 ,1,2
,1,2The Effect of Krüppel-Like Factor 3 (KLF3) Gene on Bovine Fat Deposition
GUO HongFang1, NING Yue1, CHENG Gong1,2, ZAN LinSen ,1,2
,1,2通讯作者:
收稿日期:2018-10-10接受日期:2019-01-22网络出版日期:2019-04-01
| 基金资助: |
Received:2018-10-10Accepted:2019-01-22Online:2019-04-01
作者简介 About authors
郭红芳,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (648KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
郭红芳, 宁越, 成功, 昝林森. Krüppel-Like Factor 3(KLF3)基因对牛脂肪沉积的作用[J]. 中国农业科学, 2019, 52(7): 1272-1281 doi:10.3864/j.issn.0578-1752.2019.07.014
GUO HongFang, NING Yue, CHENG Gong, ZAN LinSen.
0 引言
【研究意义】动物体内肌内脂肪沉积,影响肉的断面大理石评分,可显著提高牛肉的嫩度、多汁性和风味等感官品质,最终影响肉质等级与经济价值[1]。因此,如何提高肌内脂肪含量来满足消费者对牛肉嫩度和风味的需要,一直是牛肉脂肪沉积生物分子调控研究的一个热点[2]。脂肪沉积量主要取决于脂肪细胞增殖和分化程度,这一过程受诸多分化转录因子的协同调控,转录因子的表达量及其活性大小决定了分化过程[3]。因此,深入研究牛脂肪沉积的分子调控机制对于改善牛肉肉质性状具有重要意义。【前人研究进展】KLF家族是一类C端含有3个锌指结构的重要的转录因子,能特异性结合于靶基因启动子区发挥其调控功能,在细胞分化、细胞增殖和凋亡方面起着重要的作用[4,5,6,7]。目前哺乳动物中研究发现KLF家族成员有17个成员,大多数成员参与脂肪细胞形成。有研究表明KLF2、KLF3和KLF7在脂肪细胞分化中主要起抑制作用[7,8,9],KLF4、KLF6、KLF9、KLF11、KLF13、KLF14和KLF15则主要起促进作用[10,11,12,13,14,15,16]。KLF3基因是KLF家族中一类重要的转录因子,最早从红细胞系中作为KLF1的同源系克隆得到[17],最初体外研究发现其在3T3-L1脂肪细胞系中募集转录辅助阻遏物CtBP结合于C/EBPα的启动子区发挥抑制作用[18,19]。但在体内有大量研究表明KLF3基因敲除小鼠与同窝小鼠相比明显瘦小、脂肪垫减少,进一步研究发现主要是由于白色脂肪减少而造成的[19]。另外有研究发现干扰KLF3可增加动物肠道脂肪沉积[20],而且在鸡脂肪细胞分化过程中发现虽然KLF3基因抑制脂肪细胞形成的一些关键基因的启动子活性,但促进脂肪细胞分化标志基因PPARγ 的启动子转录活性[21]。另外有研究发现KLF3基因在调节脂质聚集和分泌中起着重要作用。它可以通过促进脂肪酸β-氧化调节脂质代谢,另外KLF3 基因突变可导致线虫中类似哺乳类的线粒体甘油三酯转移蛋白和载脂蛋白B的基因dsc-4/ vit 分别减少[22]。【本研究切入点】这些研究结果表明KLF3基因在脂肪沉积过程中发挥重要的作用。目前还没有关于KLF3基因对牛脂肪细胞分化和脂质代谢的作用研究。【拟解决的关键问题】因此,本研究以牛前脂肪细胞为研究对象,通过干扰KLF3基因的表达,确定KLF3基因在牛脂肪细胞分化和脂肪酸代谢中的作用,为进一步明确KLF3基因在调控牛脂肪沉积中的作用,改善牛肉品质提供一定的理论依据。1 材料与方法
本研究于2016年9月至2017年9月在西北农林科技大学国家肉牛改良中心实验室完成。1.1 试验动物和所需组织
秦川牛肉用新品系新生犊牛(3日龄)3头的9个组织(心、肝、脾、肺、肾、大肠、瘤胃、肾周脂、肌肉)。试验动物和所需组织均采自西北农林科技大学国家肉牛改良中心良繁场,分别采集3头牛的9个组织共计27个样本后立即置于液氮罐中迅速带回实验室,-80℃保存备用。
1.2 牛前脂肪细胞获取
选取临床检查无异常的秦川牛肉用新品系新生牛(3日龄),参照文献[23]试验方法从新生牛肾周脂肪组织分离得到牛前脂肪细胞。将得到的牛前脂肪细胞沉淀加入适量的含10% FBS的完全培养基重悬、吹匀,置37℃、5% CO2培养箱中培养,每2 d 换液1次,一般4—5 d长满,传代于6孔板中培养。当传代培养的牛前脂肪细胞生长至充分汇合时,用诱导分化培养基(含10% FBS培养基+5 μg·mL-1胰岛素+1 μg·mL-1地塞米松+0.5 mmol·L-1 3-异丁基-1甲基黄嘌呤)进行诱导分化培养2 d后,再换成脂肪细胞分化维持培养基(10% FBS+1 μg·mL-1胰岛素)进行培养2 d。1.3 SiRNA转染
牛KLF3基因的干扰RNA(SiKLF3)和对照SiRNA(NC)由上海吉玛公司合成,序列如表1所示。当牛前脂肪细胞生长至70%—90%汇合时,先用无血清培养基饥饿细胞2 h,然后分别将5 μL干扰KLF3 SiRNA 和对照组SiRNA溶解于125 μL OPTI培养基中,另外将3.75 μL lipofectin3000加入125 μL OPTI后和稀释的SiRNA混匀静置5 min后逐滴加入6孔板中。转染6 h后换成含有10% FBS的完全培养基。转染2 d后细胞生长至充分汇合时,进行诱导分化培养至第4天。Table 1
表1
表1试验中所用引物
Table 1
| 引物Primer | 引物序列(5′-3′)Sequence(5′-3′) | 片段大小Fragment size (bp) | Tm(℃) |
|---|---|---|---|
| KLF3-Si2-F | GCAAAGGAAGCGGAGAAUATT | — | — |
| KLF3-Si2-R | UAUUCUCCGCUUCCUUUGCTT | — | — |
| KLF3-Si3-F | GGAAACACACUGGAAUCAATT | — | — |
| KLF3-Si3-R | UUGAUUCCAGUGUGUUUCCTT | — | — |
| PPARγ-F | GAGATCACAGAGTACGCCAAG | 216 | 60 |
| PPARγ-R | GGGCTCCATAAAGTCACCAA | ||
| CEBPα-F | ATCTGCGAACACGAGACG | 73 | 60 |
| CEBPα-R | CCAGGAACTCGTCGTTGAA | ||
| ACCα-F | CTCCAACCTCAACCACTACGG | 171 | 60 |
| ACCα-R | GGGGAATCACAGAAGCAGCC | ||
| FAS-F | TAAGGTTCAAATTGCTGCGT | 138 | 60 |
| FAS-R | TCCAGAGCGAAGGAGAGATT | ||
| FABP4-F | TGAGATTTCCTTCAAATTGGG | 101 | 60 |
| FABP4-R | CTTGTACCAGAGCACCTTCATC | ||
| GAPDH-F | CCAACGTGTCTGTTGTGGAT | 80 | 60 |
| GAPDH-R | CTGCTTCACCACCTTCTTGA | ||
| KLF3-F | CAGTCCCTGTCAAGCAAGAG | 111 | 60 |
| KLF3-R | ACAATGGCGTGGAGTAGATG |
新窗口打开|下载CSV
1.4 RNA提取和qRT-PCR
按照TRIZOL试剂说明书分别提取新生牛9种组织RNA和诱导分化第0天和第4天的秦川牛脂肪细胞的总RNA并分别反转录为cDNA。根据NCBI数据库提供牛KLF3、PPARγ、C/EBPα、ACCα、FAS和 FABP4基因 mRNA 序列,利用Primer premier 5.0设计基因表达分析引物,以牛GAPDH基因为内参(NM_001034034)采用qRT-PCR方法检测各组织和基因的表达,引物见表1。20μL反应体系:2×SYBR Premix EX TaqⅡ 10 μL, 上、下游引物各0.8 μL,ROX Reference DyeⅡ 0.4 μL, 稀释至50 ng·μL-1的cDNA 2 μL,RNase-freedd-H2O 补至20 μL。反应程序为:94℃ 30 s 预变性后 95℃ 5 s, 60℃ 34 s,40个循环。每个样本3个生物学重复。用2-ΔΔCt法计算各组织和细胞中基因的相对表达量。1.5 油红O染色和甘油三酯含量测定
将诱导分化培养至第4天的脂肪细胞用PBS洗2—3次后用4%多聚甲醛固定培养板贴壁细胞30 min 后, PBS漂洗。吸取油红O 染液1 mL 浸染培养板, 30 min 后倒掉油红O 染液, PBS漂洗培养板2—3次, 至完全漂洗干净。置于倒置相差显微镜下观察, 并照相, 采集油红O染色图片。甘油三酯含量利用牛甘油三酯测定ELISA试剂盒按说明书步骤进行,经过样品和标准品准备,分别加样于96孔板,37℃ 反应30 min,洗板5次后加入酶标试剂,37℃ 30 min,显色10 min后加入终止液,在490 nm波长测OD值并计算。分别测定诱导分化第4 天,干扰KLF3和对照组两组之间甘油三酯的含量。每个样本设3个重复。
1.6 Western blot
将转染Si-KLF3后2 d的牛前脂肪细胞弃去培养基,室温下用PBS洗细胞3次,然后向6孔板中加入细胞裂解液,冰上裂解30 min,将细胞裂解液转至1.5 mL 离心管中12 000 r/min 4℃离心10 min后,将上清(细胞总裂解物)转至新的离心管,与蛋白上样buffer混合,在100℃煮沸10 min使蛋白质变性。每个样品取10—20 μL进行常规SDS-PAGE电泳。电泳结束后,采用BIO-RAD的MiNi Trans-Blot将样品转移至PVDF膜,将膜置于封闭液(5%脱脂奶粉的PBST),室温封闭2 h,或4℃过夜。洗去膜上的封闭液,将膜孵育在含羊抗兔的KLF3多克隆抗体(ab154531,Abcam,Cambridge,UK)或GAPDH兔单克隆抗体(ab181603,Abcam,Cambridge,UK)的PBST溶液,置于摇床上,室温反应2 h或4℃过夜;洗膜后将膜孵育在含羊抗兔IGg二抗(ab6721,Abcam,Cambridge,UK)的PBST溶液;再次洗膜后常规ECL显色。1.7 KLF3基因生物学特征分析
应用NCBI数据库中的Blastp(http://blast. ncbi. nlm.nih.gov/Blast.cgi)分析基因的同源性;应用MEAG7.0软件构建系统进化发育树。1.8 数据分析
数据采用Student-T和ANVOA 方法进行分析,数据表示为“平均数±标准差(Mean±SD)”以P<0.05表示差异显著,P<0.01表示差异极显著。2 结果
2.1 牛KLF3基因的生物学表达特征
将牛KLF3 基因蛋白序列和GenBank公布的其他物种的序列进行Blastp比对,结果发现KLF3 的蛋白序列不但与反刍动物具有较高的同源性高达98%,而且与人和家鼠也具有很高的同源性,比对见表2。另外,构建系统进化树后发现KLF3基因与反刍动物亲缘关系较近,结果见图1。Table 2
表2
表2牛KLF3基因蛋白Blastp比对
Table 2
| 物种 Species | 序列号 GenBank accession | 相似度 Similarity |
|---|---|---|
| 水牛Bubalusbubalis | XP 006055965.1 | 99% |
| 绵羊Ovisaries | XP_014962453.1 | 99% |
| 野猪 Sus scrofa | NP_001127824.1 | 98% |
| 野骆驼Camelus ferus | XP_006185663.1 | 98% |
| 智人 Homo sapiens | NP_05057615.3 | 97% |
| 小家鼠Mus musculus | NP_032479.1 | 94% |
新窗口打开|下载CSV
图1
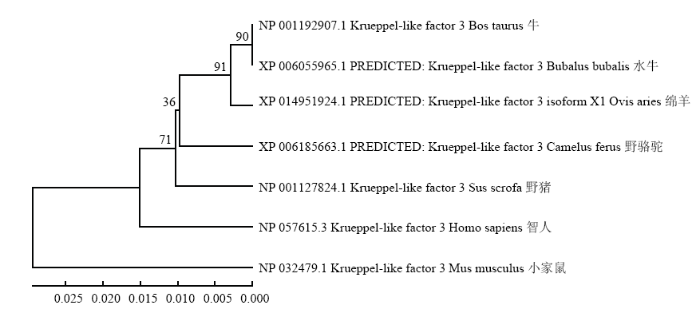 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1牛KLF3的系统进化树
Fig. 1Phylogenetic tree of bovine KLF3
2.2 牛KLF3基因组织和脂肪细胞中的表达规律分析
qRT-PCR 结果表明 KLF3 基因在新生牛的肝脏、瘤胃和脂肪中有比较高的表达量与其他组织表达量相比达到了差异显著(P<0.05)(图2-A),而在肌肉中表达量最低。而KLF3 基因在牛前脂肪细胞分化中的规律发现,KLF3 基因随着脂肪细胞分化进程推进,其表达量逐渐增加(图2-B)。这表明 KLF3 基因在脂肪组织形成和脂肪细胞分化中也许有比较重要的作用。图2
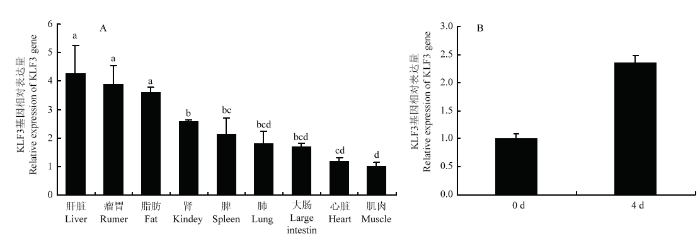 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2KLF3基因在秦川牛新生牛中组织表达谱分析(A)和牛脂肪细胞中时序表达谱分析(B)
Fig. 2KLF3 gene expression pattern in Qinchuan newborn cattle tissues (A) and bovine adipocyte(B)
2.3 干扰KLF3基因对牛前脂肪细胞分化和脂肪酸代谢的影响
为进一步研究 KLF3 基因在牛脂肪细胞分化中的作用,我们合成SiRNA干扰 KLF3 基因表达,转染牛前脂肪细胞。在干扰 KLF3 基因2 d 后分别提取牛 KLF3 基因干扰处理组和对照组(NC)RNA检测其干扰效率。我们发现Si2与对照组相比具有最高的干扰效率达到了73%。另外蛋白水平也发现干扰 KLF3 基因处理组其蛋白水平也明显下调(图3)。并且在牛前脂肪细胞分化过程中用Si2干扰 KLF3 基因后发现,在脂肪细胞分化的第0 和第 4 天 KLF3 基因的表达量分别下调了73% 和 54%与对照组相比达到了极显著差异(P<0.01,图4)。因此,后续试验中转染Si2用于干扰 KLF3 基因的表达,并检测脂肪细胞分化标志基因PPARγ和C/EBPα 以及脂质代谢基因 ACCα、FAS 和 FABP4 的表达量变化。图3
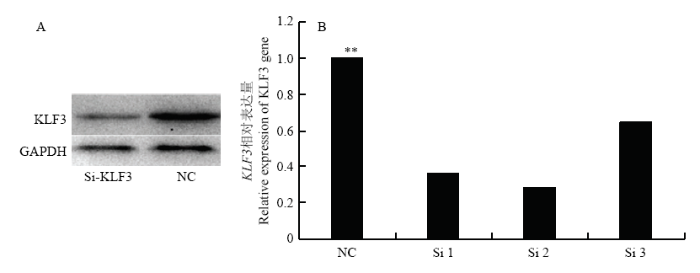 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3KLF3 基因干扰RNA筛选
A:干扰si2- KLF3 后蛋白表达量;B:不同Si处理后 KLF3 的相对表达量
Fig. 3KLF3 siRNA screening
A. Protein expression when treat with Si2-KLF3; B. KLF3 expression when treat with different SiRNA
图4
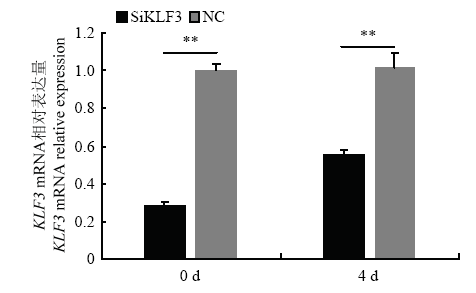 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4牛脂肪细胞分化过程中干扰KLF3 基因后KLF3基因相对表达量
** 表示差异极显著,P<0.01。下同
Fig. 4KLF3 relative expression after interfering with bovine KLF3 gene during adipocyte differentiation
** represented P<0.01. The same as below
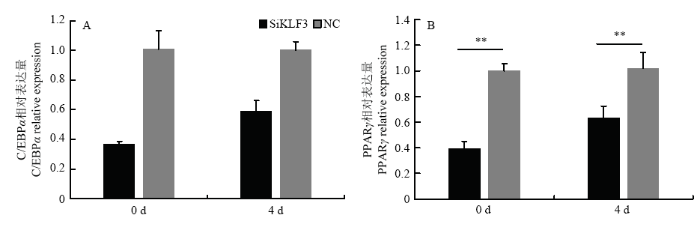
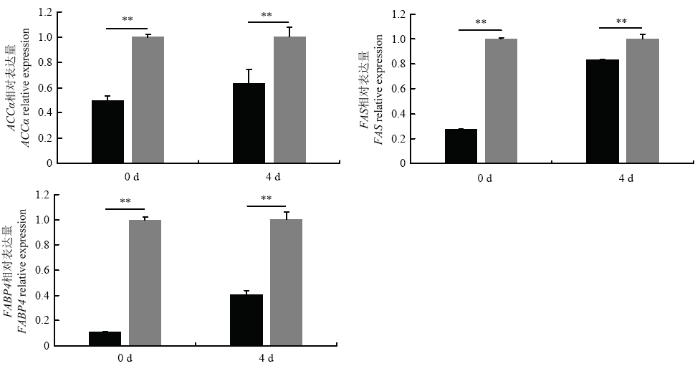
研究发现,转染Si2干扰 KLF3 基因表达后 PPARγ 的表达量在牛脂肪细胞诱导分化的第0和第4 天与对照组相比分别下调了58%和37%,达到了差异极显著(P<0.01),C/EBPα 的表达量也分别下调了64% 和41%,达到了差异极显著(P<0.01,图5)。同样干扰 KLF3 基因检测对脂质代谢的关键基因的表达量变化发现脂肪细胞基因 FABP4 的表达量与对照组相比在脂肪细胞诱导分化的第0天和第4天分别下调了89%和60%,而 ACCα 的表达量干扰 KLF3 处理组和对照组相比在脂肪细胞诱导分化第0和第4天分别下调了50%和37%, 而FAS的表达量则分别下调了73% 和19%,都达到了差异极显著(P<0.01,图6)。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5牛脂肪细胞分化过程中干扰牛 KLF3 基因 PPARγ 和 C/EBP α 的相对表达量
Fig. 5The relative expression of PPARγ and C/EBPα when silence bovine KLF3 gene during bovine adipocyte differentiation
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6牛脂肪细胞分化过程中干扰牛KLF3 基因 ACCα、FAS 和 FABP4 的相对表达量
Fig. 6The relative expression of ACCα, FAS and FABP4 when silence bovine KLF3 gene during bovine adipocyte differentiation
2.4 油红O染色和细胞脂肪含量的测定
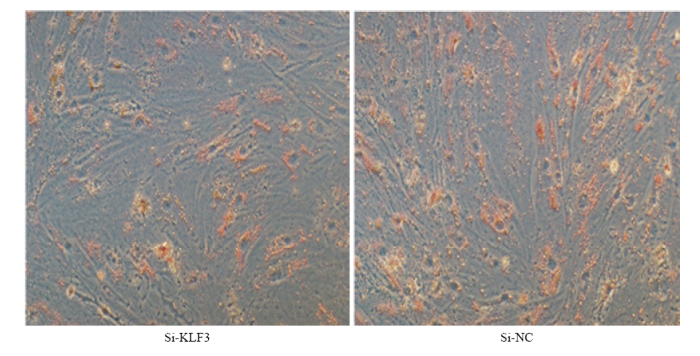
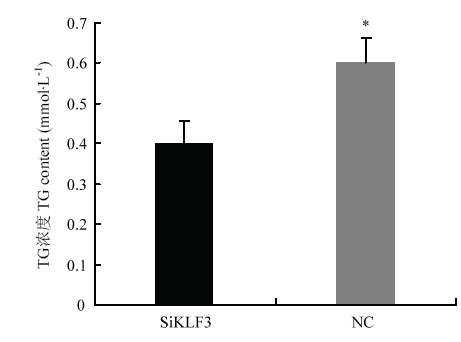
为进一步从表型上确定KLF3基因对牛脂肪细胞分化的影响,干扰牛KLF3 基因处理后并诱导分化至第4天,从形态学上用油红O染色法测定干扰KLF3 基因处理组和对照组脂滴的差异。另外用牛甘油三酯测定试剂盒测定干扰 KLF3 处理组和对照组中甘油三酯的含量。结果表明干扰 KLF3 基因抑制牛脂肪细胞分化,与对照组相比干扰 KLF3 处理组的脂滴较少,干扰 KLF3 和对照组相比甘油三酯含量明显减少并达到显著差异(P<0.05,图7,图8)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7干扰 KLF3 基因并诱导牛脂肪细胞分化至第4天油红O染色图
Fig. 7Oil O staining when silence KLF3 gene at bovine adipocyte induced differentiation 4 days
图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8干扰KLF3基因并诱导分化至第4天脂肪细胞中甘油三酯含量测定
* P<0.05
Fig. 8The content of triglyceride in bovine adipocyte when silence KLF3 gene at induced differentiation 4 days
3 讨论
牛肉品质主要受脂肪沉积的影响,而脂肪沉积主要与脂肪细胞分化和脂肪酸代谢密切相关[1,24]。大量研究表明KLF家族在脂肪细胞分化中具有重要作用,并且其家族成员大多数位于PPARγ 上游网络调控脂肪细胞分化。KLF3 基因是KLF家族重要一员,大量研究表明其在调控脂肪细胞分化及脂质代谢中具有重要作用[17,22]。本研究通过不同物种蛋白比对和基因进化树构建发现 KLF3 基因蛋白序列不但与反刍动物具有较高同源性,高达98% 以上,而且其与人和家鼠蛋白序列同源性也高达94% 以上,这说明 KLF3 基因在不同物种中都具有重要作用,而且系统进化树比对结果发现,KLF3 基因与水牛和绵羊这类反刍动物具有更近的亲缘关系,这也侧面反映了KLF3 基因在进化过程中比较保守。另外研究发现 KLF3 基因在新生牛中具有广泛表达特性并且在脂肪组织中相对表达水平较高这与在其他物种上的研究一致[19,21]。KFL3 基因的广泛表达性也表明其在机体生命活动中有着不可或缺的作用,而且其在脂肪组织中有较高表达量,并且随着脂肪细胞分化进程中其表达量逐渐增加也说明 KLF3 基因也许在脂肪细胞分化和脂质沉积中起着重要作用,这也与前人关于KLF3基因在脂肪细胞分化和脂质代谢中发挥重要作用研究相一致[9,21-22]。因此,深入研究 KLF3 基因在牛脂肪细胞分化和脂质沉积作用对改善肉牛肉质性状具有重要的意义。PPARγ 和 C/EBPα 作为脂肪细胞分化的中后期标志基因,在脂肪细胞分化中具有关键的作用,其表达量的变化影响着脂肪细胞的成熟[25,26]。目前关于 KLF3 基因在脂肪细胞分化中的作用还不明确,ZHANG等[21]研究发现在鸡脂肪细胞中过表达 KLF3 基因可促进 PPARγ 基因的启动子转录活性,而在3T3-L1细胞中有研究发现KLF3基因通过抑制 C/EBPα基因的启动子转录活性从而抑制脂肪细胞分化[19]。本研究通过筛选最佳干扰牛 KLF3 基因的SiRNA,转染至牛前体脂肪细胞并进行诱导分化,结果发现,牛脂肪细胞分化标志基因PPARγ 和 C/EBPα 表达量都随着 KLF3 基因表达量下调明显下降(P<0.01)。这表明 KLF3基因在牛脂肪细胞分化过程中抑制脂肪细胞分化关键基因 PPARγ 和 C/EBPα 表达从而抑制牛前脂肪细胞分化。不同物种中 KLF3 基因对脂肪细胞分化关键基因调控的差异也许是由于物种的差异性,因此,后续还需进一步在转录调控机制方面进行更深入的研究。
脂肪细胞的功能主要是合成并储存甘油三酯,而甘油三酯的合成在基因水平主要涉及脂肪酸的转运和合成。FABP4 是脂肪酸结合蛋白,主要负责长链脂肪酸的摄取,转运及代谢调节,在脂肪酸合成过程中起关键作用[27,28]。FAS 即脂肪酸合成酶,是脂肪酸从头合成过程中的催化乙酰辅酶A和丙二酸单酰辅酶A聚合成长链脂肪酸的主要限速酶,该酶的活性及数量对动物体脂的沉积有重要影响,其表达量升高能显著增加甘油三酯的沉积[29,30]。而 ACCα与FAS 一样也是动物体内脂肪酸合成的限速酶,它催化合成的丙二酰COA是长链脂肪酸合成的前体物质[31]。有研究发现 KLF3 基因通过调控β-氧化调节脂肪酸代谢,另外,突变KLF3基因可使脂肪酸代谢关键基因下调[20,22],这说明KLF3基因在脂肪酸代谢中也具有重要作用。本研究发现干扰牛KLF3基因转染牛脂肪细胞后发现脂肪酸代谢基因 FABP4、ACCα 及 FAS 的表达量也明显下调(P<0.01),干扰 KLF3 基因抑制脂肪酸代谢关键基因的下调,说明 KLF3 基因在牛脂肪细胞中通过调节脂肪酸代谢关键基因的表达参与脂肪酸代谢从而调控甘油三酯的积累。
随后通过干扰牛 KLF3 基因后,进行油红O染色并测定甘油三酯含量,发现与对照组相比,干扰牛 KLF3 基因组脂滴减少,甘油三酯含量下调,并且达到了差异显著(P<0.05)。这进一步说明干扰 KLF3 基因能够抑制牛脂肪形成并且综合脂肪细胞分化关键基因和脂肪酸代谢关键基因表达量的下调,说明干扰牛KLF3 基因抑制牛脂肪细胞分化,抑制脂滴积累。这与敲除 KLF3 小鼠脂肪减少结果一致 [19]。总之,在牛脂肪细胞分化过程中,干扰 KLF3 基因抑制脂滴形成和甘油三酯积累。
4 结论
牛 KLF3 基因通过抑制牛脂肪细胞分化标志基因PPARγ和C/EBPα及脂肪酸代谢关键基因 FABP4、ACCα及FAS 的表达抑制牛脂肪细胞分化和脂肪酸代谢,从而影响甘油三酯的积累和脂质沉积。为进一步研究牛 KLF3 基因调控脂质沉积、脂肪酸代谢和改善牛肉品质的转录调控提供了依据。(责任编辑 林鉴非)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.2527/jas.2013-7282PMID:24492542 [本文引用: 2]

Abstract Shiga-toxigenic Escherichia coli, such as E. coli O157:H7, are foodborne zoonotic pathogens that can cause severe illness and death in humans. The gastrointestinal tract of ruminant animals has been identified as a primary habitat for E. coli O157:H7 and, in cattle, the hindgut tract appears to be a primary site for colonization. This pathogen has been found in cattle feces, on cattle hides, and in the production environment, and transmission to humans has occurred as a result of consumption of contaminated ground beef, water, and produce. Interventions to reduce the pathogen at beef harvest have significantly reduced the occurrence of the pathogen, but outbreaks and recalls due to the pathogen still occur for beef products. Interventions in the feedyard before harvest have had little success, but critical control points for implementing interventions are limited compared with the beef abattoir. The percentage of animals shedding E. coli O157:H7 in the feces can be highly variable from pen to pen, and the levels in the feces can vary from animal to animal. Animals colonized and shedding E. coli O157:H7 at high levels are a small fraction of animals in a pen but are important source for transferring the pathogen amongst the penmates. Recent research has indicated that diet may greatly influence the shedding of E. coli O157:H7. In addition, diet can influence the microbiota composition of the feces. However, little is known about the interaction between the indigenous microbiota and fecal shedding of E. coli O157:H7. Understanding the influence of indigenous microbiota on the colonization and shedding of E. coli O157:H7 will provide a potential avenue for intervention in the preharvest production environment not yet exploited.
DOI:10.2527/jas.2012-5799URLPMID:23230118 [本文引用: 1]

This paper reviews results of studies on effects of fetal programming and maternal nutrition during pregnancy on growth, efficiency, carcass, muscle, and meat quality characteristics of cattle. It includes results from our Australian Beef Cooperative Research Centre studies on factors such as chronic severe nutritional restriction from approximately d 80 of pregnancy to parturition and/or throughout lactation used to create early-life growth differences in the offspring of cows within pasture-based systems and the effect of these treatments on production characteristics to 30 mo of age. Fetal programming and related maternal effects are most pronounced and explain substantial amounts of variation for growth-related production characteristics such as BW, feed intake, carcass weight, muscle weights, meat yield, and fat and bone weights at any given age but are less evident when assessed at the same BW and carcass weight. Some effects of maternal and early-life factors in our studies were evident for efficiency traits but fewer affected beef quality characteristics at 30 mo of age, explaining only small amounts of variation in these traits. It is difficult to uncouple maternal nutritional effects specific to prenatal life from those that carry over to the postnatal period until weaning, particularly the effects of maternal nutrition during pregnancy on subsequent lactational performance. Hence, experimental design considerations for studying fetal programming effects on offspring during later life are discussed in relation to minimizing or removing prenatal and postnatal confounding effects. The relative contribution of fetal programming to the profitability of beef production systems is also briefly discussed. In this regard, the importance of health and survival of cows and calves, the capacity of cows to rebreed in a timely manner, and the efficiency with which feed and other resources are used cannot be overemphasized in relation to economics, welfare, and the environment.
DOI:10.2527/jas.2011-4237URLPMID:21908644 [本文引用: 1]

Angus × Gelbvieh cows with 2 to 3 previous pregnancies were used to evaluate effects of maternal nutrient restriction on offspring adipose tissue morphology at standard production endpoints. At 45 d after AI to a single sire, pregnancy was confirmed and cows randomly allotted into groups and fed a control (Con, 100% of NRC recommendations), nutrient-restricted (NR, 70% of Con diet), or nutrient-restricted + protein-supplemented (NRP, 70% of Con + essential AA supply to the small intestine equal to Con) diet. At d 185 of gestation, cows were commingled and received the Con diet thereafter. Bull calves were castrated at 2 mo of age. Calves were weaned at 210 d, backgrounded for 28 d, and then placed in the feedlot for 195 d. Steers and heifers were slaughtered at an average 12th-rib fat thickness of 7.6 mm. Adipose tissue from selected depots was collected for adipocyte size analysis. There was no significant difference in BW or BCS between Con, NRP, and NR cows at d 45 of gestation, which averaged 489.7 ± 17.7 kg and 5.35 ± 0.13, respectively. At d 185 of gestation, Con and NRP groups had similar BW (566.1 ± 14.8 and 550.2 ± 14.8 kg) and BCS (6.34 ± 0.27 and 5.59 ± 0.27), but NR cows exhibited reduced (P < 0.05) BW (517.9 ± 14.8 kg) and BCS (4.81 ± 0.27). Among offspring (steers and heifers) at slaughter, there were no significant differences in BW or organ weights among treatment groups. Yield grade was reduced (P < 0.05) and semitendinosus weight/HCW tended (P = 0.09) to be reduced in NR offspring compared with Con and NRP offspring. Average adipocyte diameter was increased (P < 0.05) in subcutaneous, mesenteric, and omental adipose tissue and tended (P = 0.09) to increase in perirenal adipose tissue in NR compared with Con offspring with NRP offspring adipocyte diameter being either intermediate or similar to Con calves. The adipocyte size alterations observed in NR offspring were confirmed by DNA concentration of the adipose tissue depots. There also was an increased mRNA expression (P < 0.05) of fatty acid transporter 1 in subcutaneous adipose tissue from NR offspring compared with Con and NRP offspring. Nutritional restriction during early and mid gestation increased or tended to increase (P < 0.09) adipocyte diameter in all adipose tissue depots in finished steer and heifer calves.
DOI:10.1046/j.1365-294X.2003.02032.xPMID:11443140 [本文引用: 1]

J Biol Chem. 2001 Sep 14;276(37):34355-8. Epub 2001 Jul 6. Review
DOI:10.1042/BJ20100773PMID:3130109 [本文引用: 1]

Abstract SP/KLF (Specificity protein/Kr眉ppel-like factor) transcription factors comprise an emerging group of proteins that may behave as tumour suppressors. Incidentally, many cancers that display alterations in certain KLF proteins are also associated with a high incidence of KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homologue) mutations. Therefore in the present paper we investigate whether SP/KLF proteins suppress KRAS-mediated cell growth, and more importantly, the potential mechanisms underlying these effects. Using a comprehensive family-wide screening of the 24 SP/KLF members, we discovered that SP5, SP8, KLF2, KLF3, KLF4, KLF11, KLF13, KLF14, KLF15 and KLF16 inhibit cellular growth and suppress transformation mediated by oncogenic KRAS. Each protein in this subset of SP/KLF members individually inhibits BrdU (5-bromo-2-deoxyuridine) incorporation in KRAS oncogenic-mutant cancer cells. SP5, KLF3, KLF11, KLF13, KLF14 and KLF16 also increase apoptosis in these cells. Using KLF11 as a representative model for mechanistic studies, we demonstrate that this protein inhibits the ability of cancer cells to form both colonies in soft agar and tumour growth in vivo. Molecular studies demonstrate that these effects of KLF11 are mediated, at least in part, through silencing cyclin A via binding to its promoter and leading to cell-cycle arrest in S-phase. Interestingly, similar to KLF11, KLF14 and KLF16 mechanistically share the ability to modulate the expression of cyclin A. Collectively, the present study stringently defines a distinct subset of SP/KLF proteins that impairs KRAS-mediated cell growth, and that mechanistically some members of this subset accomplish this, at least in part, through regulation of the cyclin A promoter.
DOI:10.1007/s13205-011-0016-6URLPMID:3339616 [本文引用: 1]

The abnormalities caused by excess fat accumulation can result in pathological conditions which are linked to several interrelated diseases, such as cardiovascular disease and obesity. This set of conditions, known as metabolic syndrome, is a global pandemic of enormous medical, economic, and social concern affecting a significant portion of the world population. Although genetics, physiology and environmental components play a major role in the onset of disease caused by excessive fat accumulation, little is known about how or to what extent each of these factors contributes to it. The worm,Caenorhabditis elegansoffers an opportunity to study disease related to metabolic disorder in a developmental system that provides anatomical and genomic simplicity relative to the vertebrate animals and is an excellent eukaryotic genetic model which enable us to answer the questions concerning fat accumulation which remain unresolved. The stored triglycerides (TG) provide the primary source of energy during periods of food deficiency. In nature, lipid stored as TGs are hydrolyzed into fatty acids which are broken down through -oxidation to yield acetyl-CoA. Our recent study suggests that a member ofC. elegansKr ppel-like factor,klf-3regulates lipid metabolism by promoting FA 尾-oxidation and in parallel may contribute in normal reproduction and fecundity. Genetic and epigenetic factors that influence this pathway may have considerable impact on fat related diseases in human. Increasing number of studies suggest the role of mammalian KLFs in adipogenesis. This functional conservation should guide our further effort to exploreC. elegansas a legitimate model system for studying the role of KLFs in many pathway components of lipid metabolism.
DOI:10.1210/me.2005-0138URL [本文引用: 2]
DOI:10.1074/jbc.M210859200URLPMID:12426306 [本文引用: 1]

Obesity is an important public health problem associated with a number of disease states such as diabetes and arteriosclerosis. As such, an understanding of the mechanisms governing adipose tissue differentiation and function is of considerable importance. We recently reported that the Krüppel-like zinc finger transcription factor KLF15 can induce adipocyte maturation and GLUT4 expression. In this study, we identify that a second family member, KLF2/Lung Krüppel-like factor (LKLF), as a negative regulator of adipocyte differentiation. KLF2 is highly expressed in adipose tissue, and studies in cell lines and primary cells demonstrate that KLF2 is expressed in preadipocytes but not mature adipocytes. Constitutive overexpression of KLF2 but not KLF15 potently inhibits peroxisome proliferator-activated receptor-γ (PPARγ) expression with no effect on the upstream regulators C/EBPβ and C/EBPδ. However, the expression of C/EBPα and SREBP1c/ADD1 (adipocyte determination and differentiation factor-1/sterol regulatory element-binding protein-1), two factors that feedback in a positive manner to enhance PPARγ function, was also markedly reduced. In addition, transient transfection studies show that KLF2 directly inhibits PPARγ2 promoter activity (70% inhibition;< 0.001). Using a combination of promoter mutational analysis and gel mobility shift assays, we have identified a binding site within the PPARγ2 promoter, which mediates this inhibitory effect. These data identify a novel role for KLF2 as a negative regulator of adipogenesis.
DOI:10.2337/db12-1745URLPMID:23633521 [本文引用: 2]

Kruppel-like factor 3 (KLF3) is a transcriptional regulator that we have shown to be involved in the regulation of adipogenesis in vitro. Here, we report that KLF3-null mice are lean and protected from diet-induced obesity and glucose intolerance. On a chow diet, plasma levels of leptin are decreased, and adiponectin is increased. Despite significant reductions in body weight and adiposity, wild-type and knockout animals show equivalent energy intake, expenditure, and excretion. To investigate the molecular events underlying these observations, we used microarray analysis to compare gene expression in Klf3(+/+) and Klf3(-/-) tissues. We found that mRNA expression of Fam132a, which encodes a newly identified insulin-sensitizing adipokine, adipolin, is significantly upregulated in the absence of KLF3. We confirmed that KLF3 binds the Fam132a promoter in vitro and in vivo and that this leads to repression of promoter activity. Further, plasma adipolin levels were significantly increased in Klf3(-/-) mice compared with wild-type littermates. Boosting levels of adipolin via targeting of KLF3 offers a novel potential therapeutic strategy for the treatment of insulin resistance. Diabetes 62:2728-2737, 2013
.
DOI:10.1016/j.cmet.2008.02.001URLPMID:18396140 [本文引用: 1]

While adipogenesis is known to be controlled by a complex network of transcription factors, less is known about the transcriptional cascade that initiates this process. We report here the characterization of Krüppel-like factor 4 (KLF4) as an essential early regulator of adipogenesis. Klf4 is expressed in 3T3-L1 cells within 30 min after exposure to a standard adipogenic cocktail of insulin, glucocorticoids, and IBMX. Knockdown of KLF4 inhibits adipogenesis and downregulates C/EBPβ levels. KLF4 binds directly to the C/EBPβ ( Cebpb) promoter as shown by chromatin immunoprecipitation and gel shift assays and, together with Krox20, cooperatively transactivates a C/EBPβ reporter. C/EBPβ knockdown increases levels of KLF4 and Krox20, suggesting that C/EBPβ normally suppresses Krox20 and KLF4 expression via a tightly controlled negative feedback loop. KLF4 is specifically induced in response to cAMP, which by itself can partially activate adipogenesis. These data suggest that KLF4 functions as02an immediate early regulator of adipogenesis to induce C/EBPβ.
DOI:10.1074/jbc.M410515200URLPMID:101584 [本文引用: 1]

Abstract Kr ppel-like zinc finger transcription factors (KLFs) play diverse roles during cell differentiation and development in mammals. We have now shown by microarray analysis that expression of the KLF15 gene is markedly up-regulated during the differentiation of 3T3-L1 preadipocytes into adipocytes. Inhibition of the function of KLF15, either by expression of a dominant negative mutant or by RNA interference, both reduced the expression of peroxisome proliferator-activated receptor gamma (PPARgamma) and blocked adipogenesis in 3T3-L1 preadipocytes exposed to inducers of adipocyte differentiation. However, the dominant negative mutant of KLF15 did not affect the expression of CCAAT/enhancer-binding protein beta (C/EBPbeta) elicited by inducers of differentiation in 3T3-L1 preadipocytes. In addition, ectopic expression of KLF15 in NIH 3T3 or C2C12 cells triggered both lipid accumulation and the expression of PPARgamma in the presence of inducers of adipocyte differentiation. Ectopic expression of C/EBPbeta, C/EBPdelta, or C/EBPalpha in NIH 3T3 cells also elicited the expression of KLF15 in the presence of inducers of adipocyte differentiation. Moreover, KLF15 and C/EBPalpha acted synergistically to increase the activity of the PPARgamma2 gene promoter in 3T3-L1 adipocytes. Our observations thus demonstrate that KLF15 plays an essential role in adipogenesis in 3T3-L1 cells through its regulation of PPAR gamma expression.
DOI:10.1038/cdd.2010.100URLPMID:20725087 [本文引用: 1]

Krüppel-like factors (KLFs) as a family of zinc-finger transcription factors involve in the regulation of many physiological processes. In these studies, KLF9 was characterized for its role in adipogenesis. The expression of KLF9 was markedly upregulated during the middle stage of 3T3-L1 adipocyte differentiation, and inhibition of KLF9 by RNAi impaired adipogenesis. Using promoter deletion and mutation analysis, we identified two KLF9-binding sites within the 0.6-kb region of the PPARγ2 proximal promoter, indicating that KLF9 interacts with the PPARγ2 promoter. Furthermore, we found that KLF9 could synergistically activate PPARγ2 promoter by directly interacting with C/EBPα. In addition, overexpression of PPARγ2 rescued the impairment of adipocyte differentiation induced by KLF9 knockdown, which supports that PPARγ2 is a downstream target of KLF9. Collectively, our results indicate KLF9 as a key pro-adipogenic transcription factor through regulation of PPARγ2 expression with C/EBPα at the middle stage of adipogenesis.
[本文引用: 1]
.
DOI:10.1074/jbc.M500463200URLPMID:15917248 [本文引用: 1]

Abstract Preadipocyte differentiation occurs during distinct periods of human development and is a key determinant of body mass. Transcriptional events underlying adipogenesis continue to emerge, but the link between chromatin remodeling of specific target loci and preadipocyte differentiation remains elusive. We have identified Kr眉ppel-like factor-6 (KLF6), a recently described tumor suppressor gene, as a repressor of the proto-oncogene Delta-like 1 (Dlk1), a gene encoding a transmembrane protein that inhibits adipocyte differentiation. Forced expression of KLF6 strongly inhibits Dlk1 expression in preadipocytes and NIH 3T3 cells in vivo, whereas down-regulation of KLF6 in 3T3-L1 cells by small interfering RNA prevents adipogenesis. Repression of Dlk1 requires HDAC3 deacetylase activity, which is recruited to the endogenous Dlk1 promoter where it interacts with KLF6. Our studies identify the interaction between HDAC3 and KLF6 as a potential mechanism underlying human adipogenesis, and highlight the role of KLF6 as a multifunctional transcriptional regulator capable of mediating adipocyte differentiation through gene repression.
[本文引用: 1]
DOI:10.1186/s13578-015-0016-zURLPMID:26085920 [本文引用: 1]

Background Adipogenesis is tightly controlled by a complex network of transcription factors acting at different stages of differentiation. Kruppel-like factors (KLFs) as a family of zinc-finger...
DOI:10.1002/iub.422URL [本文引用: 2]

http://doi.wiley.com/10.1002/iub.422
DOI:10.1074/jbc.M115.638338URLPMID:25659434 [本文引用: 1]

Kr ppel-like factor 3 (KLF3/BKLF), a member of the Kr眉ppel-like factor (KLF) family of transcription factors, is a widely expressed transcriptional repressor with diverse biological roles. Although there is considerable understanding of the molecular mechanisms that allow KLF3 to silence the activity of its target genes, less is known about the signal transduction pathways and post-translational modifications that modulate KLF3 activity in response to physiological stimuli. We observed that KLF3 is modified in a range of different tissues and found that the serine/threonine kinase homeodomain-interacting protein kinase 2 (HIPK2) can both bind and phosphorylate KLF3. Mass spectrometry identified serine 249 as the primary phosphorylation site. Mutation of this site reduces the ability of KLF3 to bind DNA and repress transcription. Furthermore, we also determined that HIPK2 can phosphorylate the KLF3 co-repressor C-terminal binding protein 2 (CtBP2) at serine 428. Finally, we found that phosphorylation of KLF3 and CtBP2 by HIPK2 strengthens the interaction between these two factors and increases transcriptional repression by KLF3. Taken together, our results indicate that HIPK2 potentiates the activity of KLF3.
DOI:10.1128/MCB.01942-07URLPMID:2423134 [本文引用: 5]

Abstract Kr ppel-like factors (KLFs) recognize CACCC and GC-rich sequences in gene regulatory elements. Here, we describe the disruption of the murine basic Kr ppel-like factor gene (Bklf or Klf3). Klf3 knockout mice have less white adipose tissue, and their fat pads contain smaller and fewer cells. Adipocyte differentiation is altered in murine embryonic fibroblasts from Klf3 knockouts. Klf3 expression was studied in the 3T3-L1 cellular system. Adipocyte differentiation is accompanied by a decline in Klf3 expression, and forced overexpression of Klf3 blocks 3T3-L1 differentiation. Klf3 represses transcription by recruiting C-terminal binding protein (CtBP) corepressors. CtBPs bind NADH and may function as metabolic sensors. A Klf3 mutant that does not bind CtBP cannot block adipogenesis. Other KLFs, Klf2, Klf5, and Klf15, also regulate adipogenesis, and functional CACCC elements occur in key adipogenic genes, including in the C/ebpalpha promoter. We find that C/ebpalpha is derepressed in Klf3 and Ctbp knockout fibroblasts and adipocytes from Klf3 knockout mice. Chromatin immunoprecipitations confirm that Klf3 binds the C/ebpalpha promoter in vivo. These results implicate Klf3 and CtBP in controlling adipogenesis.
DOI:10.1016/j.jmb.2013.04.020PMID:4371790 [本文引用: 2]

61Mammals regulate lipid secretion and distribution to maintain normal levels of TG in adipocytes.61Mutation in klf-3 reduces the number of mitochondria probably leading to lower β-oxidation.61Vitamin D increases fat levels in klf-3 mutant by lowering the rate of β-oxidation.61klf-3 may have an important role in regulating lipid assembly and secretion.61We establish that klf-3 controls lipid storage, secretion and mitochondrial proliferation.
.
DOI:10.1080/09168451.2014.896735URLPMID:25036958 [本文引用: 4]

Studies in mammalian species showed that Krüppel-like factor 3 (KLF3) regulated adipose tissue development. However, it was not reported in chicken. In the current study, we found that during the growth and development of abdominal fat tissue, chicken KLF3 (Gallus gallus KLF3, gKLF3) was consecutively expressed, and its transcripts were higher at 765weeks of age and lower at 1065weeks of age in lean broilers than in fat broilers. In addition, gKLF3 overexpression suppressed chicken CCAAT/enhancer binding protein alpha (C/EBPα), fatty acid binding protein 4 (FABP4), fatty acid synthase (FASN), and lipoprotein lipase (LPL) promoter activities, but increased chicken peroxisome proliferator-activated receptor gamma (PPARγ) promoter activity. Additionally, point mutagenesis analysis showed that the substitution of Asp by Gly within the Pro-Val-Asp-Leu-Thr (PVDLT) motif of gKLF3 significantly reduced the ability of gKLF3 to regulate the promoter activities of FABP4, FASN, LPL, C/EBPα, and PPARγ. Effects of Overexpression of KLF3 and Its Mutant on the Promoter Activities of Chicken FABP4, FASN, LPL, C/EBPα, and PPARγ in DF1 Cells.
DOI:10.1016/j.jmb.2011.06.011URLPMID:21704635 [本文引用: 4]

78 In vertebrates, adipose tissue is the major site for storing energy in the form of fat. 78 KLFs are crucial regulators of adipogenesis in mammals. 78 FA β-oxidation is central to organismal energy homeostasis. 78 C. elegans klf-3 regulates fat storage by promoting β-oxidation. 78 In parallel, klf-3 may contribute to normal reproduction and fecundity in C. elegans.
DOI:10.13207/j.cnki.jnwafu.2014.02.069URL [本文引用: 1]

【目的】建立牛前体脂肪细胞的体外分离培养方法,研究牛前体脂肪细胞的生物学特性和分化特征,为研究牛脂肪发育和脂肪沉积的机制提供一种简便有效的细胞模型。【方法】采用Ⅰ型胶原酶消化法自新生牛脂肪组织中获得前体脂肪细胞,对培养的牛前体脂肪细胞进行形态学观察、生长曲线测定、油红O染色及脂肪细胞标志基因LPL、PPAR-r和脂联素表达研究。【结果】80%的牛前体脂肪细胞接种后12h开始贴壁,4d后开始向脂肪细胞转化,10d后绝大部分细胞脂滴融合,细胞脂肪含量增加;分离的牛前体脂肪细胞接种后1~2d生长缓慢,3~8d进入对数生长期,9d后进入平台期,之后细胞数目开始下降。经胰岛素诱导分化过程中,LPL在牛前体脂肪细胞分化的早期开始表达,PPAR-γ在分化的中期开始高度表达,而脂联素在牛前体脂肪细胞分化的前期未检测到,在细胞分化后期高度表达。【结论】用Ⅰ型胶原酶消化法可获得大量的牛前体脂肪细胞。培养的牛前体脂肪细胞成分均一、生长旺盛,经胰岛素诱导后能稳定的向脂肪细胞分化。
DOI:10.13207/j.cnki.jnwafu.2014.02.069URL [本文引用: 1]

【目的】建立牛前体脂肪细胞的体外分离培养方法,研究牛前体脂肪细胞的生物学特性和分化特征,为研究牛脂肪发育和脂肪沉积的机制提供一种简便有效的细胞模型。【方法】采用Ⅰ型胶原酶消化法自新生牛脂肪组织中获得前体脂肪细胞,对培养的牛前体脂肪细胞进行形态学观察、生长曲线测定、油红O染色及脂肪细胞标志基因LPL、PPAR-r和脂联素表达研究。【结果】80%的牛前体脂肪细胞接种后12h开始贴壁,4d后开始向脂肪细胞转化,10d后绝大部分细胞脂滴融合,细胞脂肪含量增加;分离的牛前体脂肪细胞接种后1~2d生长缓慢,3~8d进入对数生长期,9d后进入平台期,之后细胞数目开始下降。经胰岛素诱导分化过程中,LPL在牛前体脂肪细胞分化的早期开始表达,PPAR-γ在分化的中期开始高度表达,而脂联素在牛前体脂肪细胞分化的前期未检测到,在细胞分化后期高度表达。【结论】用Ⅰ型胶原酶消化法可获得大量的牛前体脂肪细胞。培养的牛前体脂肪细胞成分均一、生长旺盛,经胰岛素诱导后能稳定的向脂肪细胞分化。
.
DOI:10.2527/jas.2013-7308URLPMID:24669007 [本文引用: 1]

Pathogenic bacteria can live asymptomatically within and on cattle and can enter the food chain but also can be transmitted to humans by fecal or direct animal contact. Reducing pathogenic bacterial incidence and populations within live cattle represents an important step in improving food safety. A broad range of preslaughter intervention strategies are being developed, which can be loosely classified as 1) directly antipathogen strategies, 2) competitive enhancement strategies (that use the microbiome's competitive nature against pathogens), and 3) animal management strategies. Included within these broad categories are such diverse methods as vaccination against foodborne pathogens, probiotics and prebiotics, bacterial viruses (i.e., bacteriophages), sodium chlorate feeding, and dietary and management changes that specifically alter the microbiome. The simultaneous application of 1 or more preharvest strategies has the potential to reduce human foodborne illnesses by erecting multiple hurdles preventing entry into humans. However, economic factors that govern producer profitability must be kept in mind while improving food safety.
DOI:10.1210/en.2004-0180PMID:15284209 [本文引用: 1]

TNFalpha is known to inhibit adipocyte differentiation and induce insulin resistance. Moreover, TNFalpha is known to down-regulate peroxisome proliferator-activated receptor (PPAR)gamma2, an adipocyte-specific nuclear receptor of insulin-sensitizer thiazolidinediones. To clarify molecular mechanisms of TNFalpha- mediated PPARgamma2 down-regulation, we here examined the effect of TNFalpha on transcription regulation of PPARgamma2 gene expression during the early stage of adipocyte differentiation. 3T3-L1 preadipocytes (2 d after 100% confluent) were incubated in a differentiation mixture (dexamethasone, insulin, 3-isobutyl-1-methlxanthine), with or without 50 ng/ml TNFalpha, for 24 h. TNFalpha significantly decreased PPARgamma2 expression both at mRNA and protein levels (to approximately 40%), as well as aP2 mRNA expression. The mouse PPARgamma2 gene promoter region (2.2-kb) was isolated and was used for luciferase reporter assays by transient transfection. TNFalpha significantly suppressed PPARgamma2 gene transcription (to approximately 50%), and deletion analyses demonstrated that the suppression was mediated via CCAAT/enhancer-binding protein (C/EBP) binding elements at the -320/-340 region of the promoter. Moreover, TNFalpha significantly decreased expression of C/EBPdelta mRNA and protein levels (to approximately 40%). EMSA, using 3T3-L1 cells nuclear extracts with the -320/-340 region as a probe, demonstrated the binding of C/EBPdelta to the element, which was significantly decreased by TNFalpha treatment. Overexpression of CEBP/delta prevented the TNFalpha-mediated suppression of PPARgamma2 transactivation. Taken together, TNFalpha suppresses PPARgamma2 gene transcription by the inhibition of C/EBPdelta expression and its DNA binding during the early stage of adipocyte differentiation, which may contribute to the inhibition of adipocyte differentiation, as well as the induction of insulin resistance.
.
DOI:10.1016/S0163-7827(01)00005-4URLPMID:11412892 [本文引用: 1]

Adipose tissue development takes place primarily around birth but adipose cell number can increase throughout life in response to nutritional changes. At the molecular level, adipogenesis is the result of transcriptional remodeling that leads to activation of a considerable number of genes. Several transcription factors act cooperatively and sequentially in this process. This article attempts to review the roles of peroxisome proliferator-activated receptors 纬 and 未 in the control of preadipocyte proliferation and differentiation during adipose tissue development or during the adaptive response of adipose tissue mass to high-fat feeding.
DOI:10.2337/db13-0436PMID:24319114 [本文引用: 1]

Fatty acid binding protein 4 (FABP4, also known as aP2) is a cytoplasmic fatty acid chaperone expressed primarily in adipocytes and myeloid cells and implicated in the development of insulin resistance and atherosclerosis. Here we demonstrate that FABP4 triggers the ubiquitination and subsequent proteasomal degradation of peroxisome proliferator–activated receptor γ (PPARγ), a master regulator of adipogenesis and insulin responsiveness. Importantly, FABP4-null mouse preadipocytes as well as macrophages exhibited increased expression of PPARγ, and complementation of FABP4 in the macrophages reversed the increase in FABP4 expression. The FABP4-null preadipocytes exhibited a remarkably enhanced adipogenesis compared with wild-type cells, indicating that FABP4 regulates adipogenesis by downregulating PPARγ. We found that the FABP4 level was higher and PPARγ level was lower in human visceral fat and mouse epididymal fat compared with their subcutaneous fat. Furthermore, FABP4 was higher in the adipose tissues of obese diabetic individuals compared with healthy ones. Suppression of PPARγ by FABP4 in visceral fat may explain the reported role of FABP4 in the development of obesity-related morbidities, including insulin resistance, diabetes, and atherosclerosis.
DOI:10.1002/oby.20954URLPMID:25521833 [本文引用: 1]

Objective Fatty acid-binding protein 4 (FABP4) is expressed in adipocytes, and elevated plasma FABP4 level is associated with obesity-mediated metabolic phenotype. Postprandial regulation and secretory signaling of FABP4 has been investigated. Methods Time courses of FABP4 levels were examined during an oral glucose tolerance test (OGTT; n 65=6553) or a high-fat test meal eating ( n 65=6535). Effects of activators and inhibitors of adenyl cyclase (AC)-protein kinase A (PKA) signaling and guanylyl cyclase (GC)-protein kinase G (PKG) signaling on FABP4 secretion from mouse 3T3-L1 adipocytes were investigated. Results FABP4 level significantly declined after the OGTT or a high-fat meal eating, while insulin level was increased. Treatment with low and high glucose concentration or palmitate for 2 h did not affect FABP4 secretion from 3T3-L1 adipocytes. FABP4 secretion was increased by stimulation of lipolysis using isoproterenol, a β3-adrenoceptor agonist (CL316243), forskolin, dibutyryl-cAMP and atrial natriuretic peptide, and the induced FABP4 secretion was suppressed by insulin or an inhibitor of PKA (H-89), PKG (KT5823) or hormone sensitive lipase (CAY10499). Conclusions FABP4 is secreted from adipocytes in association with lipolysis regulated by AC-PKA- and GC-PKG-mediated signal pathways. Plasma FABP4 level declines postprandially, and suppression of FABP4 secretion by insulin-induced anti-lipolytic signaling may be involved in this decline in FABP4 level.
.
DOI:10.1016/S0163-7827(97)00003-9URLPMID:9373620 [本文引用: 1]

Prog Lipid Res. 1997 Mar;36(1):43-53. Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.; Review
URL [本文引用: 1]

动物体脂沉积所需要的脂肪酸大多由脂肪酸合成酶(fatty acid synthase,FAS)催化乙酰辅酶和丙二酸单酰辅酶合成,FAS是动物脂肪合成过程中的关键酶,其活性和表达的变化都会影响体内脂肪的合成。近年来对FAS的研究越来越受到重视,也取得了一些进展。本文对FAS的生理功能、组织表达和定位、发育性表达变化、日粮因素对其表达影响等几个方面的研究进展进行了综述。
URL [本文引用: 1]

动物体脂沉积所需要的脂肪酸大多由脂肪酸合成酶(fatty acid synthase,FAS)催化乙酰辅酶和丙二酸单酰辅酶合成,FAS是动物脂肪合成过程中的关键酶,其活性和表达的变化都会影响体内脂肪的合成。近年来对FAS的研究越来越受到重视,也取得了一些进展。本文对FAS的生理功能、组织表达和定位、发育性表达变化、日粮因素对其表达影响等几个方面的研究进展进行了综述。
DOI:10.1073/pnas.0603115103URLPMID:16717184 [本文引用: 1]

In animals, liver and white adipose are the main sites for the de novo fatty acid synthesis. Deletion of fatty acid synthase or acetyl-CoA carboxylase (ACC) 1 in mice resulted in embryonic lethality, indicating that the de novo fatty acid synthesis is essential for embryonic development. To understand the importance of de novo fatty acid synthesis and the role of ACCI-produced malonyl-CoA in adult mouse tissues, we generated liver-specific ACC1 knockout (LACC1KO) mice. LACCIKO mice have no obvious health problem under normal feeding conditions. Total ACC activity and malonyl-CoA levels were 70-75% lower in liver of LACCIKO mice compared with that of the WT mice. In addition, the livers of LACC1KO mice accumulated 40-70% less triglycerides. Unexpectedly, when fed fat-free diet for 10 days, there was significant up-regulation of PPAR and several enzymes in the lipogenic pathway in the liver of LACC1KO mice compared with the WT mice. Despite the significant up-regulation of the lipogenic enzymes, including a >2-fold increase in fatty acid synthase mRNA, protein, and activity, there was significant decrease in the de novo fatty acid synthesis and triglyceride accumulation in the liver. However, there were no significant changes in blood glucose and fasting ketone body levels. Hence, reducing cytosolic malonyl-CoA and, therefore, the de novo fatty acid synthesis in the liver, does not affect fatty acid oxidation and glucose homeostasis under lipogenic conditions.
