 ,1, 程婷婷1, 惠小涵1, 陈章玉1, 王瑞红2, 柯卫东3, 郭宏波
,1, 程婷婷1, 惠小涵1, 陈章玉1, 王瑞红2, 柯卫东3, 郭宏波 ,1
,1Screening of Polyphenol Oxidase Interaction Proteins from Nelumbo nucifera and Their Verification
YUAN XinBo ,1, CHENG TingTing1, XI XiaoHan1, CHEN ZhangYu1, WANG RuiHong2, KE WeiDong3, GUO HongBo
,1, CHENG TingTing1, XI XiaoHan1, CHEN ZhangYu1, WANG RuiHong2, KE WeiDong3, GUO HongBo ,1
,1通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-02-22接受日期:2020-07-13网络出版日期:2020-09-16
| 基金资助: |
Received:2020-02-22Accepted:2020-07-13Online:2020-09-16
作者简介 About authors
原新博,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (7422KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
原新博, 程婷婷, 惠小涵, 陈章玉, 王瑞红, 柯卫东, 郭宏波. 莲藕多酚氧化酶互作蛋白的筛选及验证[J]. 中国农业科学, 2020, 53(18): 3777-3791 doi:10.3864/j.issn.0578-1752.2020.18.013
YUAN XinBo, CHENG TingTing, XI XiaoHan, CHEN ZhangYu, WANG RuiHong, KE WeiDong, GUO HongBo.
0 引言
【研究意义】莲藕(Nelumbo nucifera Gaertn.)是我国最大的水生蔬菜种类[1],药食两用,其整个植株10个部位中有6个部位入药,具有降脂减肥、抗氧化、抗衰老、安神助眠等药理学功效。莲藕具有很高的营养价值和药用功效[2],但采后褐变严重影响了其商品性及加工产业的发展。为解决莲藕采后贮藏和加工过程中由多酚氧化酶(PPO)引起的褐变问题,研究筛选、验证PPO与何蛋白互作,逐步摸清其在莲藕中的作用机制,对于采后控制莲藕褐变,延长其产业链具有重要意义。【前人研究进展】PPO是由核基因编码的一种含铜金属酶[3],可将其分为单酚氧化酶(酪氨酸氧化酶)、双酚氧化酶(儿茶酚氧化酶)和漆酶3类[4],在动植物、真菌和昆虫的质体中广泛存在[5,6]。PPO通常由3部分组成:一个N端导肽区,一个高度保守的CuA和CuB区以及一个C端疏水区域[7];PPO的主要功能区是Cu结合区,其他部分对酶空间构象、高级结构形成和维持起作用[8]。研究发现PPO在植物中有多种重要功能,如抗病虫害、抗机械损伤、参与花色的形成、参与光合作用、酶促褐变等[9,10,11,12,13]。在植物细胞中,PPO和多酚是相互独立的两类物质,前者主要分布于类囊体中,后者存在于液泡中,当细胞受到损伤时,PPO会在氧气的参与下,催化多酚类物质生产醌类黑色素,使莲藕等果蔬褐变[7,14]。草酸处理莲藕切片可延缓切面褐变,并且显著降低PPO的活性[15]。抗坏血酸和芦荟凝胶联合使用抑制贮藏期间莲藕表面褐变,并抑制过氧化物酶(POD)和PPO的活性[16]。通过人工microRNAs基因沉默技术,同时下调马铃薯多酚氧化酶基因家族中StuPPO2、StuPPO3和StuPPO4,会产生低活性的PPO和低褐变的马铃薯[17]。洋葱提取物中的低分子量化合物对芋头多酚氧化酶和酶促褐变具有抑制作用[18]。番木瓜粗提物中存在的低分子量热稳定阴离子化合物可与PPO催化活性双核位点结合,抑制PPO的活性[19]。通过农杆菌介导法将反义PPO转入苹果愈伤组织中,PPO活性和愈伤组织的褐变潜力均发生变化[20]。【本研究切入点】目前关于PPO导致莲藕等果蔬褐变的分子机制未见报道,仅有关于酶学性质、体外抑制PPO活性、生理活性等的研究。有研究表明SOD、CAT和POD等抗氧化酶也参与了莲藕的褐变过程[21]。笔者课题组前期发现NnPPO1与多个蛋白存在互作[22],本研究进一步筛选、验证与莲藕NnPPO1互作的褐变相关酶。【拟解决的关键问题】利用Y2H技术,从抗氧化酶保护系统中筛选出NnCAT1与NnPPO1存在蛋白互作;利用BiFC技术进一步验证NnPPO1与NnCAT1之间是否存在互作,通过截短蛋白寻找互作关键位点;并对NnPPO1与NnCAT1进行组织特异性表达分析;对NnCAT1进行生物信息学分析、亚细胞定位分析。为深入研究莲藕中NnPPO1与NnCAT1互作的分子机制奠定基础,为精准控制NnPPO1酶活性、降低褐变提供理论依据。1 材料与方法
试验于2019年在西北农林科技大学化学与药学院进行。1.1 试验材料
1.1.1 植物材料 试验材料为莲藕‘鄂莲5号’品种;烟草为本氏烟草(Nicotiana benthamiana),种子播种于营养土中,萌发后7 d左右开始浇营养液,每隔2 d浇一次,温室温度为25℃,湿度约为70%,光照周期为14 h光照、10 h黑暗。1.1.2 试验试剂及载体 植物总RNA提取试剂盒、反转录试剂盒、DNA聚合酶和限制性内切酶均购自宝生物工程有限公司;荧光定量试剂盒购自南京诺唯赞生物科技有限公司;大肠杆菌感受态、酵母菌感受态、农杆菌感受态、同源重组试剂盒Quick-Clone Mix购自陕西普因特生物公司;DNA marker、DNA胶回收纯化试剂盒和质粒提取试剂盒均购自OMEGA公司;X-α-Gal、Aureobasidin A购自Clontech公司;PCR引物合成及载体测序均由上海生工生物工程股份有限公司完成。
构建所有载体使用的大肠杆菌是EPI300感受态,酵母双杂试验所用载体为pGADT7和pGBKT7,酵母菌株为Y2HGold感受态,亚细胞定位试验使用的载体是p35S-eGFP[23],双分子荧光互补试验所使用的载体是35S-SPYNER173与35S-SPYCEM[24]。注射烟草叶片所使用的农杆菌菌株是GV3101感受态。
1.2 RNA提取及基因克隆
使用Plant RNA Extraction Kit(Takara)提取莲藕叶片总RNA;利用PrimeScript? II 1st Strand cDNA Synthesis Kit(Takara)反转录合成cDNA。以莲藕叶片cDNA为模板,利用基因特异性引物(表1)对抗坏血酸过氧化物酶(APX)(GenBank: GU174022)[25]、过氧化氢酶(NnCAT1)、胞质铜-锌超氧化物歧化酶(CuZnSOD)(GenBank: GQ149102)[26]、半胱氨酸过氧化物酶(PER1)(GenBank: KU923323)[27]、过氧化物酶3(POD3L)(GenBank: XM_010245037)、过氧化物酶43(POD43L)(GenBank: XM_010247537)、过氧化物酶P7(PODP7L)(GenBank: XM_010248161)等5种抗氧化酶的7个基因进行PCR扩增;反应体系为25 μL:cDNA 1 μL、PrimeSTAR Max Premix(2×)12.5 μL、上下游引物各1 μL和ddH2O 9.5 μL。反应程序为98℃ 10 s;60℃ 15 s,72℃ 2 min,34个循环,16℃保存。PCR产物经琼脂糖凝胶电泳检测,使用胶回收试剂盒纯化,得到目的片段。Table 1
表1
表1PCR 引物列表
Table 1
| 引物名称Name | 序列Sequence | 用途Use |
|---|---|---|
| APX-EcoR I-F | ggaggccagtgaattcATGGCTTGTCTGGGCGGTGC | 酵母双杂交 Yeast two hybrid |
| APX-Xho I-R | tcatctgcagctcgagTCATGTTGAGAAACCCTCAGGAGG | |
| NnCAT1-EcoR I-F | ggaggccagtgaattcATGGATCCTTACAAGTATCGCCC | |
| NnCAT1-Xho I-R | tcatctgcagctcgagTCACATGCTCGGCTTCAC | |
| cytCuZnSOD-EcoR I-F | ggaggccagtgaattcATGGTGAAGGCTGTTGCGG | |
| cytCuZnSOD-Xho I-R | tcatctgcagctcgagTTAACCCTGCAAGCCAATAATACC | |
| PER1-EcoR I-F | ggaggccagtgaattcATGCCTGGACTGACGATCGG | |
| PER1-Xho I-R | tcatctgcagctcgagTCAGACGTTGGTAAAACGGAG | |
| POD3L-EcoR I-F | ggaggccagtgaattcATGAGGACTACTTATCTTCCGTT | |
| POD3L-Xho I-R | tcatctgcagctcgagTTAAGGGTTCACAACCGCACACT | |
| POD43L-EcoR I-F | ggaggccagtgaattcATGGCTCTGGTTTTTGCTC | |
| POD43L-Xho I-R | tcatctgcagctcgagTCAATTGAAAGACCTGCAGGAC | |
| PODP7L-EcoR I-F | ggaggccagtgaattcATGGCCTCCATCATCACCC | |
| PODP7L-Xho I-R | tcatctgcagctcgagTCAGTTCACTCTCCTGCAATTC | |
| NnCAT116-398-EcoR I-F | ggaggccagtgaattcATGTTCTGGAGCACAAATTCTGG | |
| NnCAT116-398-Xho I-R | tcatctgcagctcgagTCAATCATACCTTGAAGGAAAGTAA | |
| NnCAT116-493-EcoR I-F | ggaggccagtgaattcATGTTCTGGAGCACAAATTCTGG | |
| NnCAT116-493-Xho I-R | tcatctgcagctcgagTCACATGCTCGGCTTCAC | |
| NnPPO1175-380-Nco I-F | ctgcatatggccatgATGCCACGTAACTTCACGCA | |
| NnPPO1175-380-BamH I-R | gcaggtcgacggatcTCATATTGTCCACATCCGGTCGAC | |
| NnPPO1381-468-NcoI-F | ctgcatatggccatgATGTGGAAAAAGCTGGGAGG | |
| NnPPO1381-468-BamH I-R | gcaggtcgacggatcTCACTCACTGACGGTTGGC | |
| NnPPO1469-597-Nco I-F | ctgcatatggccatgATGTTCCCCAAAGAACTTGATGCG | |
| NnPPO1469-597-BamH I-R | gcaggtcgacggatcTCACGAAGCGAACACTATCTTG | |
| NnCAT1-BD-Nco I-F | ctgcatatggccatgATGGATCCTTACAAGTATCGCCC | 自激活验证 Self-activated tast |
| NnCAT1-BD-BamH I-R | gcaggtcgacggatcTCACATGCTCGGCTTCAC | |
| NnPPO1-BiFC-N-BamH I-F | cccaggcctactagtggatccATGGCGTCGCTGTCTCCC | 双分子荧光标记 BiFC |
| NnPPO1-BiFC-N-Xho I-R | cccgggagcggtaccctcgagTCACGAAGCGAACACTATCTTGA | |
| NnCAT1-BiFC-C-BamH I-F | tggcgcgccactagtggatccATGGATCCTTACAAGTATCGCCC | |
| NnCAT1-BiFC-C-Xho I-R | cccgggagcggtaccctcgagCATGCTCGGCTTCACATTGA | |
| NnCAT1-GFP-BamH I-F | ggactctagaggatccATGGATCCTTACAAGTATCGCCC | 亚细胞定位 Subcellulcar locatization |
| NnCAT1-GFP-Kpn I-R | cccttgctcaccatggtaccCATGCTCGGCTTCACATTGAGAC | |
| β-actin-F | GCCATCCAGGCCGTTCTCTC | qRT-PCR |
| β-actin-R | GGGACAGTGTGGCTGACACC | |
| NnPPO1-qPCR-F | CAAACTCCGCGATGCCAAGC | |
| NnPPO1-qPCR-R | CGTCGAGCCATTGGACACCA | |
| NnCAT1-qPCR-F | TTTGCCCTGGCGTTGTGGTC | |
| NnCAT1-qPCR-R | GGTGAGCACACTTGGGAGCA |
新窗口打开|下载CSV
1.3 共转化验证
按照同源重组Quick-Clone Mix试剂盒的操作步骤,将APX、NnCAT1、CuZnSOD、PER1、POD3L、PODP7L、POD43L等的编码区分别克隆到pGADT7 (AD)载体上,课题组前期构建多酚氧化酶诱饵载体NnPPO1-BD和去除N端导肽部分的诱饵载体JDPPO1-BD[22];通过PEG/LiAc介导的方法,将质粒组合APX-AD+JDPPO1-BD、NnCAT1-AD+JDPPO1-BD、CuZnSOD-AD+JDPPO1-BD、PER1-AD+JDPPO1-BD、POD3L-AD+JDPPO1-BD、POD43L-AD+JDPPO1-BD、PODP7L-AD+JDPPO1-BD、pGBKT7-53+pGADT7-T(阳性对照)和pGBKT7-Lam+pGADT7-T(阴性对照)分别转入酵母Y2HGold感受态细胞中,转化后的菌液涂于DDO(SD/-Trp/-Leu)二缺平板上,进行筛选含质粒的酵母,28℃培养3—5 d。从DDO平板上挑取直径为2—3 mm的阳性克隆于DDO液体培养基中,在30℃、180 r/min摇床振荡培养过夜(12—16 h)。吸取1.5 μL酵母菌液均匀点播于提前标记好的QDO(SD/-Trp/-Leu/-His/-Ade)/X-α-Gal/AbA和DDO(SD/ -His/-Ade)培养基上,28—30℃生长3—5 d后,分析菌落的生长状况。1.4 互作蛋白的毒性和自激活检测
前期已做过NnPPO1的毒性与自激活检测[22],利用基因特异性引物将NnCAT1的编码区克隆至pGBKT7(BD)载体上,将重组载体NnCAT1-BD和空载体pGBKT7分别转化酵母Y2HGold感受态中,转化后的菌液涂于SD/-Trp培养基上筛选,挑取单克隆,稀释100倍分别涂布在SD/-Trp培养基上。将质粒组合NnCAT1-BD+AD、pGBKT7-53+pGADT7-T(阳性对照)、pGBKT7-53+pGADT7-Lam(阴性对照)分别转入酵母Y2HGold菌株的感受态细胞中,转化后的菌液涂于DDO(SD/-Trp/-Leu)二缺平板上,挑菌后均匀点播于SD/-Trp和SD/-Trp/-Leu/-His培养基上,28—30℃生长3—5 d后,分析菌落的生长状况。1.5 双分子荧光互补技术验证互作蛋白
利用同源重组技术,选择BamH I和Xho I酶切位点,将NnPPO1和NnCAT1的编码区分别构建至35S-SPYNER173和35S-SPYCEM载体上,通过冻融法转入农杆菌GV3101感受态中,28℃生长2 d后挑取阳性菌落于YEB液体培养基(含20 μg?mL-1 Rif和50 μg?mL-1 Kana)中,28℃振荡培养12—16 h。离心收集菌体,用烟草注射渗透液(10 mmol?L-1 MES-KOH (pH 5.6)、10 mmol?L-1 MgCl2和150 μmol?L-1乙酰丁香酮)重悬菌体至OD600=0.8,将含有35S-NnPPO1- SPYNER173、35S-NnCAT1-SPYCEM、P19和35S- NnPPO1-SPYNER173、35S-SPYCEM、P19的菌液分别按照1﹕1﹕1混合后黑暗静止4 h,然后注射到生长约35 d的烟草叶片中。2—5 d内,使用生物激光共聚焦显微镜(LECIA TCSSP8)观察表皮细胞的黄色荧光信号。1.6 蛋白序列分析和互作结构域分析
通过两种方法证实NnPPO1和NnCAT1之间是否存在互作及两者间如何互作,对NnPPO1和NnCAT1序列进行分析,用NCBI的Conserved Domains服务器分析NnPPO1和NnCAT1蛋白氨基酸序列的保守结构域;使用DNAMAN软件对NnCAT1与其他物种CAT序列进行多序列比对;根据NnPPO1和NnCAT1的结构域分析结果,将NnCAT1的主要结构域Catalase Domain(16—398 aa)和Heme-binding Enzyme Domain(16—493 aa)分别构建到AD载体上,将NnPPO1的主要结构域Tyrosinase Domain(175—380 aa)、PPO1_KFDV Domain(381—468 aa)和PPO1_DWL Domain(469—597 aa)分别构建到BD载体上,利用酵母双杂的技术分析互作关键结构域。1.7 亚细胞定位分析
设计引物(表1),使用PrimeSTAR扩增出NnCAT1的编码区,选择BamH I和Kpn I酶切位点,然后构建到p35S-eGFP载体上,与GFP蛋白融合表达。通过农杆菌注射本氏烟草。2—5 d,在生物激光共聚焦显微镜下观察绿色荧光以及红色荧光信号。1.8 NnPPO1与NnCAT1的组织特异性表达
启动子不仅调控着基因的表达水平,也调控着基因表达的时空顺序。用PlantCARE对NnPPO1和NnCAT1的启动子区域进行分析;然后对它们的组织特异性表达进行分析,先将鄂莲5号的莲子在试验室发芽,待芽长至约10 cm时,选取大小基本一致的莲藕幼苗,种植于塑料桶中,桶内装土高度约10 cm,桶内装满水。待莲藕叶片完全展开,并长出藕带时,采收叶、叶柄、根、藕带、茎尖等部位,用液氮速冻,放于–80℃冰箱储存。使用RNA提取试剂盒提取样品总RNA并反转录为cDNA,设计引物(表1)进行PCR扩增,莲藕β-actin为内部参照基因[28],在QuantStudio(TM)6 Flex System实时PCR仪上进行实时荧光定量PCR。反应体系:2×ChamQ SYBR qPCR Master Mix 10 μL,正、反向引物各0.8 μL,50×ROX Reference Dye 2 0.4 μL,稀释5倍的cDNA 2 μL,以ddH2O补足至20 μL;扩增程序为:95℃,30 s;95℃,10 s,60℃,30 s,72℃,1 min,35个循环;72℃,4 min。并通过SPSS的t检验确定显著性(P≤0.05)[29]。各部位样品均设3个技术性和3个生物学重复1.9 NnCAT1的生物信息学分析
为进一步探讨NnCAT1的生物学功能及其在莲藕褐变中的作用机制,本研究使用Expasy(2 结果
2.1 酵母双杂交筛选NnPPO1的互作蛋白
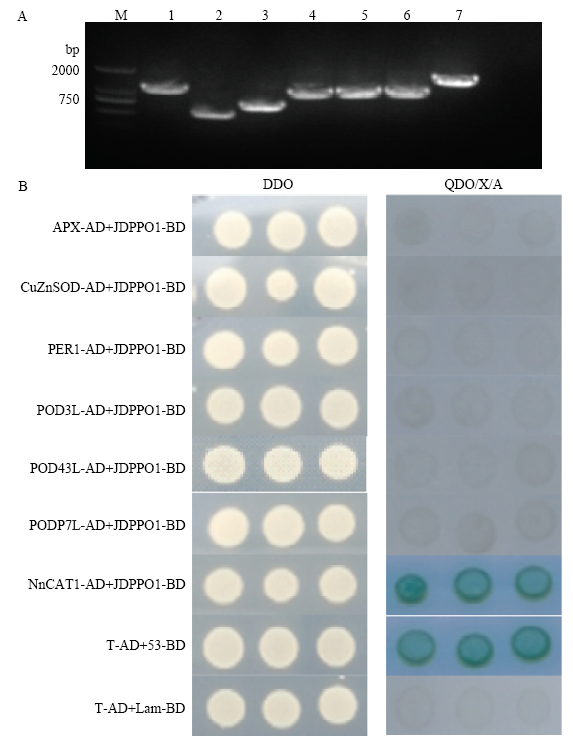
以莲藕cDNA为模板扩增7个基因(APX、CuZnSOD、PER1、POD3L、POD43L、PODP7L和NnCAT1)的编码区,分别得到1 044、459、660、981、963、957和1 479 bp的条带(图1-A)。将连接产物分别转化至EPI300大肠杆菌后,进行菌落PCR检测分析,得到的条带与预期的目的片段大小相同,将阳性菌液测序比对成功后,进行Y2H配对分析,结果显示,转化质粒的酵母菌在DDO平板上生长良好,只有含NnCAT1-AD+JDPPO1-BD的酵母菌和阳性对照能在QDO/X/A平板上生长(图1-B),表明JDPPO1与NnCAT1之间可能存在蛋白互作。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1基因的克隆及互作蛋白筛选
A:以莲藕cDNA为模板扩增7个抗氧化基因的电泳图。M:DL2000分子标记;1:APX;2:CuZnSOD;3:PER1;4:POD3L;5:POD43L;6:PODP7L;7:NnCAT1 B:酵母双杂交筛选与莲藕JDPPO1互作的蛋白
Fig. 1Gene cloning and screening of interacting proteins
A: Amplification of seven genes using Nelumbo nucifera cDNA as template. M:DL2000 marker; 1: APX; 2: CuZnSOD; 3: PER1; 4: POD3L; 5: POD43L; 6: PODP7L; 7: NnCAT1; B: Screening of JDPPO1 interacting proteins by yeast two-hybrid
2.2 互作蛋白的毒性和自激活检测
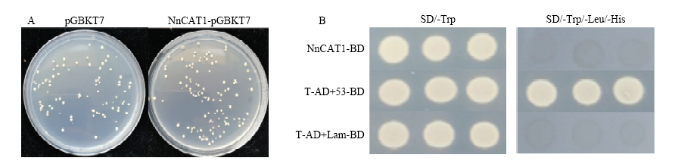
本研究对NnCAT1进行自激活和毒性检测。将含有NnCAT1-pGBKT7和对照组空载体pGBKT7的酵母,稀释相同倍数后分别涂布在SD/-Trp平板上,结果显示,两个平板上的菌落大小和数目基本相同(图2-A),表明重组载体表达的NnCAT1蛋白对酵母的生长没有影响,对酵母菌无毒性。将含有NnCAT1-pGBKT7、阳性对照和阴性对照的酵母菌,稀释相同倍数后分别涂布在一缺SD/-Trp和三缺SD/-Trp/-Ade/-His平板上,只有阳性对照在一缺和三缺平板上正常生长,含NnCAT1-pGBKT7载体的酵母菌和阴性对照在三缺平板上不能生长(图2-B),说明NnCAT1无自激活作用,无法将报告基因激活,JDPPO1与NnCAT1在酵母系统中确实存在互作。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2莲藕NnCAT1蛋白的毒性和自激活检测
A:毒性检测;B:自激活检测
Fig. 2Detection of Toxicity and self-activation of NnCAT1 Protein
A: Detection of toxicity; B: Detection of self-activation
2.3 双分子荧光互补验证蛋白互作的真实性
35S-NnPPO1-SPYNER173与35S-NnCAT1-SPYCEM的组合有很强的黄色荧光信号,并且黄色荧光主要集中在细胞膜和细胞核上,而35S-NnPPO1-SPYNER173与空载体35S-SPYCEM的组合中未观察到黄色荧光信号(图3),说明在BiFC系统中NnPPO1与NnCAT1存在蛋白互作,并且互作位置在细胞膜和细胞核上,也进一步证实了酵母双杂交结果。图3
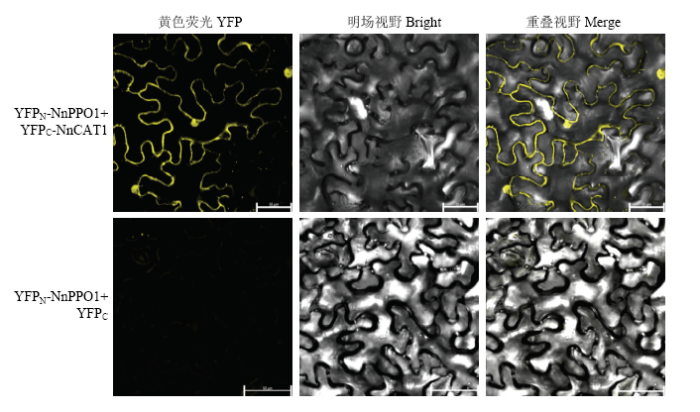 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3双分子荧光互补检测NnPPO1与NnCAT1互作
Fig. 3Interaction between NnPPO1 and NnCAT1 detected by bimolecular fluorescence complementarity
2.4 蛋白序列分析和互作结构域分析
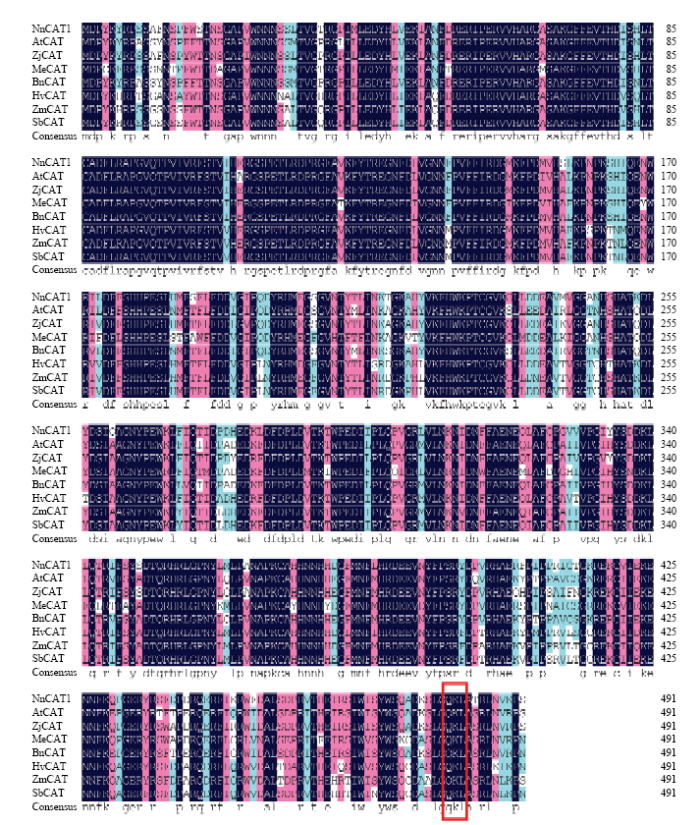
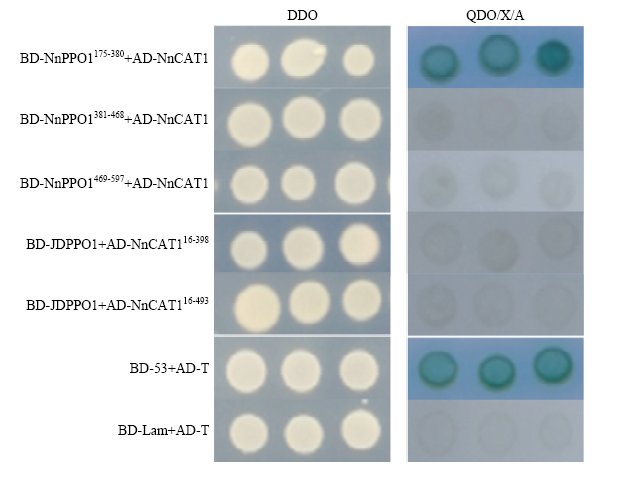
利用DNAMAN软件将NnCAT1与其他物种CAT氨基酸序列进行同源比对,发现该蛋白C末端包含保守的三肽模体(QKL)(图4),该模体可以与PTS1受体结合,说明莲藕NnCAT1可能是通过PTS1系统进入细胞内的过氧化物酶体。NnCAT1氨基酸序列与冬枣ZjCAT的相似度最高,达到91.67%,其次是与油菜、拟南芥、玉米、高粱的相似度分别为87.40%、86.79%、86.18%和86.18%,与大麦、木薯的相似度分别为83.94%和80.49%;证明该序列为莲藕的CAT,故命名为NnCAT1。保守结构域分析发现,NnPPO1主要有Tyrosinase Domain(175—380 aa)、PPO1_DWL Domain(381—468 aa)和PPO1_KFDV Domain(469—597 aa)等3个结构域;NnCAT1主要有Catalase Domain(16—398 aa)和Heme-binding Enzyme Domain(16—493 aa)结构域。将NnPPO1和NnCAT1的主要结构域分别构建到BD和AD载体上,BD-NnPPO1175-380、BD-NnPPO1381-468、BD-NnPPO1469-597分别与AD-NnCAT1互作的结果显示,只有BD- NnPPO1175-380与AD-NnCAT1发生蛋白互作(图5),说明NnPPO1与NnCAT1的互作关键部位在其保守的酪氨酸酶结构域(Tyrosinase domain);而AD-NnCAT116-398、AD-NnCAT116-493与BD-JDPPO1均不发生蛋白互作,在酵母系统中NnCAT1必须具有完整的氨基酸序列才可以与JDPPO1的互作。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4莲藕NnCAT1与其他物种CAT蛋白的氨基酸序列比对
AtCAT:拟南芥 Arabidopsis thaliana,X64271.1;ZjCAT:冬枣 Ziziphus jujuba,JN831452.1;MeCAT:木薯 Manihot esculenta,AF170272.1;BnCAT:油菜 Brassica napus,XM_013869534.2;HvCAT:大麦Hordeum vulgare,U20777.1;ZmCAT:玉米 Zea mays,NM_001254879.2;SbCAT:高粱 Sorghum bicolor,XM_002437586.1
Fig. 4Amino acid sequence alignment of NnCAT1 with other CAT proteins
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5酵母双杂交验证互作关系
Fig. 5Results of the yeast two-hybrid
2.5 NnCAT1的亚细胞定位
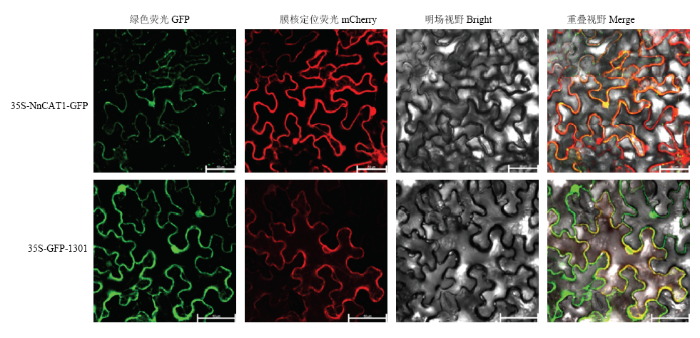
将NnCAT1编码区构建至p35S-eGFP载体上,与载体中的eGFP蛋白融合表达,将重组质粒与空载体转入农杆菌,通过在烟草叶片中瞬时表达NnCAT1与GFP的融合蛋白,发现绿色荧光信号存在于细胞核和细胞膜上,并且与膜核定位信号(mCherry)红色荧光重合,空载体GFP显示了相同的定位情况(图6)。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6NnCAT1蛋白的亚细胞定位分析
Fig. 6Subcellular localization analysis of NnCAT1 protein
2.6 NnPPO1与NnCAT1的组织特异性表达
利用PlantCARE对NnPPO1和NnCAT1起始密码子上游约2 000 bp的启动子序列进行分析,结果显示,NnPPO1序列中含有AAAC-motif、Box 4、GT1-motif、TCCC-motif、TCT-motif、chs-CMA1a等光响应元件,ARE逆境响应元件,以及CGTCA-motif、TGACG- motif、P-box等激素响应元件,推测NnPPO1的表达可能受到上述各因素的调控。NnCAT1序列中含有ATCT-motif、Box 4、G-box、GATA-motif、GT1-motif、I-box、TCT-motif等光响应性元件,ARE、MBS、TC-rich repeats等逆境响应元件,以及ABRE、CGTCA-motif、TCA-element、TGACG-motif等激素响应元件,推测NnCAT1的表达可能受到上述各因素的调控,部分响应元件见表2。Table 2
表2
表2NnPPO1与NnCAT1启动子响应元件
Table 2
| 元件名称Elements name | 功能Function | 所属序列Owned sequence |
|---|---|---|
| GT1-motif | 光响应元件Light responsive element | NnPPO1、NnCAT1 |
| ARE | 厌氧诱导必不可少的顺式作用调节元件 Cis-acting regulatory element essential for the anaerobic induction | NnPPO1、NnCAT1 |
| CGTCA-motif、TGACG-motif | MeJA反应性中涉及的顺式作用调节元件 Cis-acting regulatory element involved in the MeJA-responsiveness | NnPPO1、NnCAT1 |
| CAAT-box | 启动子和增强子区域常见的顺式作用元件 Common cis-acting element in promoter and enhancer regions | NnPPO1、NnCAT1 |
| Box 4 | 涉及光响应性的保守DNA模块的一部分 Part of a conserved DNA module involved in light responsiveness | NnPPO1、NnCAT1 |
| TCT-motif | 光响应元件的一部分Part of a light responsive element | NnPPO1、NnCAT1 |
| TATA-box | 转录启动子周围-30个核心启动子 Core promoter element around -30 of transcription start | NnPPO1、NnCAT1 |
| P-box | 赤霉素反应元件Gibberellin-responsive element | NnPPO1 |
| A-box | 顺式作用调节元件Cis-acting regulatory element | NnCAT1 |
| ABRE | 脱落酸反应性涉及的顺式作用元件 Cis-acting element involved in the abscisic acid responsiveness | NnCAT1 |
| MBS | MYB结合位点参与干旱诱导MYB binding site involved in drought-inducibility | NnCAT1 |
| TC-rich repeats | 参与防御和应激反应的顺式作用元件 Cis-acting element involved in defense and stress responsiveness | NnCAT1 |
| TCA-element | 水杨酸反应性涉及的顺式作用元件 Cis-acting element involved in salicylic acid responsiveness | NnCAT1 |
| G-box | 参与光响应的顺式作用调节元件 Cis-acting regulatory element involved in light responsiveness | NnCAT1 |
新窗口打开|下载CSV
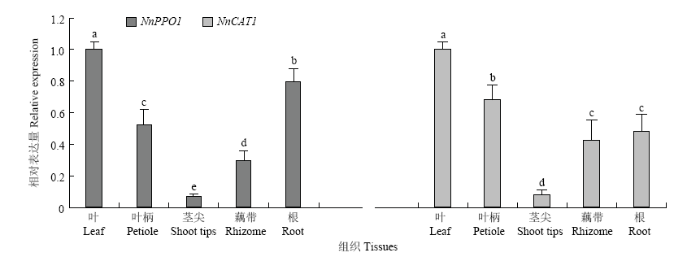
在莲藕幼苗的不同组织中两个基因均有表达。以叶中的表达量为标准进行比较,NnPPO1在叶中表达水平最高,根中其次,在茎尖中最低,并且NnPPO1在叶中的表达量是茎尖中的15.19倍;NnCAT1在叶中表达水平最高,然后依次是叶柄、根、藕带,在茎尖中的表达量最低。另外,NnPPO1与NnCAT1的表达模式基本相同,都在叶中有最高的表达量,在茎尖中表达量最低。方差分析结果表明,5个部位中的NnPPO1表达量均有显著差异;根中NnCAT1的表达量与藕带中的差异不显著,其余部位之间均差异显著(图7)。由此推测NnPPO1与NnCAT1可能在叶、叶柄和根的颜色与生长发育中发挥重要作用。
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7NnPPO1与NnCAT1的组织特异性表达
不同小写字母表示差异显著(P<0.05)
Fig. 7Tissue-specific expression of NnPPO1 and NnCAT1
Different lowercases indicate significant difference (P<0.05)
2.7 NnCAT1的生物信息学分析
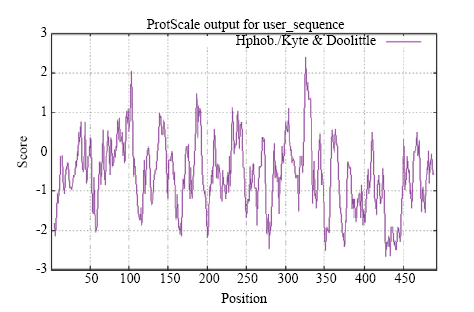
莲藕NnCAT1的相对分子质量约为57.0 kD,理论等电点(PI)为6.93。正电荷残基(Arg+Lys)总数为60个,负电荷残基(Asp+Glu)总数为62个,其分子式可写为C2573H3874N716O727S17,不稳定系数为33.03,属于稳定蛋白;跨膜结构和信号肽预测结果显示NnCAT1无跨膜区、无信号肽;叶绿体转运肽预测结果表明,NnCAT1存在叶绿体转运肽,前21 aa为转运肽区域。NnCAT1的亲疏水性分析,结果如图8所示,分值在0以上为疏水性氨基酸,在0以下为亲水性氨基酸,得分最大值为2.389,最小值为-2.678,平均值为-0.587,证明NnCAT1是亲水性蛋白质。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8NnCAT1蛋白亲疏水性分析
Fig. 8Hydrophilicity of NnCAT1 Protein
蛋白质二级结构预测结果显示,NnCAT1蛋白由27.64% α-螺旋、15.65%延长链、6.30% β-转角和50.41%无规则卷曲构成(图9-A)。NnCAT1的三维空间结构预测显示,其序列的一致性为47.64%,为同源四聚体蛋白,序列与模板序列的相似度为0.43,覆盖为0.99,为Catalase-like Superfamily家族CATALASE蛋白,与目标基因相吻合(图9-B)。
图9
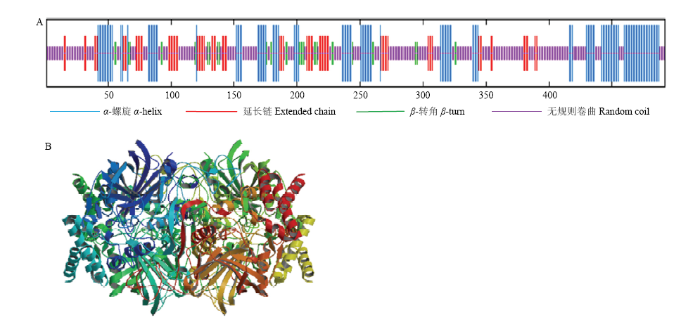 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9NnCAT1的二级结构和三级结构预测
Fig. 9Secondary structure and tertiary structure prediction of NnCAT1
3 讨论
3.1 NnPPO1与NnCAT1存在蛋白互作
蛋白互作是当前的研究热点之一,但关于PPO的蛋白互作,除本课题组研究结果外,未见其他文献报道。张海星[31]通过酵母双杂和BIFC证实了丹参PPO和酪氨酸氨基转移酶存在互作关系,证明丹参PPO对丹酚酸类的代谢有一定的调控作用。拟南芥AtNCA1与AtCAT2相互作用,可以将过氧化氢酶的折叠保持在功能状态,AtNCA1对于过氧化氢酶活性至关重要[32]。水稻叶片中GLO和OsCAT的互作-解离是调节水稻中H2O2水平的一种特定机制[33]。SOD和CAT酶活性是莲藕褐变的第二主因子,所以在研究莲藕褐变机理时,可以从SOD和CAT方面着手[21]。为了深入探索PPO导致莲藕等果蔬褐变的分子机制,本研究利用莲藕NnPPO1成员,通过酵母双杂方法,从抗氧化酶保护系统中筛选出NnCAT1蛋白与NnPPO1互作,NnCAT1具有CAT蛋白家族的所有主要特征氨基酸残基、基序和元件[34],主要功能是催化过氧化氢分解为H2O和O2,以防止活性氧自由基对植物造成的伤害[35]。同时过氧化氢酶在植物中有很多作用,如抗高(低)温胁迫、盐胁迫、干旱胁迫、抗病性、延缓植物衰老等[36,37,38,39,40,41]。本研究在烟草叶片细胞膜和细胞核上检测到强烈的黄色荧光信号,更加确定了NnPPO1与NnCAT1之间存在相互作用关系。推测NnPPO1与NnCAT1协同互作导致果蔬褐变,NnCAT1将H2O2分解产生的O2进入质体中,此时在NnPPO1的强烈催化下,产生的O2直接与多酚类物质反应形成醌类,这时果肉逐渐变色,具体还需要蛋白水平和遗传水平的进一步验证。
多序列比对发现NnCAT1与其他物种的CAT具有很高的相似度,表明不同物种间CAT序列具有很强的保守性;NnPPO1与NnCAT1的互作关键部位在其保守的酪氨酸酶结构域,而此结构域包含CuA和CuB的活性中心,互作结果与前人所述的Tyrosinase Domain是NnPPO1主要功能区一致[8];NnCAT1的完整氨基酸序列才能与JDPPO1发生互作,说明除结构域以外的部分对NnCAT1的互作过程中有不可或缺的作用,为进一步研究互作机理及构建cat1、ppo1突变体植株提供了参考。
3.2 亚细胞定位与组织表达特异性分析
本研究结果表明,NnCAT1定位在细胞膜和细胞核,而前期研究表明NnPPO1存在于叶绿体上[22],结合它们的互作位置在细胞膜和细胞核上,说明正常状态时NnCAT1与NnPPO1并不接触,推测在莲藕组织或细胞受到损伤,这种空间隔离打破后,它们会到共同的位置上协同作用导致莲藕褐变。研究表明CAT定位于过氧化物酶体中,但也有分布于其他部位,玉米的CAT3定位于线粒体中,它可能在替代氧化酶途径中起作用[42]。拟南芥AtCAT3亚细胞定位研究发现,AtCAT3定位在细胞膜、细胞质、过氧化物酶体中,说明AtCAT3也参与了细胞膜、细胞质中H2O2的代谢[43]。槟榔过氧化氢酶(ArCAT)亚细胞定位结果表明,ArCAT1蛋白定位于过氧化物酶体中,而ArCAT2和ArCAT3定位于过氧化物酶体和细胞核中[44]。红苋菜过氧化氢酶-酚氧化酶(AcCATPO)位于过氧化物酶体和细胞核中[45]。CAT的定位与其在细胞中的功能有着密切的关系。NnPPO1启动子序列中发现了很多光响应、厌氧诱导响应和激素响应元件,NnCAT1启动子序列中发现了很多光响应、厌氧诱导响应、激素响应、干旱响应和参与防御和应激反应元件,推测其可能参与了植物体内各种逆境胁迫应答,为深入揭示莲藕NnPPO1与NnCAT1的表达调控机制提供了理论依据[46]。组织特异性结果表明NnPPO1与NnCAT1的表达模式基本相同,暗示NnCAT1与NnPPO1协同互作使莲藕等果蔬褐变,推测NnPPO1与NnCAT1在植物中可能具有广谱的互作关系,以发挥更多的作用。莲藕中含有大量的多酚氧化酶,因此,莲藕是研究多酚氧化酶比较理想的植物材料。本研究结果为深入探讨莲藕NnPPO1与NnCAT1的生物学功能,研究莲藕PPO的分子作用机制,精准抑制其活性奠定了基础。
4 结论
运用酵母双杂交技术,从抗氧化酶保护系统中筛选出NnCAT1与NnPPO1存在蛋白互作;利用BiFC进一步证实NnPPO1与NnCAT1之间的互作关系,且NnPPO1保守的酪氨酸酶结构域在互作中发挥主要作用;亚细胞定位发现,NnCAT1蛋白定位于细胞核和细胞膜上;组织特异性表达结果显示NnPPO1与NnCAT1的表达模式基本相同,都在叶中有最高的表达量,在茎尖中表达量最低。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1038/srep44622URLPMID:28304398 [本文引用: 1]

Salvia miltiorrhiza is a well-known material of traditional Chinese medicine. Understanding the regulatory mechanisms of phenolic acid biosynthesis and metabolism are important for S. miltiorrhiza quality improvement. We report here that S. miltiorrhiza contains 19 polyphenol oxidases (PPOs), forming the largest PPO gene family in plant species to our knowledge. Analysis of gene structures and sequence features revealed the conservation and divergence of SmPPOs. SmPPOs were differentially expressed in plant tissues and eight of them were predominantly expressed in phloem and xylem, indicating that some SmPPOs are functionally redundant, whereas the others are associated with different physiological processes. Expression patterns of eighteen SmPPOs were significantly altered under MeJA treatment, and twelve were yeast extract and Ag(+)-responsive, suggesting the majority of SmPPOs are stress-responsive. Analysis of high-throughput small RNA sequences and degradome data showed that miR1444-mediated regulation of PPOs existing in P. trichocarpa is absent from S. miltiorrhiza. Instead, a subset of SmPPOs was posttranscriptionally regulated by a novel miRNA, termed Smi-miR12112. It indicates the specificity and significance of miRNA-mediated regulation of PPOs. The results shed light on the regulation of SmPPO expression and suggest the complexity of SmPPO-associated phenolic acid biosynthesis and metabolism.
[本文引用: 1]
[本文引用: 1]
DOI:10.1080/09168451.2014.940828URLPMID:25051980 [本文引用: 1]

The polyphenol oxidase (PPO) is involved in undesirable browning in many plant foods. Although the PPOs have been studied by several researchers, the isolation and expression profiles of PPO gene were not reported in rubber tree. In this study, a new PPO gene, HbPPO, was isolated from Hevea brasiliensis. The sequence alignment showed that HbPPO indicated high identities to plant PPOs and belonged to dicot branch. The cis-acting regulatory elements related to stress/hormone responses were predicted in the promoter region of HbPPO. Real-time RT-PCR analyses showed that HbPPO expression varied widely depending on different tissues and developmental stages of leaves. Besides being associated with tapping panel dryness, the HbPPO transcripts were regulated by ethrel, wounding, H2O2, and methyl jasmonate treatments. Moreover, the correlation between latex coagulation rate and PPO activity was further confirmed in this study. Our results lay the foundation for further analyzing the function of HbPPO in rubber tree.
DOI:10.1186/1471-2164-13-395URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.phytochem.2011.08.028URL [本文引用: 1]

Though polyphenol oxidase (PPO) genes from tomato and potato have been extensively studied, information about PPO genes in eggplant (Solanum melongena) is lacking. The main objective of this study is to understand the structural and functional aspects of eggplant PPO genes. Six eggplant PPO genes (SmePPO1-6) cloned by RACE and genome walking were found to be intronless and correspond to eight eggplant unigenes. Comprehensive sequence analyses indicated that the eggplant PPO genes exhibit considerable variation in the transit peptide regions, copper-binding domains and UTRs, and fall into two distinct structural classes. Further, PPO gene members appear to exist in clusters on eggplant chromosome 8 as seen in the case of tomato and potato PPOs. During normal growth and development, SmePPO1 and 2 are expressed in roots, whereas the transcript levels of all the eggplant PPO genes vary considerably in leaves, flowers and fruits. SmePPO1 was expressed in Escherichia coli as a GST fusion protein, and immunoblot using rabbit polyclonal antiserum to GST-SmePPO1 detected a major protein band (similar to 70 kDa) and a minor band (similar to 67 kDa) in eggplant fruit extract. Tissue printing indicated the predominant presence of PPO in the exocarp and the areas surrounding the seeds in the mesocarp of eggplant fruits. Immunolocalization of PPOs in eggplant infested with shoot-and-fruit borer revealed localization of the PPO at the site of infection in tender shoots and fruits, and further inside the mature tissues. The upregulation of eggplant PPO gene transcripts following mechanical injury shows that all the genes except SmePPO2 are induced in the fruit over 6 h. On the contrary, the transcripts of SmePPO2 and PPO3 are not detectable in the stem, and expression seems to be prominent over a 2 h period for SmePPO1 and SmePPO4-6. Our results show that eggplant PPO genes are structurally different, and are differentially expressed in various tissues of eggplant indicating their functional diversity. (C) 2011 Elsevier Ltd.
DOI:10.1094/MPMI-04-11-0082URL [本文引用: 1]

Dandelion (Taraxacum officinale) possesses an unusually high degree of disease resistance. As this plant exhibits high polyphenol oxidase (PPO) activity and PPO have been implicated in resistance against pests and pathogens, we analyzed the potential involvement of five PPO isoenzymes in the resistance of dandelion against Botrytis cinerea and Pseudomonas syringae pv. tomato. Only one PPO (ppo-2) was induced during infection, and ppo-2 promoter and beta-gincuronidase marker gene fusions revealed strong induction of the gene surrounding lesions induced by B. cinerea. Specific RNAi silencing reduced ppo-2 expression only, and concomitantly increased plant susceptibility to P. syringae pv. tomato. At 4 days postinoculation, P syringae pv. tomato populations were strongly increased in the ppo-2 RNAi lines compared with wild-type plants. When the dandelion ppo-2 gene was expressed in Arabidopsis thaliana, a plant having no PPO gene, active protein was formed and protein extracts of the transgenic plants exhibited substrate-dependent antimicrobial activity against P. syringae pv. tomato. These results clearly indicate a strong contribution of a specific, single PPO isoform to disease resistance. Therefore, we propose that specific PPO isoenzymes be included in a new family of pathogenesis-related (PR) proteins.
DOI:10.1126/science.290.5494.1163URLPMID:11073455 [本文引用: 1]

Aurones are plant flavonoids that provide yellow color to the flowers of some popular ornamental plants, such as snapdragon and cosmos. In this study, we have identified an enzyme responsible for the synthesis of aurone from chalcones in the yellow snapdragon flower. The enzyme (aureusidin synthase) is a 39-kilodalton, copper-containing glycoprotein catalyzing the hydroxylation and/or oxidative cyclization of the precursor chalcones, 2',4',6',4-tetrahydroxychalcone and 2',4',6',3,4-pentahydroxychalcone. The complementary DNA encoding aureusidin synthase is expressed in the petals of aurone-containing varieties. DNA sequence analysis revealed that aureusidin synthase belongs to the plant polyphenol oxidase family, providing an unequivocal example of the function of the polyphenol oxidase homolog in plants, i.e., flower coloration.
DOI:10.3390/ijms19123897URL [本文引用: 1]
DOI:10.1111/j.1365-313X.2005.02625.xURLPMID:16367960 [本文引用: 1]

Aureusidin synthase, a polyphenol oxidase (PPO), specifically catalyzes the oxidative formation of aurones from chalcones, which are plant flavonoids, and is responsible for the yellow coloration of snapdragon (Antirrhinum majus) flowers. All known PPOs have been found to be localized in plastids, whereas flavonoid biosynthesis is thought to take place in the cytoplasm [or on the cytoplasmic surface of the endoplasmic reticulum (ER)]. However, the primary structural characteristics of aureusidin synthase and some of its molecular properties argue against localization of the enzyme in plastids and the cytoplasm. In this study, the subcellular localization of the enzyme in petal cells of the yellow snapdragon was investigated. Sucrose-density gradient and differential centrifugation analyses suggested that the enzyme (the 39-kDa mature form) is not located in plastids or on the ER. Transient assays using a green fluorescent protein (GFP) chimera fused with the putative propeptide of the PPO precursor suggested that the enzyme was localized within the vacuole lumen. We also found that the necessary information for vacuolar targeting of the PPO was encoded within the 53-residue N-terminal sequence (NTPP), but not in the C-terminal sequence of the precursor. NTPP-mediated ER-to-Golgi trafficking to vacuoles was confirmed by means of the co-expression of an NTPP-GFP chimera with a dominant negative mutant of the Arabidopsis GTPase Sar1 or with a monomeric red fluorescent protein (mRFP)-fused Golgi marker (an H+-translocating inorganic pyrophosphatase of Arabidopsis). We identified a sequence-specific vacuolar sorting determinant in the NTPP of the precursor. We have demonstrated the biosynthesis of a flavonoid skeleton in vacuoles. The findings of this metabolic compartmentation may provide a strategy for overcoming the biochemical instability of the precursor chalcones in the cytoplasm, thus leading to the efficient accumulation of aurones in the flower.
URL [本文引用: 1]

为获得莲藕中多酚氧化酶(PPO)基因序列信息及其表达情况,运用RACE技术从莲藕(Nelumbo nucifera Gaertn. ssp. nucifera)茎尖中克隆到多酚氧化酶基因全长序列,其cDNA全长2 074 bp,完整的编码框为1 503 bp,编码501个氨基酸,5'端有160 bp非编码区,3'端有411 bp非编码区(GenBank 登陆号为HQ380894)。采用生物信息学方法发现该蛋白分子量约为56.8 kD,pI为8.60,具典型的酪氨酸家族结构域,无信号肽结构,但有跨膜螺旋的亲水性蛋白,α-螺旋和β-折叠分散于整个多肽链中。半定量RT-PCR结果显示,该基因在莲藕茎尖、幼叶、莲藕鲜切片、花瓣、茎杆5种组织中均有表达。方差分析表明,茎尖中PPO表达量与幼叶差异不显著,但显著高于其余三个部位;幼叶中表达量与莲藕鲜切片之间差异不显著,但显著高于花瓣和茎杆;花瓣和茎杆中PPO表达量最低,两者无显著差异。上述结果说明作为重要分生组织的莲藕茎尖产生的PPO可能不具活性或极不稳定,随着植株的生长发育被分配到各组织中,在特定时空下被激活并担负相应的职责功能。
URL [本文引用: 1]

为获得莲藕中多酚氧化酶(PPO)基因序列信息及其表达情况,运用RACE技术从莲藕(Nelumbo nucifera Gaertn. ssp. nucifera)茎尖中克隆到多酚氧化酶基因全长序列,其cDNA全长2 074 bp,完整的编码框为1 503 bp,编码501个氨基酸,5'端有160 bp非编码区,3'端有411 bp非编码区(GenBank 登陆号为HQ380894)。采用生物信息学方法发现该蛋白分子量约为56.8 kD,pI为8.60,具典型的酪氨酸家族结构域,无信号肽结构,但有跨膜螺旋的亲水性蛋白,α-螺旋和β-折叠分散于整个多肽链中。半定量RT-PCR结果显示,该基因在莲藕茎尖、幼叶、莲藕鲜切片、花瓣、茎杆5种组织中均有表达。方差分析表明,茎尖中PPO表达量与幼叶差异不显著,但显著高于其余三个部位;幼叶中表达量与莲藕鲜切片之间差异不显著,但显著高于花瓣和茎杆;花瓣和茎杆中PPO表达量最低,两者无显著差异。上述结果说明作为重要分生组织的莲藕茎尖产生的PPO可能不具活性或极不稳定,随着植株的生长发育被分配到各组织中,在特定时空下被激活并担负相应的职责功能。
DOI:10.1111/jfbc.13136URLPMID:31907949 [本文引用: 1]

The effect of ascorbic acid [AA (1%)] and Aloe vera gel [AVG (50%)] coating alone and in combination was investigated on enzymatic browning and quality of lotus root slices during storage at 20 +/- 1 degrees C. The combined application of AA and AVG coating delayed surface browning, reduced increase in relative electrolyte leakage (REL) and showed higher overall visual quality (OVQ). Similarly, AA and AVG combined treatment reduced superoxide anion ( O 2 - . ) and hydrogen peroxide (H2 O2 ) production and malondialdehyde (MDA) content, and suppressed peroxidase (POD) and polyphenol oxidase (PPO) activities. In addition, AA and AVG treatment conserved higher AA content, ascorbate peroxidase (APX), superoxide dismutase (SOD) and catalase (CAT) enzymes activities along with higher total phenolics and radical scavenging activity. In conclusion, the combined application of AA and AVG coating could be an appropriate treatment to delay surface browning and quality loss of lotus root slices. PRACTICAL APPLICATIONS: Lotus root is an aquatic rhizome vegetable. The fresh-cut slices of lotus roots are prone to post-cut enzymatic browning and quality deterioration during postharvest storage. Browning induced loss of visual quality and microbial infestations are the leading constraints in extending storage and/or shelf life of lotus root slices. Surface browning results in loss of characteristic color eventually leading to significant reduction in market potential and visual quality. However, quality deterioration and development of browning could be delayed with some suitable postharvest treatments. So, the effect of AA and Aloe vera gel based coating was investigated for quality conservation of lotus root slices. The findings of the current work are of global importance in reducing browning and conserving visual quality of lotus root slices in particular and fresh-cut produce in general.
DOI:10.1016/j.foodchem.2019.126051URLPMID:31891888 [本文引用: 1]

Post-cut surface browning is one of the major constraints for shelf-life extension of lotus root slices. In the present study, lotus roots slices were treated with 0, 5 and 10 mmol L(-1) oxalic acid and stored at 20 +/- 1 degrees C for 5 days. Results showed that 10 mmol L(-1) oxalic acid treated lotus slices exhibited reduced browning, superoxide anion, hydrogen peroxide, electrolyte leakage and malondialdehyde content than control. The 10 mmol L(-1) treated slices had better visual quality and higher ascorbic acid and total phenolic contents. In addition, 10 mmol L(-1) treated slices showed reduced total bacterial count along with lower soluble quinones, peroxidase and polyphenol oxidase activities in contrast to control. Similarly, 10 mmol L(-1) treatment showed higher superoxide dismutase, catalase and ascorbate peroxidase activities as compared to control. In conclusion, 10 mmol L(-1) oxalic acid application could be considered suitable to delay post-cut browning of lotus root slices.
DOI:10.1186/1471-2229-14-62URL [本文引用: 1]
DOI:10.1016/j.foodchem.2007.04.010URL [本文引用: 1]
[本文引用: 1]
DOI:10.1271/bbb.65.383URLPMID:11302173 [本文引用: 1]

Polyphenol oxidase (PPO) is responsible for enzymatic browning of apples. Apples lacking PPO activity might be useful not only for the food industry but also for studies of the metabolism of polyphenols and the function of PPO. Transgenic apple calli were prepared by using Agrobacterium tumefaciens carrying the kanamycin (KM) resistant gene and antisense PPO gene. Four KM-resistant callus lines were obtained from 356 leaf explants. Among these transgenic calli, three calli grew on the medium containing KM at the same rate as non-transgenic callus on the medium without KM. One callus line had an antisense PPO gene, in which the amount and activity of PPO were reduced to half the amount and activity in non-transgenic callus. The browning potential of this line, which was estimated by adding chlorogenic acid, was also half the browning potential of non-transgenic callus.
[D].
[本文引用: 2]
[D].
[本文引用: 2]
[D].
[本文引用: 4]
[D].
[本文引用: 4]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s12010-010-9074-1URL [本文引用: 1]

Two cytosolic copper-zinc superoxide dismutase (cytCuZnSOD) complementary deoxyribonucleic acid were achieved in Nelumbo nucifera (Elian). The active sites and common characteristics of cytCuZnSOD family were showed by homology modeling. The two recombinant proteins expressed by PET-32a vector showed the similar SOD activity (89.94+/-0.54 U/mg) and could maintain more than 90% activity after incubation at 65 degrees C. The subcellular location by green fluorescent protein revealed that these two isoforms were all located in cytosol and nucleus. The cytCuZnSODs were expressed in various parts of N. nucifera, which were expressed highest in the leafstalks and young leaves and lowest in the roots. The cytCuZnSOD messenger ribonucleic acids isolated from wounded leaves significantly increased at 1.5 h after treatment (HAT) with the highest expression at 3 HAT, after which the level decreased.
DOI:10.1111/tpj.13286URLPMID:27464651 [本文引用: 1]

Seed longevity, the maintenance of viability during storage, is a major factor for conservation of genetic resources and biodiversity. Seed longevity is an important trait of agriculture crop and is impaired by reactive oxygen species (ROS) during seed desiccation, storage and germination (C. R. Biol., 331, 2008 and 796). Seeds possess a wide range of systems (protection, detoxification, repair) allowing them to survive during storage and to preserve a high germination ability. In many plants, 1-cys peroxiredoxin (1-Cys Prx, also named PER1) is a seed-specific antioxidant which eliminates ROS with cysteine residues. Here we identified and characterized a seed-specific PER1 protein from seeds of sacred lotus (Nelumbo nucifera Gaertn.). Purified NnPER1 protein protects DNA against the cleavage by ROS in the mixed-function oxidation system. The transcription and protein accumulation of NnPER1 increased during seed desiccation and imbibition and under abiotic stress treatment. Ectopic expression of NnPER1 in Arabidopsis enhanced the seed germination ability after controlled deterioration treatment (CDT), indicating that NnPER1 improves seed longevity of transgenic plants. Consistent with the function of NnPER1 on detoxifying ROS, we found that the level of ROS release and lipid peroxidation was strikingly lower in transgenic seeds compared to wild-type with or without CDT. Furthermore, transgenic Arabidopsis seeds ectopic-expressing NnPER1 displayed enhanced tolerance to high temperature and abscisic acid (ABA), indicating that NnPER1 may participate in the thermotolerance and ABA signaling pathway.
DOI:10.1186/1471-2148-14-100URL
DOI:10.1016/j.bbrc.2014.07.064URL [本文引用: 1]

Calcium is a ubiquitous intracellular secondary messenger in plants. Calcineurin B-like proteins (CBLs), which contain four Ca2+-binding EF hand motifs, are Ca2+ sensors and regulate a group of Ser/Thr protein kinases called CBL-interacting protein kinases (CIPKs). Although the CBL-CIPK network has been demonstrated to play crucial roles in plant development and responses to various environmental stresses in Arabidopsis, little is known about their function in glucose signaling.
In the present study, we identified CIPK14 gene from Arabidopsis that play a role in glucose signaling. The subcellular localization of CIPK14 was determined using green fluorescence protein (GFP) as the reporter. Furthermore, the expression levels of CIPK14 in response to salt, drought, cold, heat, ABA, methyl viologen (MV) and glucose treatments were examined by quantitative RT-PCR and it was found to respond to multiple stimuli, suggesting that CIPK14 may be a point of convergence for several different signaling pathways. Moreover, knock-out mutation of CIPK14 rendered it more sensitive to glucose treatment. Yeast two-hybrid assay demonstrated that CIPK14 interacted with three CBLs and also with two key kinases, sucrose non-fermenting 1-related kinase (SnRK) 1.1 and SnRK1.2 implicated in glucose signaling. This is the first report to demonstrate that CIPK also plays a role in glucose signaling. (C) 2014 Elsevier Inc.
DOI:10.1093/nar/gku340URL [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1105/tpc.114.135095URLPMID:25700484 [本文引用: 1]

Catalases are key regulators of reactive oxygen species homeostasis in plant cells. However, the regulation of catalase activity is not well understood. In this study, we isolated an Arabidopsis thaliana mutant, no catalase activity1-3 (nca1-3) that is hypersensitive to many abiotic stress treatments. The mutated gene was identified by map-based cloning as NCA1, which encodes a protein containing an N-terminal RING-finger domain and a C-terminal tetratricopeptide repeat-like helical domain. NCA1 interacts with and increases catalase activity maximally in a 240-kD complex in planta. In vitro, NCA1 interacts with CATALASE2 (CAT2) in a 1:1 molar ratio, and the NCA1 C terminus is essential for this interaction. CAT2 activity increased 10-fold in the presence of NCA1, and zinc ion binding of the NCA1 N terminus is required for this increase. NCA1 has chaperone protein activity that may maintain the folding of catalase in a functional state. NCA1 is a cytosol-located protein. Expression of NCA1 in the mitochondrion of the nca1-3 mutant does not rescue the abiotic stress phenotypes of the mutant, while expression in the cytosol or peroxisome does. Our results suggest that NCA1 is essential for catalase activity.
DOI:10.1016/j.molp.2016.02.002URLPMID:26900141 [本文引用: 1]

Rapid and dynamic change in hydrogen peroxide (H2O2) levels can serve as an important signal to regulate various biological processes in plants. The change is realized by tilting the balance between its production and scavenging rates, in which membrane-associated NADPH oxidases are known to play a crucial role. Functioning independently from NADPH oxidases, glycolate oxidase (GLO) was recently demonstrated as an alternative source for H2O2 production during both gene-for-gene and non-host resistance in plants. In this study, we show that GLO physically interacts with catalase (CAT) in rice leaves, and that the interaction can be deregulated by salicylic acid (SA). Furthermore, the GLO-mediated H2O2 accumulation is synergistically enhanced by SA. Based on the well-known mechanism of substrate channeling in enzyme complexes, SA-induced H2O2 accumulation likely results from SA-induced GLO-CAT dissociation. In the GLO-CAT complex, GLO-mediated H2O2 production during photorespiration is very high, whereas the affinity of CAT for H2O2 (measured Km approximately 43 mM) is extraordinarily low. This unique combination can further potentiate the increase in H2O2 when GLO is dissociated from CAT. Taken together, we propose that the physical association-dissociation of GLO and CAT, in response to environmental stress or stimuli, seems to serve as a specific mechanism to modulate H2O2 levels in rice.
DOI:10.1002/yea.1077URLPMID:14755642 [本文引用: 1]

Within the context of studies on genes from Paracoccidioides brasiliensis (Pb) potentially associated with fungus-host interaction, we isolated a 61 kDa protein, pI 6.2, that was reactive with sera of patients with paracoccidioidomycosis. This protein was identified as a peroxisomal catalase. A complete cDNA encoding this catalase was isolated from a Pb cDNA library and was designated PbcatP. The cDNA contained a 1509 bp ORF containing 502 amino acids, whose molecular mass was 57 kDa, with a pI of 6.5. The translated protein PbCATP revealed canonical motifs of monofunctional typical small subunit catalases and the peroxisome-PTS-1-targeting signal. The deduced and the native PbCATP demonstrated amino acid sequence homology to known monofunctional catalases and was most closely related to catalases from other fungi. The protein and mRNA were diminished in the mycelial saprobic phase compared to the yeast phase of infection. Protein synthesis and mRNA levels increased during the transition from mycelium to yeast. In addition, the catalase protein was induced when cells were exposed to hydrogen peroxide. The identification and characterization of the PbCATP and cloning and characterization of the cDNA are essential steps for investigating the role of catalase as a defence of P. brasiliensis against oxygen-dependent killing mechanisms. These results suggest that this protein exerts an influence in the virulence of P. brasiliensis.
[本文引用: 1]
DOI:10.1016/j.postharvbio.2016.04.003URL [本文引用: 1]
DOI:10.1016/j.plaphy.2012.04.012URLPMID:22609456 [本文引用: 1]

Pretreatment in plants is recognized as a valuable strategy to stimulate plant defenses, leading to better plant development. This study evaluated the effects of H(2)O(2) leaf spraying pretreatment on plant growth and investigated the antioxidative mechanisms involved in the response of maize plants to salt stress. It was found that salinity reduced maize seedling growth when compared to control conditions, and H(2)O(2) foliar spraying was effective in minimizing this effect. Analysis of the antioxidative enzymes catalase (EC 1.11.1.6), guaiacol peroxidase (EC 1.11.1.7), ascorbate peroxidase (EC 1.11.1.1) and superoxide dismutase (EC 1.15.1.1) revealed that H(2)O(2) spraying increased antioxidant enzyme activities. Catalase (CAT) was the most responsive of these enzymes to H(2)O(2), with higher activity early (48 h) in the treatment, while guaiacol peroxidase (GPX) and ascorbate peroxidase (APX) were responsive only at later stages (240 h) of treatment. Increased CAT activity appears linked to gene expression regulation. Lower malondialdehyde levels were detected in plants with higher CAT activity, which may result from the protective function of this enzyme. Overall, we can conclude that pretreatment with H(2)O(2) leaf spraying was able to reduce the deleterious effects of salinity on seedling growth and lipid peroxidation. These responses could be attributed to the ability of H(2)O(2) to induce antioxidant defenses, especially CAT activity.
DOI:10.1038/s41598-017-08744-xURLPMID:28819160 [本文引用: 1]

In arid and semiarid regions, low precipitation rates lead to soil salinity problems, which may limit plant establishment, growth, and survival. Herein, we investigated the NaCl stress effect on chlorophyll fluorescence, photosynthetic-pigments, movement and chloroplasts ultrastructure in chlorenchyma cells of Opuntia streptacantha cladodes. Cladodes segments were exposed to salt stress at 0, 100, 200, and 300 mM NaCl for 8, 16, and 24 h. The results showed that salt stress reduced chlorophyll content, F v /F m , PhiPSII, and qP values. Under the highest salt stress treatments, the chloroplasts were densely clumped toward the cell center and thylakoid membranes were notably affected. We analyzed the effect of exogenous catalase in salt-stressed cladode segments during 8, 16, and 24 h. The catalase application to salt-stressed cladodes counteracted the NaCl adverse effects, increasing the chlorophyll fluorescence parameters, photosynthetic-pigments, and avoided chloroplast clustering. Our results indicate that salt stress triggered the chloroplast clumping and affected the photosynthesis in O. streptacantha chlorenchyma cells. The exogenous catalase reverted the H2O2 accumulation and clustering of chloroplast, which led to an improvement of the photosynthetic efficiency. These data suggest that H2O2 detoxification by catalase is important to protect the chloroplast, thus conserving the photosynthetic activity in O. streptacantha under stress.
[本文引用: 1]
DOI:10.1016/j.plaphy.2016.04.047URLPMID:27139585 [本文引用: 1]

Glomerella leaf spot (GLS) caused by Glomerella cingulata is a newly emergent disease that results in severe defoliation and fruit spots in apple. Currently, there are no effective means to control this disease except for the traditional fungicide sprays. Induced resistance by elicitors against pathogens infection is a widely accepted eco-friendly strategy. In the present study, we investigated whether exogenous application of salicylic acid (SA) could improve resistance to GLS in a highly susceptible apple cultivar (Malus domestica Borkh. cv. 'Gala') and the underlying mechanisms. The results showed that pretreatment with SA, at 0.1-1.0 mM, induced strong resistance against GLS in 'Gala' apple leaves, with SA treated leaves showing significant reduction in lesion numbers and disease index. Concurrent with the enhanced disease resistance, SA treatment markedly increased the total antioxidant capacity (T-AOC) and defence-related enzyme activities, including catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), phenylalanine ammonia-lyase (PAL) and polyphenol oxidase (PPO). As expected, SA treatment also induced the expression levels of five pathogenesis-related (PR) genes including PR1, PR5, PR8, Chitinase and beta-1,3-glucanase. Furthermore, the most pronounced and/or rapid increase was observed in leaves treated with SA and subsequently inoculated with G. cingulata compared to the treatment with SA or inoculation with the pathogen. Together, these results suggest that exogenous SA triggered increase in reactive oxygen species levels and the antioxidant system might be responsible for enhanced resistance against G. cingulata in 'Gala' apple leaves.
DOI:10.1111/j.1365-3040.2005.01459.xURLPMID:17080932 [本文引用: 1]

Oxygen free radicals are thought to play an essential role in senescence, especially those derived from peroxisomes. Therefore, the activities of different isoforms of the peroxisomal hydrogen peroxide (H2O2)-scavenging enzyme catalase (CAT) were analysed during senescence of Arabidopsis. CAT2 activity decreased with bolting time parallel with cytosolic ascorbate peroxidase 1 (APX1) activity before loss of chlorophyll could be measured. At the same time point, the H2O2 content increased. Subsequently, the stress-inducible CAT3 isoform was activated and APX1 activity was recovered, accompanied by a decline of the H2O2 content. In very late stages, low activities of the seed-specific CAT1 became detectable in leaves, but H2O2 increased again. Further analyses of CAT expression by promoter: beta-glucuronidase (GUS) fusions in transgenic plants revealed a vasculature-specific CAT3 expression, whereas CAT2 expression turned out to be specific for photosynthetic active tissues. CAT2 expression is down-regulated during leaf senescence, while CAT3 expression is induced with age and corresponds to an accumulation of H2O2 in the vascular bundles. CAT2 down-regulation on the transcriptional level appears as the initial step in creating the H2O2 peak during bolting time, while the decrease in APX1 activity might only be a secondary and amplifying effect.
[本文引用: 1]
DOI:10.1105/tpc.15.00144URLPMID:25966761 [本文引用: 1]

Drought is a major threat to plant growth and crop productivity. Calcium-dependent protein kinases (CDPKs, CPKs) are believed to play important roles in plant responses to drought stress. Here, we report that Arabidopsis thaliana CPK8 functions in abscisic acid (ABA)- and Ca(2+)-mediated plant responses to drought stress. The cpk8 mutant was more sensitive to drought stress than wild-type plants, while the transgenic plants overexpressing CPK8 showed enhanced tolerance to drought stress compared with wild-type plants. ABA-, H2O2-, and Ca(2+)-induced stomatal closing were impaired in cpk8 mutants. Arabidopsis CATALASE3 (CAT3) was identified as a CPK8-interacting protein, confirmed by yeast two-hybrid, coimmunoprecipitation, and bimolecular fluorescence complementation assays. CPK8 can phosphorylate CAT3 at Ser-261 and regulate its activity. Both cpk8 and cat3 plants showed lower catalase activity and higher accumulation of H2O2 compared with wild-type plants. The cat3 mutant displayed a similar drought stress-sensitive phenotype as cpk8 mutant. Moreover, ABA and Ca(2+) inhibition of inward K(+) currents were diminished in guard cells of cpk8 and cat3 mutants. Together, these results demonstrated that CPK8 functions in ABA-mediated stomatal regulation in responses to drought stress through regulation of CAT3 activity.
[本文引用: 1]
[本文引用: 1]
DOI:10.3389/fpls.2017.01345URLPMID:28824680 [本文引用: 1]

AcCATPO is a plant catalase-phenol oxidase recently identified from red amaranth. Its physiological function remains unexplored. As the starting step of functional analysis, here we report its subcellular localization and a non-canonical targeting signal. Commonly used bioinformatics programs predicted a peroxisomal localization for AcCATPO, but failed in identification of canonical peroxisomal targeting signals (PTS). The C-terminal GFP tagging led the fusion protein AcCATPO-GFP to the cytosol and the nucleus, but N-terminal tagging directed the GFP-AcCATPO to peroxisomes and nuclei, in transgenic tobacco. Deleting the tripeptide (PTM) at the extreme C-terminus almost ruled out the peroxisomal localization of GFP-AcCATPODelta3, and removing the C-terminal decapeptide completely excluded peroxisomes as the residence of GFP-AcCATPODelta10. Furthermore, this decapeptide as a targeting signal could import GFP-10aa to the peroxisome exclusively. Taken together, these results demonstrate that AcCATPO is localized to the peroxisome and the nucleus, and its peroxisomal localization is attributed to a non-canonical PTS1, the C-terminal decapeptide which contains an internal SRL motif and a conserved tripeptide P-S/T-I/M at the extreme of C-terminus. This work may further the study as to the physiological function of AcCATPO, especially clarify its involvement in betalain biosynthesis, and provide a clue to elucidate more non-canonic PTS.
DOI:10.1105/tpc.108.058974URLPMID:18984675 [本文引用: 1]

The plant signaling molecule salicylic acid (SA) and/or xenobiotic chemicals like the auxin mimic 2,4-D induce transcriptional activation of defense- and stress-related genes that contain activation sequence-1 (as-1)-like cis-elements in their promoters. as-1-like sequences are recognized by basic/leucine zipper transcription factors of the TGA family. Expression of genes related to the SA-dependent defense program systemic acquired resistance requires the TGA-interacting protein NPR1. However, a number of as-1-containing promoters can be activated independently from NPR1. Here, we report the identification of Arabidopsis thaliana SCARECROW-like 14 (SCL14), a member of the GRAS family of regulatory proteins, as a TGA-interacting protein that is required for the activation of TGA-dependent but NPR1-independent SA- and 2,4-D-inducible promoters. Chromatin immunoprecipitation experiments revealed that class II TGA factors TGA2, TGA5, and/or TGA6 are needed to recruit SCL14 to promoters of selected SCL14 target genes identified by whole-genome transcript profiling experiments. The coding regions and the expression profiles of the SCL14-dependent genes imply that they might be involved in the detoxification of xenobiotics and possibly endogenous harmful metabolites. Consistently, plants ectopically expressing SCL14 showed increased tolerance to toxic doses of the chemicals isonicotinic acid and 2,4,6-triiodobenzoic acid, whereas the scl14 and the tga2 tga5 tga6 mutants were more susceptible. Hence, the TGA/SCL14 complex seems to be involved in the activation of a general broad-spectrum detoxification network upon challenge of plants with xenobiotics.
