and Pests of Anhui Higher Education Institutes, Hefei 230036
,School of Plant Protection, Anhui Agricultural University/Anhui Province Key Laboratory of Integrated Pest Management on Crops/Key Laboratory of Biology and Sustainable Management of Plant Diseases and Pests of Anhui Higher Education Institutes, Hefei 230036通讯作者:
责任编辑: 岳梅
收稿日期:2020-09-14接受日期:2020-09-24网络出版日期:2021-05-01
| 基金资助: |
Received:2020-09-14Accepted:2020-09-24Online:2021-05-01
作者简介 About authors
张承启,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (4741KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张承启, 廖露露, 齐永霞, 丁克坚, 陈莉. 禾谷镰孢核孔蛋白基因FgNup42的功能分析[J]. 中国农业科学, 2021, 54(9): 1894-1903 doi:10.3864/j.issn.0578-1752.2021.09.007
ZHANG ChengQi, LIAO LuLu, QI YongXia, DING KeJian, CHEN Li.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】由禾谷镰孢复合种(Fusarium graminearum species complex)引起的赤霉病严重威胁麦类作物的生产安全[1,2],除了造成严重的产量损失外,病原菌还能够在谷物籽粒中产生一系列的镰刀菌毒素,包括脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON)及其乙酰化衍生物3ADON和15ADON,雪腐镰刀菌烯醇(NIV)及其乙酰化衍生物玉米赤霉烯酮(zearalenone,ZEN),严重威胁食品安全和人畜生命健康[3,4]。自公布了禾谷镰孢基因组数据以来[5],该病原菌中涉及致病、产毒相关基因的分子生物学功能得到了充分的解析,随着组学层面的研究以及A-to-I的RNA编辑的发现,禾谷镰孢的生长发育、致病和毒素生物合成调控机制得到了进一步的阐释[6,7,8,9]。真核生物细胞功能的发挥取决于mRNA准确、有效地通过嵌在核膜上的核孔复合体从细胞核内输出到细胞质中[10]。核孔复合体除了在细胞核与细胞质之间进行物质交换运输外,该复合体中元件还扮演着与运输无关的重要角色,包括基因表达调控、染色质组装、DNA修复和mRNA加工等生物学过程[11]。解析核孔复合体中相关蛋白在禾谷镰孢形态建成、致病以及毒素生物合成过程中的作用,对揭示该病原菌生长发育、抵御逆境、产毒和致病的分子机理,以及进一步探索小麦赤霉病的综合防控技术具有重要意义。【前人研究进展】遗传信息的顺利传递需要新转录和加工的mRNA通过核孔复合体(nuclear pore complexes,NPC)从细胞核输出到细胞质中。核孔复合体是贯穿核膜的大分子运输机器,每个核孔复合体约由1 000个蛋白质亚基(统称为核孔蛋白,nucleoporins)组成[12]。核孔复合体融合贯穿在核膜中形成孔道,并同时生成由富含苯丙氨酸-甘氨酸重复序列(phenylalanine-glycine repeats,FG)组成的被动扩散屏障;每个核孔复合体由约60 MDa的对称核心组成,该核心在胞质面和核质面分别被不同的蛋白质进行修饰[13]。最早发现核孔复合体具有调控基因表达功能的试验证据来自于酿酒酵母(Saccharomyces cerevisiae),MENON等首次证明了核孔复合体中支架蛋白Nup84本身可以充当转录激活因子[14],随后的研究发现酿酒酵母中其他的核孔蛋白也同样参与了基因转录激活过程[15,16]。Nup42(又名Rip1)作为酿酒酵母中最早被鉴定的核孔蛋白之一,其与具有穿梭功能的RNA结合蛋白Rev的核输出信号(nuclear export signal,NES)互作,促进了mRNA前体的剪接和输出[17]。在酿酒酵母中,Nup42与mRNA输出因子Gle1互作促使成熟的mRNA经过核孔,由具有ATPase酶活性的DEAD-box蛋白Dbp5水解ATP提供能量,进而运输至细胞质[18,19,20]。在酵母和人类细胞中研究发现,Nup42具有两个独立功能的结构域,即FG结构域和CTD(carboxy-terminal domain)结构域,其中FG结构域与运输受体Mex67-Mtr2互作招募成熟的mRNA[21,22];CTD结构域与mRNA输出因子Gle1互作,可以向细胞质中运输热激蛋白成熟的转录本[23,24]。当人体细胞中同时缺失基因NUP42以及参与肌醇六磷酸合成的激酶基因IPK1时,热激蛋白基因转录的mRNA则不能从细胞核输出至细胞质中,从而导致细胞出现对温度敏感的缺陷[25]。当在双突变体nup42Δipk1Δ中只转入并表达Nup42的CTD结构域时,能够实现热激蛋白基因转录的mRNA正常输出到细胞质中,并且恢复了突变体nup42Δipk1Δ对温度敏感的缺陷[25,26],但在细胞中只表达Nup42的FG结构域时并不能恢复突变体nup42Δ对温度敏感的缺陷[21]。关于核孔蛋白Nup42的功能研究目前仅限于人类、哺乳动物和酵母等模式生物细胞,在植物病原真菌中未见报道。【本研究切入点】禾谷镰孢中核孔蛋白Nup42由基因FgNup42(FGSG_06067)编码,核孔蛋白在真核生物基因表达调控以及mRNA加工运输等生物学过程中至关重要,但目前没有相关研究报道禾谷镰孢中核孔蛋白基因的生物学功能。【拟解决的关键问题】基于同源重组原理和PEG介导的原生质体转化的方法,获得禾谷镰孢基因FgNup42敲除突变体ΔFgNup42以及回补突变体ΔFgNup42-C,明确FgNup42在禾谷镰孢生长发育、抵抗逆境、产毒和致病等方面的作用,为进一步探究禾谷镰孢致病和毒素生物合成调控提供一定的理论依据。1 材料与方法
试验于2018—2020年在安徽农业大学国家农作物品种审定特性鉴定站完成。1.1 菌株及培养条件
禾谷镰孢野生型菌株PH-1(NRRL 31084)、基因敲除突变体ΔFgNup42和回补突变体ΔFgNup42-C、质粒PBS、neo-PYF11、酵母菌株XK-125以及大肠杆菌DH5α均保存在安徽农业大学国家农作物品种审定特性鉴定站。供试马铃薯琼脂培养基(potato dextrose agar,PDA)、完全培养基(complete medium,CM)、基本培养基(minimal medium,MM)、分生孢子诱导培养基CMC(carboxymethyl cellulose)等的配制方法参考镰孢菌试验手册(Fusarium laboratory manual)[27]。1.2 化学试剂和仪器
DNA聚合酶、反转录试剂盒、质粒小量提取试剂盒均购自南京诺唯赞生物科技有限公司;酵母转化试剂盒购自MP Biomedicals公司;酵母质粒提取试剂盒购自Solarbio公司;潮霉素B购自Roche公司;引物、胶回收试剂盒、G418等常规生化试剂均采购于上海生工生物工程有限公司。PCR仪,C1000 Touch,Bio-Rad公司;电泳仪,Powerpac HV,Bio-Rad公司;凝胶成像仪,Chemidoc,Bio-Rad公司;荧光倒置显微镜,Ti-S,尼康;移液器及台式离心机,Eppendorf公司。
1.3 基因敲除及回补突变体的获得
在禾谷镰孢基因组数据库网站(Table 1
表1
表1本研究所用到的引物
Table 1
| 引物 Primer | 序列 Sequence (5′-3′) |
|---|---|
| Nup42-up-F | GCGCTCTCAAGAGAGTCACCG |
| Nup42-up-R | CAAAATAGGCATTGATGTGTTGACCTCCCGCGGTAAGCTCTGGCTACCT |
| Nup42-down-F | CTCGTCCGAGGGCAAAGGAATAGAGTAGCATAGGTAGGTAGGTATCTAC |
| Nup42-down-R | TTGATTCCAGCCTCCAGCTG |
| HPH-F | GGAGGTCAACACATCAATGCCTATT |
| HPH-R | CTACTCTATTCCTTTGCCCT |
| Nup42-nest-F | CGAGGGCCATGTCATTCCTG |
| Nup42-nest-R | GAGTGAGATTCTAGGATCGT |
| Nup42-ID-F | GTATCTACTATGGCGATATTGC |
| Nup42-ID-R | GTATCCAACCAATCAGCGTGTC |
| Nup42-GFP-F | ACTCACTATAGGGCGAATTGGGTACTCAAATTGGTTGCGCTCTCAAGAGAGTCACCG |
| Nup42-GFP-R | CACCACCCCGGTGAACAGCTCCTCGCCCTTGCTCACAAAGTCCCACAATGTGCATTC |
| ID-Nup42-GFP-F | CCGCAAGCTAACAATCCATTC |
| ID-GFP-R | GTCAGCTTGCCGTAGGTGGCA |
| Tri1-RT-F | GATGTTCCTTCTCGACAGCGT |
| Tri1-RT-R | CACTGGTCGAAGATAGCTGG |
| Tri3-RT-F | TGTTACGATCAATGGCTTGG |
| Tri3-RT-R | TCCTCGTTGTAGTTTGCATCA |
| Tri4-RT-F | ACGTGTGGCTACTCAGGAGAA |
| Tri4-RT-R | TGGAATTGCCTTGGGGTA |
| Tri6-RT-F | AAATGCCCATTCCCTAGTTG |
| Tri6-RT-R | ATCTCGCATGTTATCCACCCT |
| Tri7-RT-F | TACCGTCGTCTTCAAAACCA |
| Tri7-RT-R | ACGCCAATGGTGTTCACAAA |
| Tri8-RT-F | ATATAACGGTACCCCCAGATG |
| Tri8-RT-R | TGTTTGTAGGACACTTCCGGT |
| Tri14-RT-F | AACTCCCGTTGTGATCAAGCA |
| Tri14-RT-R | AACAGTAATGTTGGCACCGT |
| actin-RT-F | CCACGTCACCACTTTCAACT |
| actin-RT-R | TGCTTGGAGATCCACATTTG |
新窗口打开|下载CSV
利用表1中引物Nup42-GFP-F/Nup42-GFP-R扩增基因FgNup42的启动子至开放阅读框的DNA片段,使用酵母转化试剂盒将所得的PCR产物与经XhoⅠ线性化的质粒neo-pYF11共转化进入XK1-25酵母中构建重组质粒[30,31];然后,鉴定并提取阳性酵母菌株中的质粒并将其转化至大肠杆菌DH5α中;最后,提取大肠杆菌质粒经PEG介导的原生质体转化至敲除突变体ΔFgNup42中,通过G418(100 μg·mL-1)筛选并经PCR鉴定回补转化子。
1.4 菌落形态、无性繁殖及有性生殖观测
菌落形态观察:菌株于PDA平板上培养3 d,打孔器取直径5 mm的菌碟分别接种于PDA、MM、CM的9 cm平板中,25℃培养3 d拍照,每次重复3皿,试验重复3次。边缘菌丝观察:将灭菌洁净的载玻片浸润在融化状态的CM培养基中迅速取出放置暗盒中使其表面培养基凝固,取直径3 mm的菌碟于载玻片上,每个菌株重复5个玻片,25℃培养16—20 h后取出载玻片于显微镜下观察边缘菌丝形态并拍照。
分生孢子产量及隔膜数统计:从新鲜活化的菌落边缘打孔取5个直径5 mm的菌碟接种到分装有30 mL CMC培养液的50 mL三角瓶中,每个菌株3瓶,25℃,180 r/min摇培7 d。血球计数板统计孢子数量,试验重复3次。吸取CMC培养液中的分生孢子,荧光增白剂(calcofluor white,CFW)染色,显微镜下统计不同隔膜数孢子的数量,每个菌株分别统计300个分生孢子。
有性生殖观测:将菌碟接种于胡萝卜培养基中,4个重复,25℃培养至所有菌株都长满整个培养基,刮净培养基表面的气生菌丝,加入800 μL 0.1% Tween-20均匀涂抹在培养基表面,晾干后放置黑光灯下25℃培养20 d,观察统计子囊壳数量及子囊孢子形态并拍照。
1.5 胁迫因子敏感性测定
从活化于PDA中生长3 d的菌落边缘取直径5 mm的菌碟分别接种于含1 mol·L-1 NaCl、1 mol·L-1 KCl、0.2 g·L-1刚果红、0.25 μg·mL-1戊唑醇和0.25 μg·mL-1氰烯菌酯的PDA平板上,25℃培养3 d后拍照,每次重复3个皿,试验重复3次。1.6 致病力和毒素测定
麦穗接种:在小麦(感病品种安农8455)抽穗扬花盛期,注射10 μL孢子悬浮液(106孢子/mL)到麦穗中部小穗的花药中,每天早晚各喷灌一次,15 d后统计发病情况并拍照记录,每个菌株重复30株麦穗。玉米花丝接种:将4根新鲜的玉米花丝彼此成一束,每束玉米花丝长度为10 cm,成束排列在经无菌蒸馏水润湿的滤纸上。玉米花丝束中央放置一个直径为5 mm的菌碟,5个重复,25℃保湿7 d,拍照。毒素的提取和测定方法参考JI等[32]。
1.7 TRI表达分析
于PDA中生长3 d的菌落边缘取5个直径为5 mm的菌碟,置于分装有30 mL诱导产毒培养基(trichothecene biosynthesis induction,TBI)的三角瓶中,锡箔纸包裹避光,25℃,180 r/min摇培48 h[33]。过滤收集TBI中的菌丝并在液氮中充分研磨,提取总RNA,使用反转录试剂盒进行逆转录。利用表1中相关引物,qRT-PCR确定TRI的表达水平,试验重复3次。1.8 数据统计分析
采用Excel 2010计算菌落生长抑制率、分生孢子产量、子囊壳数量以及毒素含量,数据采用Fisher’s最小显著差数法进行分析(P=0.05)。2 结果
2.1 敲除FgNup42对禾谷镰孢营养生长的影响
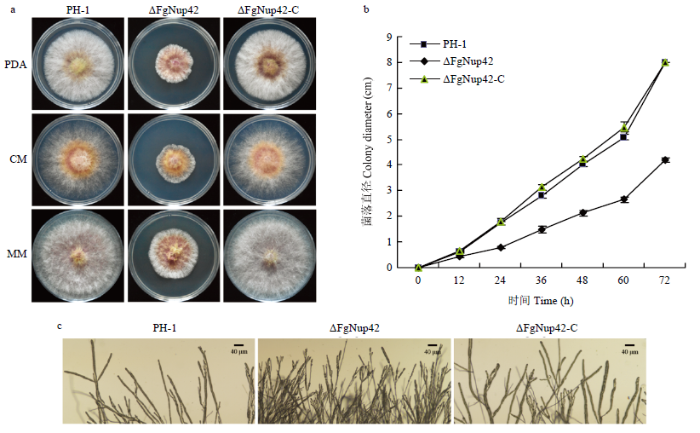
基因缺失突变体ΔFgNup42在培养基PDA、MM和CM上的生长速率与野生型PH-1、回补突变体ΔFgNup42-C相比降低了约50%(图1-a、1-b)。通过观察菌落边缘形态特征发现,敲除突变体ΔFgNup42相较野生型,边缘菌丝分枝变多且致密(图1-c),结果表明FgNup42的缺失严重影响了禾谷镰孢的生长速率以及菌落的形态特征。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1突变体ΔFgNup42的菌落形态和生长缺陷
a:PH-1、敲除突变体ΔFgNup42和回补体ΔFgNup42-C在培养基PDA、CM和MM中25℃生长3 d PH-1, ΔFgNup42 deletion mutant and ΔFgNup42-C complemented strains were grown on PDA, CM and MM at 25℃ for 3 d;b:菌株PH-1、ΔFgNup42和ΔFgNup42-C在PDA培养基中的生长速率比较Comparison of mycelial growth rates among PH-1, ΔFgNup42 and ΔFgNup42-C strains on PDA medium;c:PH-1、ΔFgNup42和ΔFgNup42-C的菌落边缘菌丝生长情况Hyphal growth at the edges of PH-1, ΔFgNup42 and ΔFgNup42-C colonies
Fig. 1Colony morphology and growth defects of the ΔFgNup42 mutant
2.2 FgNup42参与禾谷镰孢分生孢子的发育
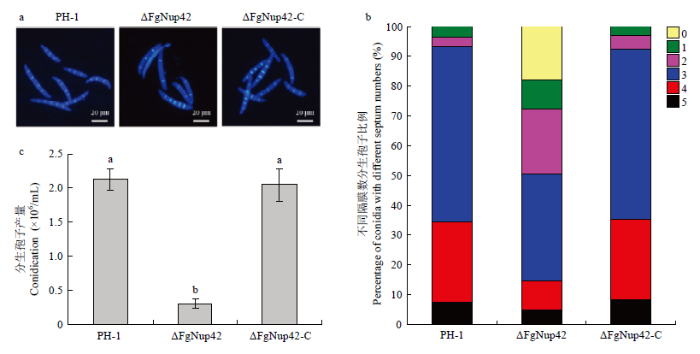
经荧光增白剂(CFW)染色,显微镜观察诱导产孢培养基CMC中分生孢子的形态发现,基因敲除突变体ΔFgNup42产生的分生孢子隔膜数与野生型相比明显变少(图2-a);通过统计分析不同隔膜数分生孢子所占的比例发现,突变体ΔFgNup42中0—2个隔膜的分生孢子比例较野生型和回补体明显增加(图2-b)。血球计数板统计各菌株的分生孢子产量发现,敲除突变体ΔFgNup42的产孢量显著降低,只有野生型PH-1的14.55%(图2-c)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2敲除基因FgNup42对禾谷镰孢分生孢子形成的影响
a:PH-1、ΔFgNup42和ΔFgNup42-C的分生孢子隔膜经荧光增白剂染色Septa of conidia produced by PH-1, ΔFgNup42 and ΔFgNup42-C visualized after staining with calcofluor white (CFW);b:PH-1、ΔFgNup42和ΔFgNup42-C中不同隔膜数的孢子比例Percentage of conidia with different septum numbers in PH-1, ΔFgNup42 and ΔFgNup42-C;c:敲除基因FgNup42导致产孢量降低Deletion of FgNup42 led to decreased conidiation
Fig. 2Impacts of FgNup42 deletion on conidiogenesis of F. graminearum
2.3 敲除基因FgNup42对有性生殖的影响
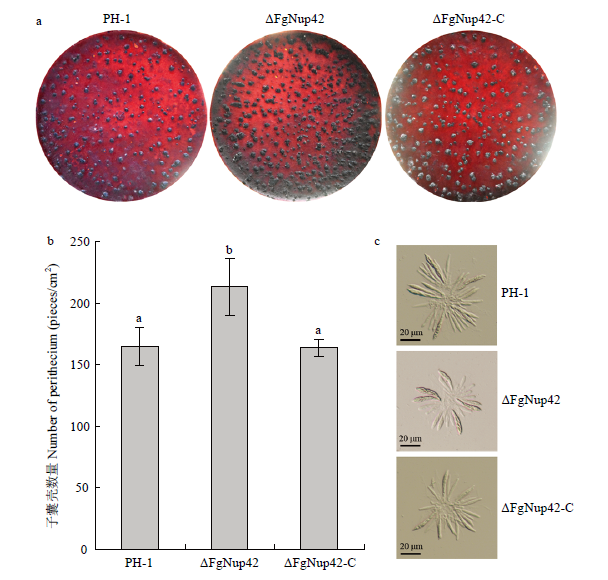
在禾谷镰孢侵染小麦的过程中,子囊孢子作为重要的初侵染源在病害循环中发挥着至关重要的作用[34]。因此,笔者观测了野生型PH-1、基因缺失突变体ΔFgNup42以及回补突变体ΔFgNup42-C子囊壳以及子囊孢子的形成状况。经过20 d的有性生殖诱导,敲除突变体ΔFgNup42产生的子囊壳数量显著多于野生型和回补体(图3-a、3-b)。于载玻片上压开子囊壳后观察发现,野生型PH-1、敲除突变体ΔFgNup42和回补突变体ΔFgNup42-C在子囊以及子囊孢子的形态特征上没有明显差异(图3-c),说明基因FgNup42的缺失不影响子囊以及子囊孢子的形态。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3突变体ΔFgNup42在有性生殖过程中的缺陷
a:PH-1、ΔFgNup42和ΔFgNup42-C在有性生殖条件下诱导20 d后的子囊壳形成情况Perithecium formation in mating cultures of PH-1, ΔFgNup42 and ΔFgNup42-C examined at 20 days post-induction;b:敲除基因FgNup42导致产子囊壳数量增加Deletion of FgNup42 led to increased perithecium;c:PH-1、ΔFgNup42和ΔFgNup42-C的子囊形态Asci rosettes of PH-1, ΔFgNup42 and ΔFgNup42-C
Fig. 3The ΔFgNup42 mutant was defective in sexual development
2.4 FgNup42参与禾谷镰孢对渗透、细胞壁以及杀菌剂胁迫的敏感性
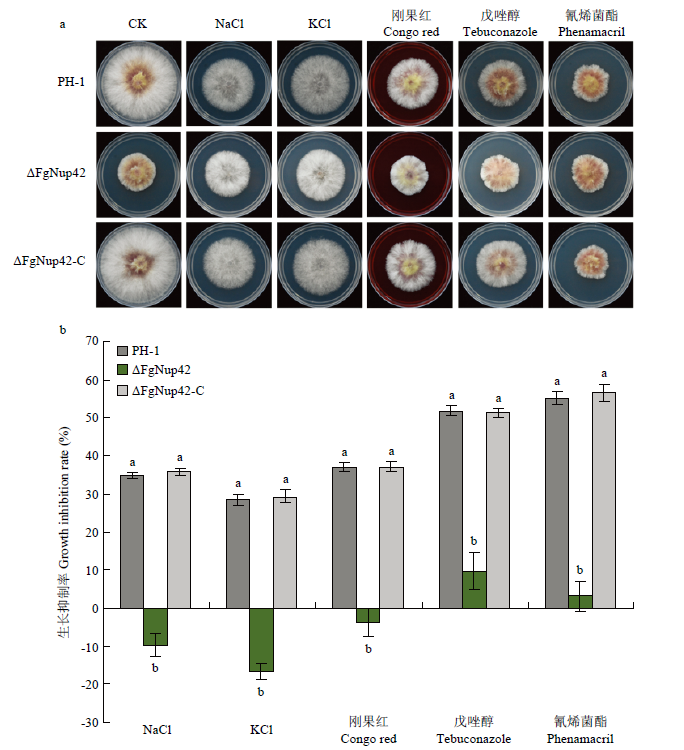
Nup42作为细胞核孔的重要组分,而核孔又是物质进出细胞核与细胞质的重要通道[13],因此测定了基因敲除突变体ΔFgNup42对渗透胁迫(1 mol·L-1 NaCl和1 mol·L-1 KCl)、细胞壁胁迫(0.2 g·L-1刚果红)以及杀菌剂(0.25 μg·mL-1戊唑醇和0.25 μg·mL-1氰烯菌酯)的敏感性,发现ΔFgNup42相较野生型对NaCl、KCl、刚果红、戊唑醇以及氰烯菌酯的抗性显著增加(图4)。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4缺失FgNup42影响渗透、细胞壁和杀菌剂胁迫的敏感性
a:PH-1、ΔFgNup42和ΔFgNup42-C在添加1 mol·L-1 NaCl、1 mol·L-1 KCl、0.2 g·L-1刚果红、0.25 μg·mL-1戊唑醇和0.25 μg·mL-1氰烯菌酯的PDA培养基中25℃生长3 d的比较Comparison of PH-1, ΔFgNup42 and ΔFgNup42-C following incubation at 25℃ for 3 d on PDA plates supplemented with 1 mol·L-1 NaCl, 1 mol·L-1 KCl, or 0.2 g·L-1 Congo red, 0.25 μg·mL-1 tebuconazole and 0.25 μg·mL-1 phenamacril;b:菌株在含有NaCl、KCl、刚果红、戊唑醇和氰烯菌酯的PDA培养基中生长3 d相较对照组的菌丝生长抑制率Mycelial growth inhibition compared with non-treated controls following incubation for 3 d on PDA containing NaCl, KCl, Congo red, tebuconazole and phenamacril
Fig. 4Effects of FgNup42 disruption on the sensitivity to osmotic stresses, cell wall-damaging agent and fungicides
2.5 FgNup42的缺失对禾谷镰孢致病和产毒的影响
为了明确FgNup42在禾谷镰孢致病过程中的功能,对野生型PH-1、敲除突变体ΔFgNup42以及回补突变体ΔFgNup42-C在麦穗和玉米花丝上进行了致病力测定。田间麦穗接种发现突变体ΔFgNup42的致病力显著下降,仅局限于小穗发病(图5-a);通过室内接种玉米花丝发现,ΔFgNup42在玉米花丝上的侵染扩展程度相比野生型和回补体同样明显下降(图5-b)。DON毒素及其衍生物是禾谷镰孢的关键致病因子[35],由于敲除基因FgNup42后导致病原菌致病力严重下降,因此笔者测定了PH-1、ΔFgNup42以及ΔFgNup42-C的产毒能力。结果显示,ΔFgNup42产生的毒素量DON、3ADON以及15ADON显著低于野生型和回补菌体(图5-c)。为了进一步证实该结果,测定了参与DON毒素生物合成途径中各TRI的表达量,与野生型相比,ΔFgNup42中7个TRI基因的转录水平明显降低(图5-d)。上述结果表明,FgNup42通过调节禾谷镰孢中TRI的表达来调控毒素的生物合成。图5
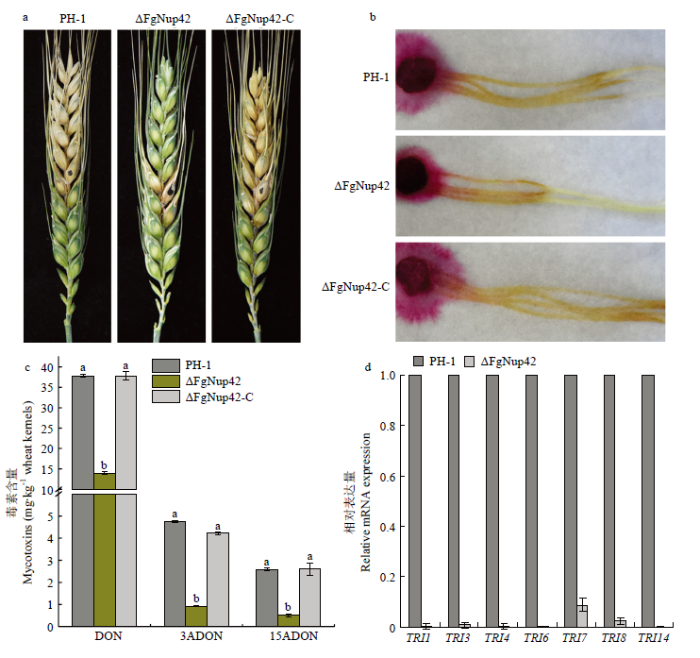 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5FgNup42参与禾谷镰孢致病力以及单端孢霉烯族毒素的合成
a:PH-1、ΔFgNup42和ΔFgNup42-C在麦穗上的发病症状Disease symptoms on wheat heads caused by PH-1, ΔFgNup42 and ΔFgNup42-C;b:PH-1、ΔFgNup42和ΔFgNup42-C接种玉米花丝致病情况Maize silks inoculated with PH-1, ΔFgNup42 and ΔFgNup42-C;c:各菌株于接种侵染的麦穗籽粒中单端孢霉烯族毒素的产量Levels of trichothecene mycotoxins produced by each strain in infected spikelets collected from inoculated wheat heads;d:7个TRI在PH-1和ΔFgNup42中的相对表达量Relative transcription levels of 7 TRIs in PH-1 and ΔFgNup42
Fig. 5Involvement of FgNup42 in pathogenicity and trichothecene mycotoxins biosynthesis of F. graminearum
3 讨论
核孔复合体控制着生物大分子在细胞核与细胞质之间的运输,在mRNA输出至细胞质的最后一步,DEAD-box解旋酶Dbp5(人类细胞中叫做DDX19)被核孔蛋白Nup42、Gle1和Nup214激活,可从mRNA颗粒中去除RNA结合蛋白Nxf1·Nxt1,从而使成熟的mRNA顺利到达细胞质完成翻译[13]。Nup42编码一个核孔蛋白,该蛋白最早被鉴定到与酿酒酵母中表达的HIV-1 Rev的效应子互作[17,36]。虽然有关Nup42的生化功能在酿酒酵母和人类细胞中研究得比较透彻[11,37],但是有关Nup42分子遗传学方面的功能尚未在植物病原真菌中研究报道。本研究发现禾谷镰孢FgNup42基因缺失突变体ΔFgNup42生长速率与野生型相比明显减慢,但是在酿酒酵母中Nup42并非其生长所必需[36]。此外,突变体ΔFgNup42在无性繁殖过程中出现严重缺陷,分生孢子产量显著减少并且不同隔膜的分生孢子比例分布发生了明显的分化。但ΔFgNup42的有性生殖能力增强了,相较野生型菌株产生了更多的子囊壳,同时子囊孢子的形态正常,可能因为敲除基因FgNup42致使禾谷镰孢有丝分裂相关基因的表达受到抑制,而减数分裂过程得到了促进,此现象背后的调控机制有待进一步研究。突变体ΔFgNup42对外界渗透压、细胞壁胁迫以及杀菌剂的敏感性发生了变化,禾谷镰孢FgNup42参与菌体抵御外界环境胁迫,很可能由于基因FgNup42的缺失导致相关信号通路的转录因子Hog1[38]、Slt2[39]、FgSR[40]以及FgTfml[41]从细胞质进入细胞核受阻,这与Nup42在酿酒酵母中同源基因缺失突变体rip1Δ抵抗逆境胁迫因子类似[11,26]。除了在生长发育过程中出现的缺陷外,ΔFgNup42在寄主植物上的致病力严重降低,虽然敲除了基因FgNup42后使菌体的生长速率减慢了50%,但是突变体ΔFgNup42在麦穗上的致病仅局限于单个小穗,没有向周围小穗扩展,说明ΔFgNup42致病力的缺陷并不是由生长速率降低引起的。通过测定菌株产毒能力的变化发现,ΔFgNup42在发病麦粒中3种毒素(DON、3ADON和15ADON)合成量显著减少,而毒素是禾谷镰孢关键的致病因子,帮助其在寄主中扩展蔓延[35,42],因此可以推断ΔFgNup42致病力的降低主要是其毒素合成能力的下降所导致的。突变体ΔFgNup42中参与DON毒素生物合成的TRI表达量的显著降低更加证实了其毒素合成能力的缺陷,关于核孔蛋白FgNup42调控DON毒素生物合成TRI的转录及出核运输机制有待于进一步研究。
4 结论
通过基因敲除与回补的方法研究了禾谷镰孢中基因FgNup42的功能,FgNup42的缺失导致禾谷镰孢生长速率降低,分生孢子产量减少,有性生殖能力变强,对渗透、细胞壁以及杀菌剂的胁迫敏感性变弱,毒素合成能力下降同时致病力严重降低。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
