 ,*青海大学农林科学院 / 青海省农林科学院 / 青海省青稞遗传育种重点实验室 / 国家麦类改良中心青海青稞分中心, 青海西宁810016
,*青海大学农林科学院 / 青海省农林科学院 / 青海省青稞遗传育种重点实验室 / 国家麦类改良中心青海青稞分中心, 青海西宁810016Differentially expressed protein analysis of different drought tolerance hulless barley leaves
LI Jie**, FU Hui**, YAO Xiao-Hua, WU Kun-Lun ,*Academy of Agricultural and Forestry Sciences, Qinghai University / Qinghai Academy of Agricultural and Forestry Sciences / Qinghai Key Laboratory of Hulless Barley Genetics and Breeding / Qinghai Subcenter of National Hulless Barley Improvement, Xining 810016, Qinghai, China
,*Academy of Agricultural and Forestry Sciences, Qinghai University / Qinghai Academy of Agricultural and Forestry Sciences / Qinghai Key Laboratory of Hulless Barley Genetics and Breeding / Qinghai Subcenter of National Hulless Barley Improvement, Xining 810016, Qinghai, China通讯作者:
收稿日期:2020-08-05接受日期:2020-12-1网络出版日期:2021-07-12
| 基金资助: |
Received:2020-08-05Accepted:2020-12-1Online:2021-07-12
| Fund supported: |
作者简介 About authors
李洁, E-mail: lij_28257@163.com

摘要
关键词:
Abstract
Keywords:
PDF (532KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李洁, 付惠, 姚晓华, 吴昆仑. 不同耐旱性青稞叶片差异蛋白分析[J]. 作物学报, 2021, 47(7): 1248-1258. doi:10.3724/SP.J.1006.2021.01062
LI Jie, FU Hui, YAO Xiao-Hua, WU Kun-Lun.
干旱是影响面积最广、危害程度最深的非生物胁迫, 每年因干旱导致作物减产高达50%以上[1]。植物为适应干旱环境, 其叶片、根系等组织感受到胁迫后, 会通过信号转导级联反应, 转录激活、调节大量基因及蛋白质的转录与表达, 从而协助植物得以在逆境中存活[2,3,4]。因此, 绝大部分基因及其功能有待于在蛋白质层面予以揭示和阐述。
青稞(Hordeum vulgare L. var. nudum Hook. f.)属于禾本科小麦族大麦属普通大麦种, 因其内外颖壳分离, 籽粒裸露, 又称为裸大麦[5], 是青藏高原等高寒地区的主要作物。青稞主要种植在高寒缺氧、环境恶劣的地区, 在长期的适应性进化过程中产生了大量的优异抗逆基因, 具有耐寒、耐旱、耐瘠薄等优异种性[6]。在青海, 青稞种植区域多在海拔2700 m以上灌溉设施不能满足的高寒农区和农牧交错区, 其受旱时期主要发生在春季及夏季, 严重影响青稞幼苗分蘖的形成与籽粒灌浆, 最终导致籽粒不饱满以及减产等问题。因此选择耐旱青稞品种, 可以使广阔、干旱、贫瘠的土地最大资源化[5,7]。
目前对青稞抗逆基因[8,9,10]的研究已经有了一些报道, 但利用差异蛋白质研究方法筛选、鉴定相关耐旱基因在青稞抗逆研究中应用鲜见报道。本研究采用同位素标记相对和绝对定量(isobaric tags for relative and absolute quantification, iTRAQ)技术, 比较两种对水分胁迫敏感程度不同的青稞在干旱胁迫下叶片蛋白表达的差异, 进行定性定量分析, 旨在从蛋白水平揭示青稞品种间的耐旱差异, 为青稞耐旱研究提供一些方法和手段。
1 材料与方法
1.1 试验材料
根据之前28个青稞品种干旱胁迫筛选结果[5], 从中选用敏感与不敏感的2个青稞品种为供试青稞品种, 材料分别为旱地紫青稞(不敏感, HDZ)、大麻青稞(敏感, DM)。1.2 试验处理
材料采用玻璃温室盆栽限量供水种植, 每个花盆中放风干土6 kg, 种植前两天用水将花盆中的土壤浇透。选择籽粒光滑饱满的种子播种, 每盆播25粒, 出芽后每盆定苗20株, 温室温度22~25℃。待青稞植株长至三叶一心时处理, 设干旱胁迫与正常供水两种处理。干旱梯度形成前确保各盆土壤相对含水量基本达到饱和, 然后停止浇水让其自然干旱至设定标准, 每处理重复3次。在干旱处理第7天(土壤相对含水量为对照的65%)及第10天(土壤相对含水量为对照的45%)上午10:00, 收取长势一致的植株倒二叶同一部位叶片约0.5 g, 放置于-80℃超低温冰箱保存。1.3 生理指标的测量
在干旱处理第7天和第10天, 采植株叶片进行叶绿素含量、蛋白质含量、相对电导率及丙二醛含量等生理生化指标进行测定。叶绿素含量采用丙酮乙醇混合液法测定[11]; 蛋白质含量采用Bradford法测定[12]; 电导率采用电导率仪法测定, 以相对电导率(%)表示, 相对电导率 = (浸泡电导率值/煮沸后电导率值)×100%, 丙二醛(MDA)含量采用硫代巴比妥酸显色法测定[13]。1.4 叶片总蛋白质提取
采用TCA-丙酮沉淀-酚/SDS-联合抽提法提取青稞叶片总蛋白[14]。提取样品为干旱处理第10天2个青稞的叶片及正常培养对照叶片。总蛋白提取时, 预冷研磨器具及其他需要接触到样品的工具; 样品加入液氮研磨, 加入含0.2% DTT的10% TCA-丙酮(w/v) (-20℃预冷), 充分混合, -20℃沉淀2 h, 20,000×g, 4℃, 离心30 min; 弃上清, 取沉淀; 沉淀用含100 mmol L-1醋酸铵的80%甲醇溶液洗1次, 80%丙酮洗1次, 通风橱内室温干燥10 min; 加入10 mL酚/SDS抽提液振荡混匀温育5 min, 4℃, 20,000×g离心10 min, 转移上层酚相至干净新管中, 加入5倍体积的预冷的含100 mmol L-1醋酸铵的甲醇溶液, -20℃沉淀4 h或过夜, 20,000×g 离心10 min, 弃上清液, 沉淀中再加入预冷的80%甲醇和80%丙酮各一次, 悬起沉淀并充分清洗后将溶液吸出, 通风橱内自然风干, -80℃冰箱保存备用。10 mL酚/SDS抽提液: pH 8.0 Tris饱和酚与SDS缓冲液(30%蔗糖、2% SDS、5% β-巯基乙醇、0.1 mol L-1 pH 8.0 Tris-HCl)等量混合。1.5 蛋白质裂解与定量
采用Bradford[12]法对蛋白进行定量。称取20 mg蛋白质干粉加入200 μL裂解缓冲液, 30℃水浴1 h, 14℃、20,000×g离心30 min, 取上清液即为叶片总蛋白溶液, 测定蛋白浓度, 每个样品测量3个生物学重复。1.6 蛋白组学测定
按照美国AB Sciex公司生产的iTRAQ Reagent 8 Plex Multi-plex试剂盒说明书, 对2组共计12个蛋白质样品进行酶解与标记。混合标记后的样品用gemini NX (Phenomemex, 00F-4453-E0)色谱柱进行分级, 随后用1100系列高效液相色谱仪(Agilent公司, 美国)和Orbi-trap Elite质谱仪(Thero Scientific公司, 美国)进行液相分离和质谱分析[15,16], 此部分工作由上海维基生物科技有限公司承担。1.7 数据处理与分析
1.7.1 原始数据 根据鉴定蛋白质的FASTA格式序列文本, 利用Compute pI/Mw工具计算每一个蛋白质的分子量和等电点, 将定量及鉴定结果进行合并处理, 采用GO数据库的注释对鉴定蛋白质的功能进行分类。MS/MS数据通过搜索NCBI和EST数据库。搜索条件: 胰酶消化, 最大遗漏酶切位点1个, 肽段质量精确度1×10-3%, MS/MS 质量精确度±0.50 Da, 固定修饰Carbamidomethyl (C)。根据蛋白质谱搜库检索和差异蛋白筛选结果, 进行Blast分析。1.7.2 GO分析及KEGG代谢通路分析 所有鉴定差异表达蛋白通过ID号到Unipro数据库中批量获取对应的注释文件(*.dat) FASTA文件, 在Customize选项中选Geneontology链接到GO数据库, 得到每个蛋白的GO注释信息。将差异表达蛋白的FASTA格式文件上传到KOBAS2.0中, 选择map到KO中, 得到差异表达蛋白所对应的KO号, 根据所得到的KO号在KEGG ORTHOLOGY数据库中做代谢通路分析, 得到差异表达蛋白质作图到各个通路上的结果。
2 结果与分析
2.1 叶绿素含量
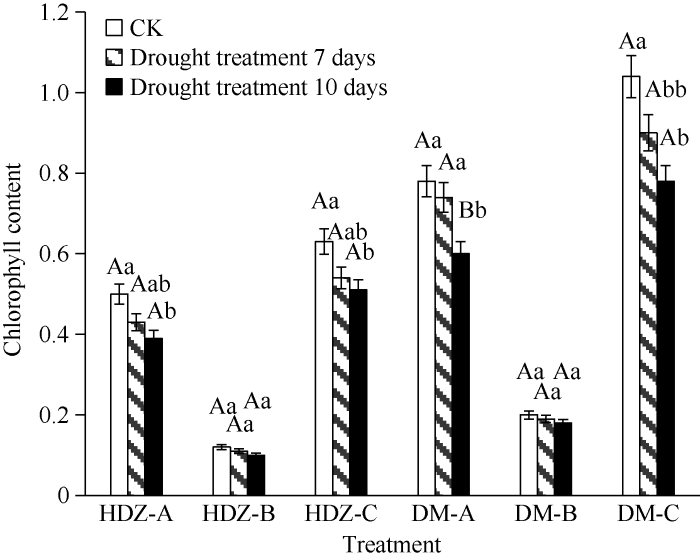
干旱胁迫处理对2个青稞品种的叶绿素含量产生了不同的影响(图1)。干旱胁迫后的2个品种的叶片叶绿素a、叶绿素b、总叶绿素含量与对照相比呈逐渐降低的趋势, 干旱胁迫时间越久, 叶绿素含量对比对照降低越多, 其中大麻青稞叶片叶绿素a、叶绿素b、总叶绿素含量的降低趋势更为明显, 说明在重度干旱胁迫时大麻青稞叶片的光合能力受到的抑制明显高于旱地紫青稞。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1HDZD-HDZCK和DMD-DMCK叶绿素含量变化
A: 叶绿素a; B: 叶绿素b; C: 总叶绿素。大写字母表示0.01极显著水平, 小写字母表示0.05显著水平。
Fig. 1Chlorophyll content of HDZD-HDZCK and DMD- DMCK
A: chlorophyll a; B: chlorophyll b; C: total chlorophyll. Uppercase letters indicate extremely significant differences at the 0.01 probability level, lowercase letters indicate significant differences at the 0.05 probability level.
2.2 可溶性蛋白质含量
干旱胁迫条件下, 旱地紫青稞和大麻青稞叶片可溶性蛋白质含量随干旱胁迫时间的延长呈逐渐降低的趋势(表1), 干旱胁迫第7天旱地紫青稞叶片可溶性蛋白质含量降低为对照的94.38%, 干旱胁迫第10天, 降低为对照的92.47%。大麻青稞叶片在干旱胁迫第7天可溶性蛋白含量降低为对照的78.70%, 干旱胁迫第10天, 降低为对照的76.50%, 降低趋势明显大于旱地紫青稞。推测可溶性蛋白与植物细胞的渗透调节有关, 高含量的可溶性蛋白可使细胞维持较低的渗透势, 抵抗水分胁迫带来的伤害, 但是当水分亏缺严重到影响了植物蛋白合成代谢时, 可溶性蛋白含量就会下降。由此可以看出, 旱地紫青稞比大麻青稞具有更强的抗渗透胁迫能力。2.3 相对电导率和丙二醛含量
干旱胁迫处理后, 旱地紫青稞叶片及大麻青稞叶片相对电导率和丙二醛含量均呈现增高趋势(表1)。但在相同干旱胁迫下大麻青稞叶片的相对电导率和丙二醛含量增加趋势更加明显, 证明其细胞膜遭到破坏更大, 导致膜透性增大, 电解质外渗更多, 同时膜脂过氧化程度更高, 释放出的丙二醛还会抑制蛋白合成, 从而增加细胞的渗透势。这些结果表明, 大麻青稞膜系统对干旱胁迫的耐受程度明显低于旱地紫青稞。Table 1
表1
表1干旱处理对青稞叶片蛋白质、相对电导率及丙二醛含量的影响
Table 1
| 处理 Treatment | 品种 Variety | 蛋白质含量 Protein content (mg g-1) | 相对电导率 Relative electrical conductivity (%) | 丙二醛含量 Malondialdehyde content (μmoL g-1) |
|---|---|---|---|---|
| 对照 CK | 旱地紫 HDZ | 7.30±0.10 Aa | 44.50±0.68 Cc | 32.92±0.45 Cc |
| 大麻 DM | 10.00±0.56 Aa | 50.10±0.34 Cc | 28.70±0.10 Cc | |
| 干旱处理7 d 7 days after drought treatment | 旱地紫 HDZ | 6.90±0.55 Aa | 45.89±0.47 Bb | 35.55±0.29 Bb |
| 大麻 DM | 7.87±0.12 Bb | 54.33±0.60 Bb | 32.21±0.38 Bb | |
| 干旱处理10 d 10 days after drought treatment | 旱地紫 HDZ | 6.75±0.26 Aa | 48.97±0.71 Aa | 38.92±0.42 Aa |
| 大麻 DM | 7.65±0.61 Bb | 60.22±0.39 Aa | 40.33±0.48 Aa |
新窗口打开|下载CSV
2.4 蛋白鉴定
分别对干旱处理10 d (HDZD)和正常水分培养(HDZCK)的旱地紫青稞叶片及干旱处理10 d (DMD)和正常水分培养(DMCK)的大麻青稞叶片, 采用iTRAQ技术进行蛋白质的定性定量工作, 共鉴定出蛋白质及多肽4163个。由表2可知, 肽段覆盖率在10%以下的蛋白占鉴定总蛋白的30.55%, 肽段覆盖率在10%~20%的蛋白占鉴定总蛋白的37.45%, 肽段覆盖率在20%~30%以上的蛋白占鉴定总蛋白的19.19%, 肽段覆盖率在30%~40%以上的蛋白占鉴定总蛋白的8.82%, 肽段覆盖率在40%以上的蛋白占鉴定总蛋白的4.01%, HDZD- HDZCK比较组叶片经过质谱分析共测出蛋白质及多肽共2150个, DMD- DMCK比较共测出蛋白质及多肽2013个。Table 2
表2
表2鉴定蛋白的覆盖率分布
Table 2
| 比较组 Comparison group | 肽段覆盖率 Protein coverage (%) | 总计 Total | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤10 | 10<X≤20 | 20<X≤30 | 30<X≤40 | 40<X≤50 | 50<X≤60 | 60<X≤70 | ||
| HDZD-HDZCK | 650 | 807 | 409 | 192 | 64 | 18 | 8 | 2150 |
| DMD-DMCK | 622 | 752 | 390 | 170 | 55 | 16 | 6 | 2013 |
新窗口打开|下载CSV
2.5 差异表达蛋白
根据实验条件筛选并注释了4163个差异蛋白, 对每个蛋白质的差异倍数以2为底数取其对数, 以此作为横坐标, 用P值以10为底取其对数的绝对值为纵坐标, 做出差异蛋白的火山图(图2)。结果显示, 2个品种青稞的差异表达蛋白总数是184个, 其中, HDZD-HDZCK比较组中表达上调的蛋白68个, 表达下调的蛋白63个, 共131个。蛋白含量相差4.0倍以上的有4个, 其中最大为7.495倍。含量相差3.0倍以上的有11个, 相差3.0~2.0倍的有17个, 相差1.9~1.0倍有31个。在DMD-DMCK比较组中表达上调蛋白21个, 表达下调蛋白32个, 共53个。蛋白含量相差3.0倍以上的有1个, 含量相差2.9~2.0倍2个, 含量相差1.9~1.0倍有13个。图2
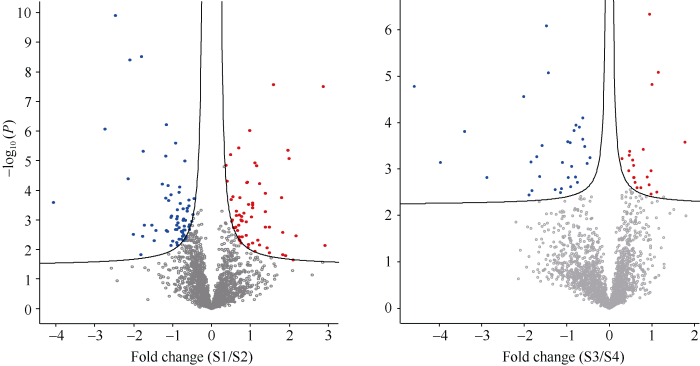 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2HDZD-HDZCK青稞(左)和DMD-DMCK青稞(右)差异表达蛋白火山图
Fig. 2Differentially expressed protein volcano map of HDZD-HDZCK barley (left) and DMD-DMCK barley (right)
2.6 差异表达蛋白的生物信息功能分析
2.6.1 青稞差异蛋白GO富集分析 对差异蛋白主要功能进行GO功能注释, 分析结果如图3, 差异蛋白的生物学功能覆盖范围分布较广。HDZD-HDZCK对照组中, 分子功能类占比66%, 主要涉及催化活性及结合等功能; 生物过程类占比17%, 主要涉及生物合成过程、细胞过程以及代谢过程; 细胞组成类占比17%, 主要涉及细胞膜、细胞器以及大分子复合物等细胞组分。DMD-DMCK比较组中, 分子功能类占比71%, 主要涉及催化活性及结合功能; 生物过程类占比14%, 主要涉及单一生物合成过程、代谢过程以及脂质转运过程等; 细胞组成类占比15%, 主要由细胞器、细胞膜、大分子复合物以及胞质溶胶四部分组成。HDZD-HDZCK和DMD-DMCK的差异蛋白共有184个, 对其行使的功能进行分类, 可以分为12类(表3), 分属于碳水化合物和能量代谢类、蛋白质生物合成类、氧化还原类、氨基酸转运与代谢类、蛋白质代谢类、应激与防御类、电子载体类等。图3
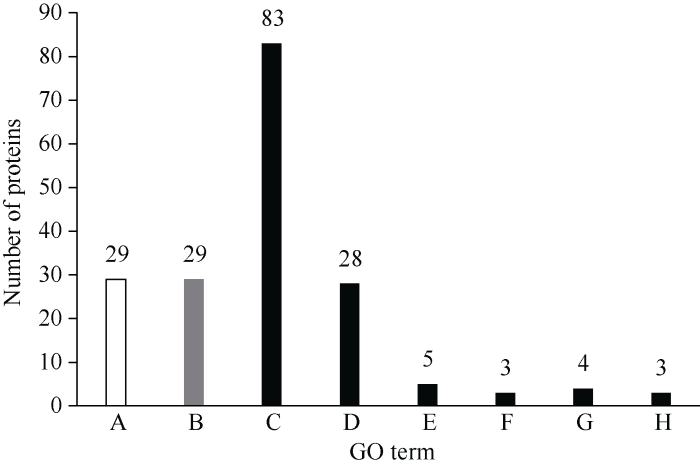 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3GO富集柱状图
A: 属于生物过程(BP); B: 属于细胞组分(CC); C、D、E、F、G属于分子功能(MF); A: 防御响应; B: 胞外部分; C: 催化活性; D: ATP结合; E: 结构分子活性; F: 转移活性; G: 运输活性; H: 其他活性。
Fig. 3GO enrichment of differentially expressed proteins
A: biological process (BP), B: cell component (cc), and C-G: molecular functions (MF). A: defense response; B: extracellular part; C: catalytic activity; D: ATP binding; E: structural molecule activity; F: transfer activity; G: transport activity; H: other activities.
Table 3
表3
表3HDZD-HDZCKA和DMD-DMCK差异表达蛋白功能分类
Table 3
| 蛋白质功能分类 Functional classification of proteins | 蛋白质数量 Number of proteins |
|---|---|
| 胁迫应答类 Stress response | 11 |
| 脂类代谢相关类 Lipid metabolism | 13 |
| 糖类代谢相关类 Carbohydrate metabolism | 10 |
| 蛋白质代谢相关类 Protein metabolism | 48 |
| 氧化还原类 Oxidoreduction | 35 |
| 能量供应类 Energy | 16 |
| 光合相关类 Photosynthetic | 15 |
| 未知蛋白 Unknown functional protein | 36 |
新窗口打开|下载CSV
2.6.2 差异表达蛋白的KEGG分析 通过对差异蛋白进行KEGG分析, 两组对照注释蛋白通路见图4。其中占比最大的3条通路分别是代谢通路、氨基酸的生物合成以及次级代谢产物的生物合成。差异表达蛋白主要涉及13条代谢通路, 包括柠檬酸循环、光合作用、半胱氨酸和蛋氨酸等通路。其中表达上调和下调蛋白共同参与的途径有6条, 半胱氨酸和蛋氨酸代谢通路在干旱胁迫的2个品种中都表现为表达上调。在HDZD-HDZCK中, 与呼吸和光合作用相关的通路均显著上调, 其中包括三羧酸循环(TCA)、碳代谢、丙酮酸代谢、乙醛酸和二羧酸的代谢以及光合作用中的碳固定通路; 在DMD-DMCK中, 关于蛋白质的合成通路均显著上调, 其中包括核糖体组装、RNA转运、内质网中的蛋白质加工以及可提供能量的氧化磷酸化通路。
图4
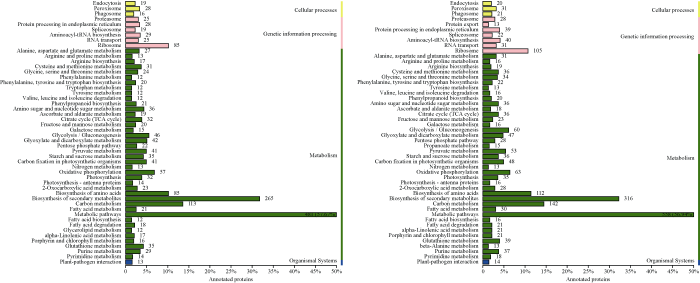 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4HDZD-HDZCK(左)和DMD-DMCK(右)差异蛋白KEGG注释
Fig. 4KEGG annotation of differentially expressed proteins of HDZD-HDZCK (left) and DMD-DMCK (right)
Table 4
表4
表4显著差异蛋白的KEGG通路
Table 4
| 通路ID Pathway ID | 通路名称 Pathway name |
|---|---|
| ko00020 | 三羧酸循环 TCA cycle |
| ko00640 | 丙酸酯代谢 Propanoate metabolism |
| ko01200 | 碳代谢 Carbon metabolism |
| ko00270 | 半胱氨酸和蛋氨酸代谢 Cysteine and methionine metabolism |
| ko00620 | 丙酮酸代谢 Pyruvate metabolism |
| ko00630 | 乙醛酸和二羧酸的代谢 Glyoxylate and dicarboxylate metabolism |
| ko00710 | 光合作用中的碳固定 Carbon fixation in photosynthetic |
| ko00195 | 光合作用 Photosynthsis |
| ko03010 | 核糖体 Ribosome |
| ko03013 | RNA转运 RNA transport |
| ko00190 | 氧化磷酸化 Oxidaxtive phosphorylation |
| ko04145 | 吞噬作用 Phagosome |
| ko04141 | 内质网的蛋白质加工 Protein processing in endoplasmic reticulum |
新窗口打开|下载CSV
3 讨论
本研究以2份干旱胁迫耐受性不同的青稞品种(旱地紫青稞和大麻青稞)为研究材料, 对其叶绿素含量、可溶性蛋白含量等进行了生理指标的测定, 其次采用同位素标记相对和绝对定量(iTRAQ)技术, 分析了干旱胁迫后的叶片全蛋白组的差异表达。3.1 干旱处理对青稞叶绿素含量的影响
在干旱处理7 d和10 d后, 旱地紫青稞和大麻青稞叶绿素a、b及叶绿素总量对比对照均呈现为持续下降的趋势, 与之前对马铃薯[17]、文冠果[18]等研究结果不同, 没有出现抗旱性强的植物叶绿素含量随着胁迫时间的延长呈现先上升再下降的趋势, 可能是由于不同植物内叶绿素响应干旱胁迫的机制存在差异, 但大麻青稞叶片叶绿素a、叶绿素b、总叶绿素含量的降低趋势更为明显, 证明在重度干旱胁迫时大麻青稞叶片的光合作用能力受到的抑制高于旱地紫青稞。干旱组的2个品种的叶绿素a含量较对照组比叶绿素b含量下降趋势明显, 说明重度干旱胁迫会造成叶绿素分解, 叶绿素含量降低, 并且叶绿素a相比较叶绿素b对干旱胁迫更加敏感, 这与对徐银萍等对大麦干旱胁迫后叶片的叶绿素研究结果一致[19]。3.2 干旱处理对青稞渗透调节物质的影响
植物受到干旱胁迫时, 会产生与抗逆活动相关的蛋白质并会水解一些结构蛋白以维持正常的生理需要, 导致细胞内可溶性蛋白含量增加[20]。在正常生理环境下, 旱地紫青稞体内的可溶性蛋白质含量低于大麻青稞。随着干旱胁迫时间的延长, 2个品种青稞内的可溶性蛋白含量均表现出先快后慢的下降趋势, 大麻青稞蛋白质含量的减少值和降低比例始终大于旱地紫青稞, 这与对甘薯研究结果相一致[21]。相对电导率在衡量植物细胞膜稳定性中具有重要作用。在正常生理环境下, 旱地紫青稞的相对电导率低于大麻青稞的相对电导率。随着干旱胁迫时间的延长, 两个品种青稞的相对电导率均表现为先慢后快的增加趋势, 这说明干旱胁迫破坏了细胞膜的稳定性, 大量溶质渗出, 导致相对电导率增大, 这种现象在易家宁等[22]对紫苏以及邓辉茗等[23]对绵毛水苏幼苗的水分胁迫的研究中都得到了印证。
丙二醛含量主要反映细胞膜脂过氧化的程度, 含量越高细胞膜脂过氧化程度越高, 细胞膜稳定性越差[24]。在正常生理环境下, 旱地紫青稞的丙二醛含量高于大麻青稞。随着干旱胁迫时间的延长, 前者的丙二醛含量持续上升, 而大麻青稞的丙二醛含量的增加呈现出先慢后快的趋势, 并且在干旱处理10 d的时候, 其丙二醛含量超过了旱地紫青稞。由此暗示, 旱地紫青稞中的细胞膜能维持相对的稳定性, 受干旱影响较小。
3.3 干旱处理对青稞蛋白表达的影响
本研究共筛选到184个差异表达蛋白, 其中上调表达蛋白89个, 下调表达蛋白95个。旱地紫青稞和大麻青稞表达差异量最高的蛋白质分别是异柠檬酸裂解酶和β-1,3-葡聚糖酶, 上调倍数分别是16.64和24.03; 最显著下调的蛋白质是DUF3700 domain-containing protein以及DUF3479 domain-containing protein, 下调倍数分别为7.69和2.25。Δ1-吡咯啉-5-羧酸合成酶(P5CS), 脱水蛋白及BURP domain-containing protein等蛋白质在2个品种的青稞中的表达量均上调, 同时脂氧合酶、类萌发蛋白等蛋白质也出现不同程度的差异表达。异柠檬酸裂解酶在旱地紫青稞中上调表达倍数最大, 为16.64倍。异柠檬酸裂解酶是乙醛酸循环体中特有的酶, 可将异柠檬酸裂解为琥珀酸和乙醛酸。后期生成的草酰乙酸可参加机体内的能量代谢过程或生成其他代谢物质, 乙醛酸循环可将油脂转化为糖类[25]。目前有关异柠檬酸裂解酶在非生物胁迫下的作用研究还较少, 而本实验中在旱地紫青稞中异柠檬酸酸裂解酶含量显著上调, 其作用可能是在干旱胁迫下为机体供能以维持正常的生理代谢, 防止植物受到损害, 同时可能是利用乙醛酸循环将油脂转化为糖类, 可增强细胞的渗透调节。
在大麻青稞中受干旱胁迫上调表达倍数最高的蛋白为β-1,3-葡聚糖酶, 上调24.03倍, 其隶属于糖基水解酶, 参与植物体的器官发育、果实成熟及衰老等重要生命活动[26]。之前的研究结果显示, β-1,3-葡聚糖酶可提高植物的抗病性[27,28]、还可受到低温胁迫[29]、水杨酸、乙烯等外源植物激素的诱导, 增强植物的抗性。徐小萍等[29]对三明野生蕉低温处理发现该酶对低温的响应活性不仅存在时间的临界点, 还存在温度临界点, 在短时间内对低温响应强烈, 且仅在某一临界温度下响应低温胁迫, 是一种低温保护酶[30]。在其他非生物胁迫中, β-1,3-葡聚糖酶的研究还知之甚少, 尤其是在干旱胁迫下, 发挥怎样的作用及其作用机理, 也是需要后期着重研究的内容。
Δ1-吡咯啉-5-羧酸合成酶(P5CS), 在旱地紫青稞和大麻青稞的干旱组中表现为表达量上调, 上调倍数分别是2.40和2.15, 在旱地紫青稞的表达量略高。P5CS是合成脯氨酸的关键酶, 而脯氨酸是植物内一种重要的渗透调节物质, 在非生物胁迫下发挥重要的作用[31]。前人已对烟草[32]、菜豆[33]、枸杞[34]、碱蓬[35]中的P5CS基因进行研究, 发现其在干旱胁迫、盐胁迫、低温胁迫以及ABA胁迫处理后表达上调、抗性增强。碱蓬的SgP5CS基因导入拟南芥并过量表达, 干旱胁迫后, 发现游离脯氨酸含量增加, 丙二醛的含量显著降低, 拟南芥抗旱性增加, 说明P5CS是一种受干旱胁迫的正调控的蛋白[35]。
DUFs虽然是一类功能还未研究透彻的蛋白家族, 但经研究发现DUFs在植物正常生长发育、生物胁迫防御以及响应环境逆境危害等方面发挥着至关重要的作用[36]。在不同作物中, DUFs受到不同非生物胁迫的调控。拟南芥的DUF6对盐、干旱胁迫敏感[37]; 玉米的DUF642受酸铝胁迫调控[38]; 小麦的DUF860响应热胁迫[39]; 沙冬青的DUF1517具有一定的抗寒作用[40]; 水稻中的DUF则受到干旱胁迫调控[41]。大量实验证据表明, DUFs受到非生物胁迫的正调控, 但在本次实验中, 受到干旱胁迫的旱地紫青稞和大麻青稞的DUF3700和DUF3479蛋白均呈下调表达, 下调倍数分别是7.69倍和2.22倍, 这与现有的研究报道结果不相一致, 可能与在青稞中的其他调控网络有关, 还需要后续在其他组学中进行比对分析其原因。
DHNs是LEA蛋白第二家族成员, 因其C端有一段保守的K片段, 在植物失水时可以形成α-螺旋, 以防止细胞膜受损以及细胞内蛋白质变形[42]。DHN5在‘旱地紫’青稞和DHN8在‘大麻’青稞的干旱组中均表现为表达量上调, 分别上调2.89倍和15.65倍。DHNs虽均属于同一个家族, 但其功能及作用机理不尽相同[43], 有主要受干旱和ABA诱导表达的, 有被低温和寒冷诱导型, 也有与植物抗旱和抗旱相关型, 在植物中多表现为组成型行使其抗逆功能。在小麦中, 表现为对干旱和盐胁迫有响应[44,45], 对冷胁迫下的大麦也表现出有增强其抗逆性的作用[46], 在拟南芥中过表达的DHN5还可参与到JA信号转导中, 下调3个JA信号转导负调控因子JAZ家族成员的蛋白表达, 从而增强其对植物病原菌的防御作用[47]。在早稻中的DHN8可受干旱和高盐诱导表达, 但不受低温诱导表达。DHN5及DHN8在这两种青稞种质中抵御胁迫时的作用机理可能存在差异, 且其表达量上调倍数相差悬殊, 但具体机理不明, 这是后续需要着重研究的部分。
脂氧合酶(LOX)催化以亚油酸、亚麻酸等多元不饱和脂肪酸为底物的膜脂过氧化反应, 生成氢过氧化物, 破坏膜的完整性[48]。马明杰等[49]通过对冷敏性蔬菜青椒低温处理, 发现LOX活性增加, 说明LOX对低温敏感, 活性增强后破坏膜脂从而破坏膜的完整性, 导致青椒的冷敏性。王成慧[50]通过转基因手段将甜瓜CmLOX08转入拟南芥, 并构建沉默植株以及过表达植株, 进行干旱处理后发现过表达植株LOX活性升高, 过氧化氢含量降低, 减少了氧化伤害, 增强了植株的抗旱性; 而沉默植株LOX活性降低, 过氧化氢积累, 对干旱胁迫敏感。孙婷婷等[51]将甘蔗的ScLOX1基因导入本氏烟草中, 在氯化钠和PEG处理后, 该基因表达量显著上升, 增强了植物的抗旱性。本次实验中, LOX的活性在旱地紫青稞中表达下调, 在大麻青稞中相反, 说明在大麻青稞中LOX的活性更容易受到干旱胁迫的调控, 推测与大麻青稞是干旱敏感型有关。通过比较二者干旱第7天与第10天的电导率结果发现, 这与干旱胁迫下大麻青稞的电导率高于同等处理时间旱地紫青稞变化的生理状态一致。
类萌发素蛋白(GLPs)是一种植物体内普遍存在的可溶性糖蛋白, 既可以作为酶和结构蛋白催化调控植物体内生理反应, 也可作为受体参加信号转导[52]。GLPs在两个品种青稞中的表达结果相反, 在旱地紫青稞中下调表达, 下调1.92倍; 在大麻青稞中表达上调, 上调倍数是2.19。之前的研究发现, GLPs既可以通过介导细胞壁增厚来阻止病原菌入侵响应生物胁迫, 也可以对盐胁迫、干旱胁迫、低温胁迫[53]以及重金属[54]等非生物胁迫做出具有组织特异性的应答。Hurkman等[55]在研究盐胁迫大麦幼苗时发现, 在大麦幼苗受到盐胁迫时, 仅在根部特异性上调GLPs表达。本次实验GLPs的差异表达说明旱地紫青稞中类萌发蛋白对干旱胁迫的敏感程度低于大麻青稞, 这也可能与旱地紫青稞的耐旱性强于大麻青稞有关。
4 结论
干旱胁迫对青稞的生长和代谢具有一定的破坏, 从而导致青稞减产减量。干旱胁迫致使青稞叶片细胞膜稳定性下降, 膜脂过氧化程度升高, 渗透调节物质大量积累, 叶绿素含量降低, 影响了青稞的正常生理代谢过程。异柠檬酸裂解酶、β-1,3-葡聚糖酶、Δ1-吡咯啉-5-羧酸合成酶、类萌发素蛋白等代谢物也出现显著差异表达的现象, 通过表达的上调或下调, 增加青稞的抗逆性, 使其在干旱环境中足以存活且保证一定的产量。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1006/abio.1976.9999URLPMID:942051 [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00216-012-5918-6URLPMID:22451173 [本文引用: 1]

The iTRAQ (isobaric tags for relative and absolute quantification) technique is widely employed in proteomic workflows requiring relative quantification. Here, we review the iTRAQ literature; in particular, we focus on iTRAQ usage in relation to other commonly used quantitative techniques e.g. stable isotope labelling in culture (SILAC), label-free methods and selected reaction monitoring (SRM). As a result, we identify several issues arising with respect to iTRAQ. Perhaps frustratingly, iTRAQ's attractiveness has been undermined by a number of technical and analytical limitations: it may not be truly quantitative, as the changes in abundance reported will generally be underestimated. We discuss weaknesses and strengths of iTRAQ as a methodology for relative quantification in the light of this and other technical issues. We focus on technical developments targeted at iTRAQ accuracy and precision, use of 4-plex over 8-plex reagents and application of iTRAQ to post-translational modification (PTM) workflows. We also discuss iTRAQ in relation to label-free approaches, to which iTRAQ is losing ground.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.2478/s11658-006-0044-0URLPMID:16983453 [本文引用: 1]

Dehydrins (DHNs) are part of a large group of highly hydrophilic proteins known as LEA (Late Embryogenesis Abundant). They were originally identified as group II of the LEA proteins. The distinctive feature of all DHNs is a conserved, lysine-rich 15-amino acid domain, EKKGIMDKIKEKLPG, named the K-segment. It is usually present near the C-terminus. Other typical dehydrin features are: a track of Ser residues (the S-segment); a consensus motif, T/VDEYGNP (the Y-segment), located near the N-terminus; and less conserved regions, usually rich in polar amino acids (the Phi-segments). They do not display a well-defined secondary structure. The number and order of the Y-, S-and K-segments define different DHN sub-classes: Y(n)SK(n), Y(n)Kn, SK(n), K(n) and K(n)S. Dehydrins are distributed in a wide range of organisms including the higher plants, algae, yeast and cyanobacteria. They accumulate late in embryogenesis, and in nearly all the vegetative tissues during normal growth conditions and in response to stress leading to cellular dehydration (e.g. drought, low temperature and salinity). DHNs are localized in different cell compartments, such as the cytosol, nucleus, mitochondria, vacuole, and the vicinity of the plasma membrane; however, they are primarily localized to the cytoplasm and nucleus. The precise function of dehydrins has not been established yet, but in vitro experiments revealed that some DHNs (YSK(n)-type) bind to lipid vesicles that contain acidic phospholipids, and others (K(n)S) were shown to bind metals and have the ability to scavenge hydroxyl radicals [Asghar, R. et al. Protoplasma 177 (1994) 87-94], protect lipid membranes against peroxidation or display cryoprotective activity towards freezing-sensitive enzymes. The SK(n)-and K-type seem to be directly involved in cold acclimation processes. The main question arising from the in vitro findings is whether each DHN structural type could possess a specific function and tissue distribution. Much recent in vitro data clearly indicates that dehydrins belonging to different subclasses exhibit distinct functions.
URLPMID:20460710 [本文引用: 1]
DOI:10.1007/s00299-007-0412-xURLPMID:17641860 [本文引用: 1]

Late Embryogenesis Abundant (LEA) proteins are associated with tolerance to water-related stress. A wheat (Triticum durum) group 2 LEA proteins, known also as dehydrin (DHN-5), has been previously shown to be induced by salt and abscisic acid (ABA). In this report, we analyze the effect of ectopic expression of Dhn-5 cDNA in Arabidopsis thaliana plants and their response to salt and osmotic stress. When compared to wild type plants, the Dhn-5 transgenic plants exhibited stronger growth under high concentrations of NaCl or under water deprivation, and showed a faster recovery from mannitol treatment. Leaf area and seed germination rate decreased much more in wild type than in transgenic plants subjected to salt stress. Moreover, the water potential was more negative in transgenic than in wild type plants. In addition, the transgenic plants have higher proline contents and lower water loss rate under water stress. Also, Na(+) and K(+) accumulate to higher contents in the leaves of the transgenic plants. Our data strongly support the hypothesis that Dhn-5, by its protective role, contributes to an improved tolerance to salt and drought stress through osmotic adjustment.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1023/a:1006123432157URLPMID:10092181 [本文引用: 1]

Germins and germin-like proteins (GLPs) constitute a ubiquitous family of plant proteins that seem to be involved in many developmental and stress-related processes. Wheat germin has been extensively studied at the biochemical level: it is found in the apoplast and the cytoplasm of germinating embryo cells and it has oxalate oxidase activity (EC 1.2.3.4). Germin synthesis can also be induced in adult wheat leaves by auxins and by a fungal pathogen but it remains to be determined whether the same gene is involved in developmental, hormonal and stress response. In this work, we have studied the expression of one of the wheat germin genes, named gf-2.8, in wheat as well as in transgenic tobacco plants transformed with either this intact gene or constructs with GUS driven by its promoter. This has allowed us to demonstrate that expression of this single gene is both developmentally and pathogen-regulated. In addition, we show that expression of the wheat gf-2.8 germin gene is also stimulated by some abiotic stresses, especially the heavy metal ions Cd2+, Cu2+ and Co2+. Several chemicals involved in stress signal transduction pathways were also tested: only polyamines were shown to stimulate expression of this gene. Because regulation of the wheat gf-2.8 germin gene is complex and because its product results in developmental and stress-related release of hydrogen peroxide in the apoplast, it is likely that it plays an important role in several aspects of plant growth and defence mechanisms.
DOI:10.1104/pp.97.1.366URLPMID:16668394 [本文引用: 1]

The 26 kilodalton, isoelectric point 6.3 and 6.5 (Gs1 and Gs2) polypeptides that increase in barley (Hordeum vulgare L.) roots during salt stress were isolated and identified. Both Gs1 and Gs2 had high sequence similarity to germin, a protein that increases significantly in germinating wheat seeds. Like germin, Gs1 and Gs2 were resistant to proteases and were glycosylated. Immunoblots were probed with antibodies to Gs1 and Gs2 to determine the distribution of these polypeptides among organs and cell-free fractions. Gs1 and Gs2 were present in roots and coleoptiles, but absent from leaves. In roots, Gs1 and Gs2 were present in the mature region, but not the tip. Gs1 and Gs2 increased in roots, but decreased in coleoptiles in response to salt stress. Gs1 and Gs2 were distributed among the soluble, microsomal, and cell wall fractions of roots, but the majority of Gs1 and Gs2 was present in the soluble fraction. Although Gs1 and Gs2 were heat stable, their synthesis was not affected by abscisic acid treatment. Gs2 accumulated during abscisic acid treatment, whereas Gs1 did not. However, a 25.5 kilodalton, isoelectric point 6.1 polypeptide that was immunologically related to Gs1 did accumulate with abscisic acid treatment.
