 ,, 梁光伟, 贺亚军
,, 梁光伟, 贺亚军 ,*, 钱伟西南大学农学与生物科技学院, 重庆 400716
,*, 钱伟西南大学农学与生物科技学院, 重庆 400716QTL mapping of salt and drought tolerance related traits in Brassica napus L.
MENG Jiang-Yu ,, LIANG Guang-Wei, HE Ya-Jun
,, LIANG Guang-Wei, HE Ya-Jun ,*, QIAN WeiCollege of Agronomy and Biotechnology, Southwest University, Chongqing 400716, China
,*, QIAN WeiCollege of Agronomy and Biotechnology, Southwest University, Chongqing 400716, China通讯作者:
收稿日期:2020-02-16接受日期:2020-10-14网络出版日期:2021-03-12
| 基金资助: |
Received:2020-02-16Accepted:2020-10-14Online:2021-03-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3491KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
蒙姜宇, 梁光伟, 贺亚军, 钱伟. 甘蓝型油菜耐盐和耐旱相关性状的QTL分析[J]. 作物学报, 2021, 47(3): 462-471. doi:10.3724/SP.J.1006.2021.04034
MENG Jiang-Yu, LIANG Guang-Wei, HE Ya-Jun, QIAN Wei.
油菜是重要的食用油料作物之一。然而, 我国当前油菜的增产率并不能满足人们对食用油不断增长的消费需求。因此, 油菜产量需要大幅的提升[1]。盐胁迫和干旱胁迫是非生物胁迫中危害农业生产的重要因素。世界盐渍土面积约10亿公顷[2], 受环境及气候变化影响, 其面积仍在不断扩大[3], 这不仅限制了农业土地的利用, 而且影响了油菜的产量[4]。无独有偶, 干旱也严重影响了油菜的正常生长和产量。我国每年旱灾受灾面积约为1325万公顷, 长江流域油菜播种期干旱尤为严重, 油菜播种后, 常常会遇到长达10~15 d的秋旱[5]。因此, 培育耐盐、耐旱油菜品种, 通过对大量具有农业潜力的盐渍地和旱灾发生地加以利用来提高油菜产量, 对保障国家粮油安全具有重要意义。
耐盐性和耐旱性是非常复杂的性状, 为多基因控制的数量性状[6]。通过利用分子标记和QTL作图, 有关油菜耐盐性和耐旱性的QTL定位研究已取得了一些进展。荐红举等[6]利用甘蓝型黄籽油菜GH06和黑籽油菜P174为亲本构建的重组自交系群体, 检测到11个与盐胁迫下发芽率相关的QTL, 分布在A01、A03、A07、A09、C06染色体上, 可解释4.90%~10.92%的表型变异; 同样利用这个重组自交系群体, 侯林涛等[7]检测到盐胁迫下与叶干重、根鲜重相关的QTL各3个, 分布在A02、A04、C03染色体上, 可解释7.16%~16.15%的表型变异。利用甘蓝型油菜耐盐品种2205和盐敏品种1423作为亲本构建的F2:3群体, Lang等[8]鉴定了与耐盐相关的10个性状, 检测到45个耐盐相关性状QTL, 这些QTL分别解释4.80%~51.14%的表型变异, 其中A05连锁群上的主效QTL qSPAD5区间内的候选基因Bra003640可能与耐盐性相关; Zhang等[9]利用这个遗传群体, 在A10染色体的15.70 Mb ~15.82 Mb发现1个主效QTL, 解释36.0%的表型变异, 在这个区间里找到一个候选基因Bra009510可能与叶片形态发育和耐盐性有关。除了传统QTL定位研究, 近年来, 利用全基因组关联分析等方法也发现了一些控制耐盐性的遗传因素。张蕊等[10]用317份甘蓝型油菜自交系材料, 利用油菜60K SNP芯片做全基因组关联分析, 检测到45个与耐盐性显著关联的SNP, 其中40个与下胚轴长度显著关联, 5个与根长显著关联, 单个SNP解释的表型变异分别为9.12%~14.46%和7.67%~8.93%。贺亚军等[11]对307个不同品系的甘蓝型油菜进行发芽试验, 结合油菜60K SNP芯片做GWAS, 共检测到225个与耐盐性状显著关联的SNP位点, 其中164个与根长显著关联, 23个与鲜重显著关联, 38个与发芽率显著关联, 其中与根长、鲜重、发芽率最显著关联的SNP位点分别位于A08、A02和A06染色体, 贡献率分别为23.84%、18.59%和31.81%。Yong等[12]用85个油菜自交系, 鉴定了与耐盐性相关的62个QTL, 并发现候选基因BnaaTSN1与甘蓝型油菜耐盐性显著相关。Wan等[13]从368份甘蓝型油菜的GWAS结果发现25个与耐盐相关的QTL, 分别解释4.21%~9.23%的表型变异, 找到与耐盐相关的38个候选基因。
在油菜耐旱QTL定位研究方面, 李真等[14]利用甘蓝型油菜DH群体的5个生长性状检测到50个与油菜耐旱的相关QTL。杨玉恒等[15]在正常灌溉和干旱胁迫2种环境下对14个耐旱性状和5个性状的耐旱系数进行QTL分析, 共定位到123个QTL, 解释的表型变异在4.07%~18.20%。王丹丹等[16]利用重组自交系F2:4家系, 在正常灌溉和干旱胁迫2种环境条件下, 对5个耐旱相关性状及其耐旱系数进行了QTL分析, 共检测到8个QTL, 单个位点解释的表型变异为6.6%~12.4%。荐红举等[6]用20%的PEG-6000溶液模拟干旱胁迫, 构建重组自交系高密度SNP遗传图谱, 找到8个与耐旱相关的QTL位点, 分布在A01、A03、A06、A09染色体上, 可解释的表型变异为3.84%~6.90%。Fletcher等[17]对干旱条件下甘蓝型油菜DH群体根系性状、开花期及产量进行QTL分析, 共检测到20个QTL, 主要集中在A10和C02连锁群上的2个QTL区段, 进一步分析表明, 这2个QTL区段通过直接影响根系来间接影响开花期。接着, Fletcher等[18]又用重测序的方法发现1个新的紧密连锁的标记, 其插入缺失位点刚好在基因Bna.FLC.A10上。许军红等[19]对苗期耐旱相关性状进行QTL初步定位, 检测到12个耐旱相关QTL, 分别位于A01、C02、A03、A08、C09染色体上, 可解释7.0%~24.3%的表型变异。黄倩等[20]利用一个甘蓝型油菜F2:3群体在抽薹期进行耐旱性鉴定和QTL分析, 共检测到28个QTL, 分别分布在14个连锁群上, 可解释1.1%~36.6%的表型变异。
尽管前人已分别检测了油菜耐盐和耐旱相关的许多QTL位点, 然而, 以往的研究很少在同一群体同时对耐盐和耐旱相关位点进行检测和比较分析。本研究利用同一个群体, 在盐胁迫和干旱胁迫处理下, 考察发芽率、根长、整株鲜重这3个耐盐、耐旱相关性状, 对这2种非生物胁迫下的相关QTL同时进行检测和比较分析, 旨在发现控制油菜耐盐和耐旱性共同的QTL位点, 为油菜耐盐、耐旱分子标记辅助育种提供更多标记信息, 同时, 为今后研究油菜在应对不同胁迫环境的相同或特异的响应机制提供一定的遗传信息。
1 材料与方法
1.1 试验材料
本研究选用的2个亲本分别是德国冬性甘蓝型油菜品种Express和中国半冬性甘蓝型油菜品种SWU07, 二者不仅在生态类型和地理来源上存在明显差异, 而且, 在盐胁迫和干旱胁迫下, 两亲本间的发芽率、根长和鲜重存在显著差异。将两亲本杂交, 获得杂种F1再通过花药培养和染色体加倍, 得到包含261个株系的DH群体[22]。取亲本和DH群体的种子做发芽试验。1.2 胁迫处理与表型考察
每份材料选取健康饱满、大小均一的种子150粒, 其中50粒作为对照组, 50粒作为试验组一, 50粒作为试验组二, 均匀放入培养皿中。培养皿底部垫3层滤纸保持水分, 盖上皿盖以保持培养皿内的湿度。试验组一用1.2% NaCl溶液25 mL作为培养液, 试验组二用20% (w/w)的PEG-6000溶液25 mL作为培养液, 对照组用去离子水25 mL作为培养液[11]。发芽试验在西南大学油菜中心组培室内进行, 昼夜温度恒定25℃, 光照/黑暗时间12 h/12 h, 光照强度87.5 μmol m-2 s-1, 相对湿度60% [23]。培养观察7 d, 第7天打开皿盖, 统计发芽数, 计算发芽率, 发芽率(%)=发芽种子数/参试种子数×100。每皿随机选取生长一致的10株幼苗, 依次测定根长, 称量整株鲜重, 并做好相关记录。试验设置3次重复。分别将盐胁迫和干旱胁迫下的发芽率、根长、鲜重相对值作为评价耐盐和耐旱的指标[24,25]。盐胁迫下各性状的相对值=1.2% NaCl处理测定值/CK测定值×100%, 干旱胁迫下各性状的相对值=20% PEG-6000处理测定值/CK测定值×100%。计算出各性状的相对值后, 分析各性状的平均数、最小值、最大值、标准差和变异系数。使用SAS V8软件分析DH群体各性状间的相关性。
1.3 遗传连锁图谱与QTL分析
利用JoinMap 3.0软件(http://www.kyazma.nl/ index.php/mc.JoinMap)构建甘蓝型油菜遗传连锁图谱。首先参照JoinMap 3.0标准数据模块的格式, 整理好群体中表现清晰带型的SSR分子标记的基因型, 在正式构建连锁图谱之前, 通过JoinMap 3.0的命令计算各单株和各标记的缺失率, 以及单株之间、标记之间的相似率, 排除缺失过多、同源相似率过高以及偏分离严重的标记和单株。构建图谱时参照软件的默认参数设置, 即重组率≤0.4, LOD值>1.0。首先采用LOD值8.0~20.0对所有标记分组。在构建连锁群的骨架图时, 剔除各连锁群上卡方值大于3.0的标记。在构建好的基本骨架图的基础上, 将分群时能分到各个连锁群, 但是却不被包含在基本骨架图上的标记逐一添加到骨架图谱上, 如果添加标记后并不改变原有的标记顺序, 表明该标记可以加入连锁群; 如果标记添加后, 改变了原有骨架图中标记的顺序, 则放弃添加该标记, 保持标记添加前原有连锁群中的标记顺序。按照该方法依次添加剩余标记直至完成所有分类群中的标记。所用遗传图谱包含293个SSR多态性标记位点, 总长1188 cM, 相邻标记间的平均距离为4.05 cM [22]。采用WinQTL Cartographer 2.5软件的复合区间作图法对耐盐和耐旱相关性状的QTL进行检测[26]。参数设置中, 背景标记数目为5, 扫描窗口10 cM, 扫描步长1 cM, 表型数据均通过采用1000次的排布检测方法来确定在P=0.05条件下显著QTL的阈值。当LOD≥2.5时, 认为这个置信区间的QTL存在[27]。按McCouch等[28]的方法命名检测到的QTL, 以“q”加相对应的性状再加染色体编号表示, 字体为斜体。用MapChart2.2软件绘制QTL在连锁群的分布图。2 结果与分析
2.1 表型分析
在1.2% NaCl或20% PEG-6000的胁迫条件下, 亲本和DH群体的种子发芽率、根长、以及整株鲜重相对值都小于1, 说明在盐胁迫或干旱胁迫下, 这3个性状都受到了抑制(表1)。在盐胁迫下, 2个亲本的发芽率差异最明显, 发芽率对盐胁迫最敏感; 在干旱胁迫下, 2个亲本的根长差异最明显, 根长对干旱胁迫最敏感。亲本Express和SWU07的耐盐相关性状分析表明, Express的发芽率相对值和鲜重相对值都比SWU07高, 但根长相对值比SWU07低。而对两亲本的耐旱相关性状分析表明, Express的发芽率相对值和根长相对值都比SWU07高, 但鲜重相对值比SWU07低。两亲本间表型差异显著性分析表明, 在盐胁迫和干旱胁迫下, 两亲本间发芽率、根长和鲜重均达到显著差异(P<0.05) (表1)。在盐胁迫或干旱胁迫下, DH群体的发芽率、根长、鲜重相对值均呈连续性分布(图1和图2), 3个性状在盐胁迫或干旱胁迫下的相对值, 均表现出广泛的表型变异, 并且存在明显的双向超亲分离现象, 表明耐盐和耐旱相关性状受多基因控制, 属于数量性状遗传。Table 1
表1
表1亲本和DH群体在不同胁迫环境下的表型分析
Table 1
| 胁迫环境 Stress environment | 性状 Trait | 亲本 Parent | DH群体 DH population | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SWU07 | 表达 Express | Pt-test | 最小值 Min. | 最大值 Max. | 平均值 Mean | 标准差 SD | 变异系数 CV (%) | ||
| 1.2% NaCl | 发芽率 Germination rate | 0.036 | 0.173 | 0 | 0 | 0.590 | 0.151 | 0.130 | 86.333 |
| 根长 Root length | 0.046 | 0.032 | 0.007 | 0.019 | 0.313 | 0.082 | 0.048 | 58.970 | |
| 鲜重 Fresh weight | 0.155 | 0.184 | 0.028 | 0.119 | 0.773 | 0.272 | 0.104 | 38.206 | |
| 20% PEG-6000 | 发芽率 Germination rate | 0.894 | 0.959 | 0.017 | 0.120 | 0.980 | 0.845 | 0.172 | 20.377 |
| 根长 Root length | 0.402 | 0.694 | 0 | 0.242 | 0.995 | 0.583 | 0.157 | 26.840 | |
| 鲜重 Fresh weight | 0.960 | 0.744 | 0.001 | 0.304 | 0.993 | 0.744 | 0.162 | 21.824 | |
新窗口打开|下载CSV
图1
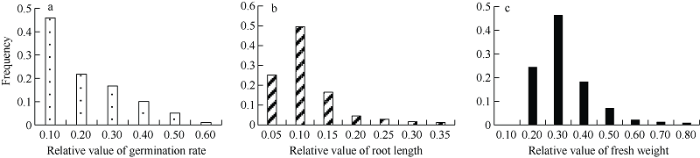 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1DH群体耐盐相关性状的频率分布图
a、b、c图分别是盐胁迫下发芽率相对值、根长相对值、鲜重相对值的频率分布图。
Fig. 1Phenotype frequency distribution of salt tolerance related traits in DH population
Figures a, b, and c show the frequency distribution of relative value of germination rate, relative value of root length, and relative value of fresh weight under salt stress, respectively.
图2
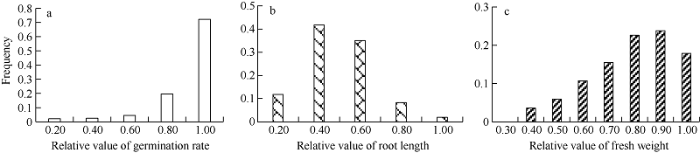 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2DH群体耐旱性状的频率分布图
a、b、c图分别是旱胁迫下发芽率相对值、根长相对值、鲜重相对值的频率分布图。
Fig. 2Phenotype frequency distribution of drought tolerance related traits in DH population
Figures a, b, and c show the frequency distribution of relative value of germination rate, relative value of root length, and relative value of fresh weight under drought stress, respectively.
2.2 相关性分析
相关性分析表明, 在盐胁迫下, DH群体的根长、鲜重、发芽率相对值两两之间的相关系数都呈显著正相关(表2), 其中根长与发芽率、根长与鲜重相对值的相关性达到极显著水平(P<0.001), 发芽率与鲜重相对值的相关性达到显著水平(P<0.05)。在干旱胁迫下, DH群体的根长、鲜重、发芽率相对值两两之间的相关系数都呈极显著正相关(P<0.001)。对2种胁迫环境下的相关性分析表明, 盐胁迫环境下的鲜重相对值与干旱胁迫下的鲜重和发芽率相对值分别呈显著正相关(P<0.05)。Table 2
表2
表2DH群体各性状的相关性分析
Table 2
| 性状 Trait | 1.2% NaCl | 20% PEG-6000 | |||||
|---|---|---|---|---|---|---|---|
| SGR | SRL | SFW | DGR | DRL | DFW | ||
| 1.2% NaCl | SGR | ||||||
| SRL | 0.2564** | ||||||
| SFW | 0.1317* | 0.4273** | |||||
| 20% PEG-6000 | DGR | 0.0439 | 0.1294 | 0.1817* | |||
| DRL | 0.0559 | 0.0166 | 0.1267 | 0.3238** | |||
| DFW | -0.0433 | 0.0032 | 0.1729* | 0.4494** | 0.2656** | ||
新窗口打开|下载CSV
2.3 耐盐相关性状QTL分析
盐胁迫环境下, 在3次重复中共检测到与盐胁迫相关的QTL 17个(表3和图3), 分布在A02、A03、A05、A09、C01和C09染色体上, 单个QTL可解释的表型变异为3.61%~10.59%。其中8个QTL与发芽率相关, 分布在A03、A05、C01和C09染色体上, 单个QTL可解释的表型变异为3.61%~10.59%; 7个QTL与根长相关, 分布在A03、A09和C09染色体上, 单个QTL可解释的表型变异为3.78%~8.53%; 2个QTL与鲜重相关, 位于A02和C09染色体上, 单个QTL可解释的表型变异为4.64%~5.79%。将每个性状在不同重复中检测到的QTL进行整合发现, 有5个QTL在2次重复中被检测到。与发芽率相关的qSGR-A03-1和qSGR-A03-3、qSGR-A03-2和qSGR-A03-4、qSGR-C01-1和qSGR-C01-2可以在重复1、3中被重叠检测到; 与根长相关的qSRL-A03-1和qSRL-A03-3在重复1、3中被重叠检测到, qSRL-C09-1和qSRL-C09-3在重复1、2中被重叠检测到。最终将这些QTL整合为 5个可重复性的位点。这样, 通过整合这些可重复性的位点, 最终得到12个与耐盐相关性状相关的位点。Table 3
表3
表3DH群体中检测到的耐盐、耐旱相关的QTL
Table 3
| 胁迫环境 Stress environment | 性状 Trait | 重复 Repetition | QTL名称 QTL name | 染色体 Chr. | 位置 Position | 加性效应 Additive | 贡献率 R2 (%) | LOD 值 LOD score | 置信区间 Confidence interval |
|---|---|---|---|---|---|---|---|---|---|
| 1.2% NaCl | 发芽率 Germination rate | REP1 | qSGR-A03-1 | A03 | 27.11 | -0.03 | 7.06 | 4.13 | 22.1-32.0 |
| REP1 | qSGR-A03-2 | A03 | 35.61 | -0.03 | 4.46 | 3.07 | 33.4-38.3 | ||
| REP1 | qSGR-A05 | A05 | 45.11 | 0.03 | 3.61 | 2.53 | 40.1-52.0 | ||
| REP1 | qSGR-C01-1 | C01 | 37.01 | 0.04 | 10.40 | 4.72 | 36.0-37.4 | ||
| REP1 | qSGR-C09 | C09 | 8.50 | -0.03 | 3.74 | 2.61 | 5.0-16.4 | ||
| REP 3 | qSGR-A03-3 | A03 | 27.11 | -0.04 | 9.37 | 5.47 | 22.7-32.0 | ||
| REP 3 | qSGR-A03-4 | A03 | 35.61 | -0.03 | 5.62 | 3.82 | 33.4-39.1 | ||
| REP 3 | qSGR-C01-2 | C01 | 37.01 | 0.04 | 10.59 | 4.75 | 36.0-39.1 | ||
| 根长 Root length | REP 1 | qSRL-A03-1 | A03 | 28.81 | -0.01 | 5.14 | 3.44 | 22.3-32.0 | |
| REP 1 | qSRL-A03-2 | A03 | 35.61 | -0.01 | 3.78 | 2.50 | 33.4-39.1 | ||
| REP 1 | qSRL-A09 | A09 | 86.41 | -0.01 | 8.53 | 5.03 | 77.8-95.0 | ||
| REP 1 | qSRL-C09-1 | C09 | 7.91 | -0.01 | 6.61 | 4.31 | 3.5-11.9 | ||
| REP 1 | qSRL-C09-2 | C09 | 14.91 | -0.01 | 4.99 | 3.06 | 11.9-17.5 | ||
| REP 2 | qSRL-C09-3 | C09 | 7.91 | -0.03 | 4.14 | 2.52 | 3.0-17.4 | ||
| REP 3 | qSRL-A03-3 | A03 | 30.81 | 0.26 | 6.30 | 3.44 | 28.4-33.4 | ||
| 鲜重 Fresh weight | REP 2 | qSFW-C09 | C09 | 19.61 | 0.00 | 4.64 | 2.94 | 17.5-31.8 | |
| REP 3 | qSFW-A02 | A02 | 46.00 | -0.03 | 5.79 | 3.52 | 44.9-50.0 | ||
| 20% PEG-6000 | 发芽率 Germination rate | REP 1 | qDGR-A02 | A02 | 47.00 | -0.01 | 4.41 | 2.57 | 44.9-51.0 |
| REP 1 | qDGR-A03 | A03 | 35.60 | 0.01 | 5.45 | 3.40 | 33.4-37.0 | ||
| REP 1 | qDGR-A05-1 | A05 | 65.00 | 0.04 | 6.05 | 3.00 | 59.0-78.6 | ||
| REP 2 | qDGR-A05-2 | A05 | 66.11 | 0.04 | 4.90 | 3.28 | 59.3-78.1 | ||
| 根长 Root length | REP 1 | qDRL-A02 | A02 | 40.80 | 0.04 | 12.90 | 2.96 | 40.2-42.1 | |
| REP 3 | qDRL-A01 | A01 | 10.31 | 0.16 | 4.92 | 2.98 | 7.9-12.7 | ||
| REP 3 | qDRL-A10 | A10 | 0.51 | 0.16 | 4.32 | 2.62 | 0-2.7 | ||
| 鲜重 Fresh weight | REP 1 | qDFW-A09 | A09 | 73.41 | 0.00 | 7.11 | 4.43 | 72.2-74.7 | |
| REP 2 | qDFW-A01-1 | A01 | 25.50 | 0.07 | 3.94 | 2.51 | 15.6-37.1 | ||
| REP 2 | qDFW-C03 | C03 | 25.60 | -0.06 | 9.21 | 3.87 | 21.4-34.1 | ||
| REP 3 | qDFW-A01-2 | A01 | 25.51 | 0.07 | 3.97 | 2.53 | 15.6-37.1 |
新窗口打开|下载CSV
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3耐盐、耐旱相关性状QTL在连锁群上的分布
白色填充的框图代表盐胁迫下检测到的QTL。黑色填充的框图代表干旱胁迫下检测到的QTL。
Fig. 3Distribution of salt and drought tolerance-related QTLs detected in DH population on linkage groups
The white-filled block diagrams represent the QTLs detected under salt stress and the black-filled block diagrams represent the QTLs detected under drought stress.
此外, 盐胁迫下不同性状间的QTL也有重叠。根长和发芽率这2个不同耐盐相关性状检测到了置信区间重叠的QTL, 与发芽率相关的qSGR-A03-1、qSGR-A03-3和与根长相关的qSRL-A03-1、qSRL-A03-3的置信区间发生重叠, 与发芽率相关的qSGR-A03-2、qSGR-A03-4和与根长相关的qSRL-A03-2的置信区间发生重叠; 与发芽率相关的qSGR-C09和与根长相关的qSRL-C09-1、qSRL-C09-2、qSRL-C09-3的置信区间发生重叠。对于这些在多次重复中检测到的QTL或不同耐盐指标在同一个区间检测到QTL, 在今后的研究工作中可以重点关注, 开发紧密连锁的分子标记用于育种的辅助选择。
2.4 耐旱相关性状QTL分析
干旱胁迫环境下, 在3次重复中共检测到与干旱胁迫相关的QTL 11个(表3和图3), 分布在A01、A02、A03、A05、A09、A10和C03染色体上, 单个QTL可解释的表型变异为3.94%~12.90%。其中4个QTL与发芽率相关, 分布在A02、A03和A05染色体上, 单个QTL可解释的表型变异为4.41%~6.05%; 3个QTL与根长相关, 位于A01、A02、A10染色体上, 可解释的表型变异为4.32%~12.90%; 4个QTL与鲜重相关, 分别位于A01、A09和C03染色体上, 可解释的表型变异分别为3.94%~9.21%。将每个性状在不同重复中检测到的 QTL 进行整合发现, 有2个QTL在2次重复中被检测到。与发芽率相关的qDGR-A05-1和qDGR-A05-2的置信区间发生重叠, 在重复1、2中被检测到; 与鲜重相关的qDFW-A01-1和qDFW-A01-2的置信区间发生重叠, 在重复2、3中被检测到。最终将这些QTL整合为2个可重复性的位点。这样, 通过整合这些可重复性的位点, 最终得到9个与耐旱相关性状相关的位点。
2.5 两种胁迫环境下重叠的QTL
对2种环境胁迫下的QTL比较分析(表3和图3)发现, 干旱胁迫下检测到的qDGR-A03与盐胁迫下检测到的qSGR-A03-2、qSGR-A03-4、qSRL-A03-2相互重叠, 干旱胁迫下检测到的qDGR-A02与盐胁迫下检测到的qSFW-A02相互重叠。3 讨论
植物对盐胁迫和干旱胁迫的响应是一个复杂的生理过程[29,30]。在油菜种子发芽期, 盐胁迫、干旱胁迫均会引起很多相关性状的改变, 如种子的发芽率、根长、植株鲜重等[6]。在相对发芽率、根长、鲜重3个油菜耐盐或耐旱指标中, 本研究发现, 种子的发芽率受盐胁迫影响最大, 株系间存在较大的差异, 因此, 相对发芽率更能体现油菜的耐盐性, 这与陈新军等[31]的研究结果一致。而在耐旱指标中, 根长受干旱胁迫影响最大, 株系间存在的差异也最大, 因此, 相对根长更能体现油菜的耐旱性。通过分别对油菜发芽期盐胁迫和干旱胁迫下种子耐盐或耐旱相关性状进行鉴定和QTL分析, 共检测到12个与盐胁迫相关的QTL和9个与干旱胁迫相关的QTL, 这些研究结果可为耐盐或耐旱油菜的分子标记辅助选择育种提供理论依据。为进一步对本研究发现的QTL与已报道的耐盐和耐旱相关QTL进行比较分析, 收集已报道的耐盐和耐旱相关QTL, 整理QTL置信区间内包含的SSR标记序列或者SSR标记的引物序列, 登陆油菜基因组数据库(http://www.genoscope.cns.fr/blatserver/ cgi-bin/colza/webBlat), 利用Blast工具[32], 对QTL区间两端的标记序列与甘蓝型油菜基因组进行比对[33], 得到QTL的物理位置。与已报道的油菜耐盐性QTL定位结果相比较, 本研究中所检测到的QTL与前人定位QTL位点有部分重叠或非常接近。其中, 位于A05染色体的qSGR-A05与张蕊等[10]通过全基因组关联分析发现的位于A05上6.1 Mb~6.3 Mb与下胚轴长相关的位点有重叠区间。Wan等[13]发现的位于A09上30.8 Mb附近的与幼苗长相关的SNP位点落在本研究中检测到的与根长相关QTL qSRL-A09区间内。贺亚军等[11]利用全基因组关联分析发现的位于A03染色体上与根长显著关联的SNP位点与本研究发现的与根长相关的QTL qSRL-A03-2和与发芽率相关的QTL qSGR-A03-2、qSGR-A03-4相距较近, 都在24.0 Mb附近; 位于C09染色体上16.0 Mb附近的与根长显著关联的SNP位点落在本研究中检测到的与根长相关QTL qSRL-C09-1、qSRL-C09-3和与发芽率相关的QTL qSGR-C09区间内, 同时发现这个SNP位点附近有3个串联重复的耐盐候选基因BnaC09g19080D、BnaC09g19090D 和BnaC09g19100D [11]。A02染色体的qSFW-A02与侯林涛等[7]利用重组自交系群体检测到的控制盐胁迫下油菜叶干重的QTL位点qSTDLA02-1相距较近, 都在24.0 Mb附近, 同时, qSFW-A02与贺亚军等[11]通过全基因组关联分析在A02上10.2 Mb附近发现的盐胁迫下鲜重相关的SNP位点相距很近。这些利用不同群体检测到的位点很可能为同一QTL区间, 但还需要进一步比较研究。与前人研究结果比较, 在盐胁迫环境下, 本研究检测到位于C01染色体上与发芽率相关的QTL qSGR-C01-1和qSGR-C01-2、位于A03染色体上与发芽率相关的QTL qSGR-A03-1和qSGR-A03-3、与根长相关的QTL qSRL-A03-1和qSRL-A03-3是未被报道过的新QTL。这些新的QTL位点信息可以帮助我们找到更多控制油菜耐盐性的遗传变异, 将有助于甘蓝型油菜耐盐性的遗传改良。
与已报道的油菜耐旱性QTL定位结果比较, 本研究中所检测到的QTL与已报道的QTL位点有重叠或非常近。其中, 干旱胁迫下整株鲜重相关的QTL qDFW-A01-1、qDFW-A01-2与李真[14]发现的与干重相关的位点tdw1b.3、rsdw1.4都在A01连锁群的BRMS096标记附近; 与发芽率相关的QTL qDGR-A03和荐红举等[6]在A03上检测到与干旱胁迫发芽率相关的位点距离很近; 本研究的qDGR-A05-1、qDGR-A05-2是与干旱胁迫发芽率相关的QTL, 和李真[14]发现的与干旱胁迫下根长相关的位点rl5.6均在A05连锁群的标记BRAS072a附近, 并且二者有重合的区间; qDFW-C03是与干旱胁迫整株鲜重相关的QTL, 位于C03连锁群, 黄倩等也在C03连锁群发现2个与干旱胁迫整株鲜重相关的QTL[20]; 在干旱胁迫环境下, 本研究检测到位于A02染色体上与干旱胁迫下发芽率相关的QTL qDGR-A02和与根长相关的QTL qDRL-A02是未被报道过的新QTL。
本研究中, 无论是盐胁迫还是干旱胁迫下, 所检测到的QTL效应值都比较小, 表现为微效多基因控制的数量遗传。值得关注的是, 对2种胁迫环境下的相关性分析发现, 盐胁迫环境下的鲜重相对值与干旱胁迫下的鲜重和发芽率相对值分别呈显著正相关(P<0.05)。而且, 通过将检测到的盐胁迫相关的QTL和干旱胁迫相关的QTL进行比较发现, 在这2种胁迫环境下检测到相互重叠的QTL, 干旱胁迫下检测到的qDGR-A03与盐胁迫下检测到的qSGR-A03-2、qSGR-A03-4、qSRL-A03-2相互重叠, 干旱胁迫下检测到的qDGR-A02与盐胁迫下检测到的qSFW-A02相互重叠, 这些研究结果表明, 植物在生长发育过程中, 可能要同时应对多种生物或非生物胁迫, 尤其是干旱和盐碱通常同时发生[34], 在进化过程中, 为了适应环境, 植物演化形成了应对不同胁迫环境的相同或特异的响应机制[35]。
4 结论
盐胁迫下, 检测到12个与油菜耐盐性状相关的QTL, 分布在A02、A03、A05、A09、C01和C09染色体上。干旱胁迫下, 检测到9个与油菜耐旱性状相关的QTL, 分布在A01、A02、A03、A05、A09、A10和C03染色体上。所检测到的耐盐性和耐旱性相关QTL效应值都比较小, 表现为微效多基因控制的数量遗传。其中, 干旱胁迫下检测到的qDGR-A03与盐胁迫下检测到的qSGR-A03-2、qSGR-A03-4、qSRL-A03-2相互重叠, 干旱胁迫下检测到的qDGR-A02与盐胁迫下检测到的qSFW-A02相互重叠。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 1]

]]>
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1080/07352689.2010.524517URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3724/SP.J.1006.2014.00629URL [本文引用: 5]

研究盐胁迫、干旱胁迫下甘蓝型油菜的发芽率,寻找与发芽率相关联的分子标记,可为油菜逆境胁迫下种子萌发的分子标记辅助育种提供理论依据。本研究以甘蓝型黄籽油菜GH06和甘蓝型黑籽油菜P174为亲本,通过单粒传法(single seed descent, SSD)连续自交9代构建重组自交系群体。采用16 g L–1的NaCl溶液进行盐胁迫,20% (W/W)的PEG-6000溶液模拟干旱胁迫,处理重组自交系种子并统计其发芽率。实验室构建的SNP遗传图谱,包含2795个SNP多态性标记位点,总长1832.9 cM,相邻标记间平均距离为0.66 cM,利用该图谱并采用复合区间作图法(CIM)分析两种胁迫条件下第3天、第4天及累计4 d后发芽率的QTL。共检测到19个QTL,分布于A01、A03、A06、A07、A09和C06染色体上。其中,11个盐胁迫相关的QTL可解释的变异为4.9%~10.9%,8个干旱胁迫相关的QTL可解释的变异为3.8%~6.9%;并且在A03和A09染色体上,盐胁迫和干旱胁迫下检测到的QTL有相近区段。研究结果表明油菜种子发芽率属于典型的数量性状,受环境影响较大;且随着胁迫时间的延长,油菜种子启动了不同的基因来响应环境胁迫。]]>
[本文引用: 5]
DOI:10.3724/SP.J.1006.2017.00179URL [本文引用: 2]

GH06与P174杂交后通过单粒传法连续自交获得的高世代重组自交系群体,以含16 g L–1 NaCl的Hoagland溶液培养幼苗进行盐胁迫处理25 d后,分别测定叶和根的鲜重及干重,根据已构建的高密度SNP遗传连锁图谱进行QTL定位,在QTL物理区间筛选耐盐相关基因并以极端表型材料进行qRT-PCR分析。采用复合区间作图法(CIM),在对照和盐胁迫处理中共检测到19个QTL,其中与盐胁迫相关的有6个,可解释的表型变异7.16%~16.15%,分布在A02、A04和C03染色体上,将QTL置信区间序列和拟南芥中与盐胁迫相关的基因比对分析,共找到8个候选基因。对其中4个候选基因在极端表型材料中的表达分析表明,BnaA02g14680D与BnaA02g14490D基因在盐胁迫处理后的48 h或72 h表达量均高于对照组,即基因的表达由盐胁迫引起,而BnaC03g64030D在敏感型材料中的相对表达量高于在耐盐型材料中,BnaC03g62830D在敏感型材料中没有明显变化,但在耐盐型材料中呈现先升高后降低的表达特征,其表达可能会增强植株对盐胁迫的耐受力。本研究为油菜耐盐基因功能挖掘和油菜耐盐品种选育奠定基础。]]>
[本文引用: 2]
DOI:10.3389/fpls.2017.01000URLPMID:28659949 [本文引用: 1]

Salinity stress is one of typical abiotic stresses that seriously limit crop production. In this study, a genetic linkage map based on 532 molecular markers covering 1341.1 cM was constructed to identify the loci associated with salt tolerance in Brassica napus. Up to 45 quantitative trait loci (QTLs) for 10 indicators were identified in the F2:3 populations. These QTLs can account for 4.80-51.14% of the phenotypic variation. A major QTL, qSPAD5 on LG5 associated with chlorophyll can be detected in three replicates. Two intron polymorphic (IP) markers in this QTL region were developed successfully to narrow down the QTL location to a region of 390 kb. A salt tolerance related gene Bra003640 was primary identified as the candidate gene in this region. The full length of the candidate gene was 1,063 bp containing three exons and two introns in B. napus L. The open reading frame (ORF) is 867 bp and encodes 287 amino acids. Three amino acid differences (34, 54, and 83) in the conserved domain (B-box) were identified. RT-qPCR analysis showed that the gene expression had significant difference between the two parents. The study laid great foundation for salt tolerance related gene mapping and cloning in B. napus L.
DOI:10.1016/j.plantsci.2018.01.005URLPMID:29606219 [本文引用: 1]

Lobed leaf is a common trait, which is related with photosynthesis and plant stress resistance in crops. In order to fine map and isolate the lobed-leaf gene in Brassica napus, an F2:3 population derived from 2205 (salt tolerance) and 1423 (salt sensitive) was constructed, and the quantitative trait locus (QTL) technology was adopted to identify the QTLs related to lobed leaf formation. As a result, one major QTL was identified on LG10, and two intron polymorphic (IP) markers and one sequence characterized amplified region (SCAR) marker were successfully developed in QTL region. The lobed-leaf gene was mapped to a region from 15.701 to 15.817M on A10. In light of annotations of the genes in candidate region, a leaf morphological development related gene, Bra009510, was primary identified as the candidate gene. The full length of the candidate gene was 1390bp containing three exons and two introns in the two parents. The open reading frame (ORF) was 693bp and encoded a protein of 229 amino acids. Eight amino acid differences between the two parents in CDS (coding sequences) region were identified. qRT-PCR analysis showed that the expression of the candidate gene was significantly different between the two parents under salt stress. These results showed that the candidate gene might be related to leaf morphological development and abiotic stresses. Our study will lay a solid foundation for studying lobed leaf mechanism in B. napus L.
DOI:10.3864/j.issn.0578-1752.2017.01.002URL [本文引用: 2]

317份具有代表性的甘蓝型油菜自交系为材料,在正常生长和盐胁迫条件下进行沙培鉴定,利用芸薹属60K SNP芯片和全基因组关联分析鉴定正常生长与盐胁迫下甘蓝型油菜发芽期根和下胚轴长度显著关联的SNP,并确定其连锁不平衡区间。通过区间内基因的功能注释及盐胁迫下油菜幼苗根和叶片转录组差异表达基因筛选连锁不平衡区间内的重要候选基因,并以实时荧光定量PCR分析候选基因的组织特异性和盐胁迫诱导表达模式,提高候选基因筛选的准确性。【结果】正常生长和盐胁迫下甘蓝型油菜发芽期下胚轴和根长在不同材料间变异较大,频次分布表明目标性状均为数量性状,受多基因调控。全基因组关联分析模型比较表明,MLM+P+K模型为最优模型。以此模型对目标性状进行全基因组关联分析,检测到45个显著关联SNP,其中40个与下胚轴长度显著关联,5个与根长显著关联,单个SNP解释的表型变异分别为9.12%—14.46%和7.67%—8.93%。重复检测的显著相关SNP中,值得注意的是C04染色体的rs8970,同时与4个性状显著关联,表型贡献率为7.67%—12.35%,是唯一在下胚轴长和根长间重复检测到的显著关联SNP。11个重要关联SNP中有6个位于10—442 kb的连锁不平衡区块中。转录组分析表明,11个连锁不平衡区间共包含447个基因,其中15个受盐胁迫诱导表达。转录组和基因功能注释综合分析表明,BnaSRO1、BnaPAGR2、BnaNPH3、BnaMYB124、BnaSAM-Mtase、BnaBIN2、BnaUMAMIT11、BnaEXPA7、BnaRPT3、BnaEF-hand和BnaF3H很可能为各自区间的候选基因。实时荧光定量PCR结果证实除BnaNPH3外,其他基因均在根或下胚轴中受盐胁迫诱导上调表达。组织特异性分析还发现BnaUMAMIT11、BnaPAGR2和BnaEXPA7主要在萌发的根和下胚轴中特异表达,BnaRPT3、BnaBIN2和BnaMYB124虽然呈组成型表达,但在萌发阶段的下胚轴中表达量最高,证实这些基因很可能参与油菜发芽期根和下胚轴生长发育及耐盐性的调节。【结论】全基因组关联分析共鉴定出45个控制油菜发芽期根和下胚轴发育及耐盐性的显著关联SNP。连锁不平衡、转录组和基因功能注释综合分析初步鉴定出11个重要候选基因。]]>
[本文引用: 2]
DOI:10.3864/j.issn.0578-1752.2017.07.002URL [本文引用: 5]

【目的】通过对甘蓝型油菜耐盐相关性状进行全基因组关联分析,寻找可能与油菜耐盐性相关的SNP位点,发掘与油菜耐盐性有关的候选基因。【方法】以1.2%NaCl溶液作为培养液,去离子水为对照,对307个不同品系的甘蓝型油菜进行发芽试验。播种后7 d测定幼苗根长、鲜重及发芽率,计算盐胁迫下各性状相对值,并作为评价耐盐的指标。结合油菜60K SNP芯片,利用SPAGeDi v1.4软件对该群体307份甘蓝型油菜进行亲缘关系分析,并计算亲缘关系值的矩阵。利用软件STRUCTURE v2.3.4对关联群体进行了群体结构分析。为有效排除假关联的影响,将群体结构和材料间的亲缘关系考虑进关联分析中,同时进行了PCA+K模型、Q+K模型以及K模型3种混合线性模型分析和比较,根据所有SNP的–lg(P)观察值和期望值,确定每个性状GWAS分析的最优模型。采用TASSEL 5.0软件,在最优模型下对307份材料耐盐各性状的相对值分别与SNP标记进行全基因组关联分析。利用油菜基因组数据库,在显著SNP位点侧翼序列200 kb范围内提取基因。根据拟南芥中已经明确功能的耐盐相关基因,筛选出目标基因组区段内与耐盐相关的油菜同源基因。【结果】全基因组关联分析共检测到164个与根长显著关联的SNP位点,23个与鲜重显著关联的SNP位点,38个与发芽率显著关联的SNP位点。其中与根长、鲜重、发芽率最显著关联的SNP位点分别位于染色体A08、A02和A06,贡献率分别为23.84%、18.59%和31.81%。在这些显著SNP位点侧翼序列200 kb范围内发掘出可能与油菜耐盐性有关的50个候选基因。这些候选基因主要包括转录因子MYB、WRKY、ABI1、bZIP、ERF1、CZF1、XERICO等以及一些下游受转录因子调控的不同功能基因NHX1、PTR3、CAT1、HKT、CAX1、ACER、STH、STO等。在根长和发芽率2个不同耐盐性状的分析结果中均筛选出位于A03染色体上的耐盐基因BnaA03g14410D。另外,这些耐盐候选基因中包含两组串联重复基因,分别是位于A03染色体上的BnaA03g18900D和BnaA03g18910D,位于C09染色体上的BnaC09g19080D、BnaC09g19090D和BnaC09g19100D。除此之外,耐盐候选基因中还包含2个距离非常近(中间只间隔2个基因)的重复基因BnaC02g39600D和BnaC02g39630D。【结论】共检测到225个与耐盐性状显著关联的SNP位点,筛选出50个可能与油菜耐盐性有关的候选基因。
[本文引用: 5]
DOI:10.1007/s00425-015-2310-8URLPMID:25921693 [本文引用: 1]

MAIN CONCLUSION: By genome-wide association study, QTLs for salt tolerance in rapeseed were detected, and a TSN1 ortholog was identified as a candidate gene responsible for genetic variation in cultivars. Dissecting the genomic regions governing abiotic stress tolerance is necessary for marker-assisted breeding to produce elite breeding lines. In this study, a world-wide collection of rapeseed was evaluated for salt tolerance. These rapeseed accessions showed a large variation for salt tolerance index ranging from 0.311 to 0.999. Although no significant correlation between salt tolerance and Na(+) content was observed, there was a significant negative correlation between shoot biomass production under a control condition and salt tolerance. These rapeseed accessions were genotyped by DArTseq for a total of 51,109 genetic markers, which were aligned with 'pseudomolecules' representative of the genome of rapeseed to locate their hypothetical order for association mapping. A total of 62 QTLs for salt tolerance, shoot biomass, and ion-homeostasis-related traits were identified by association mapping using both the P and Q+K models. Candidate genes located within the QTL regions were also shortlisted. Sequence analysis showed many polymorphisms for BnaaTSN1. Three of them in the coding region resulting in a premature stop codon or frameshift were found in most of the sensitive lines. Loss-of-function mutations showed a significant association with salt tolerance in B. napus.
DOI:10.3389/fpls.2017.00593URLPMID:28491067 [本文引用: 2]

Soil salinity is a serious threat to agriculture sustainability worldwide. Salt tolerance at the seedling stage is crucial for plant establishment and high yield in saline soils; however, little information is available on rapeseed (Brassica napus L.) salt tolerance. We evaluated salt tolerance in different rapeseed accessions and conducted a genome-wide association study (GWAS) to identify salt tolerance-related quantitative trait loci (QTL). A natural population comprising 368 B. napus cultivars and inbred lines was genotyped with a Brassica 60K Illumina Infinium SNP array. The results revealed that 75 single-nucleotide polymorphisms (SNPs) distributed across 14 chromosomes were associated with four salt tolerance-related traits. These SNPs integrated into 25 QTLs that explained 4.21-9.23% of the phenotypic variation in the cultivars. Additionally, 38 possible candidate genes were identified in genomic regions associated with salt tolerance indices. These genes fell into several functional groups that are associated with plant salt tolerance, including transcription factors, aquaporins, transporters, and enzymes. Thus, salt tolerance in rapeseed involves complex molecular mechanisms. Our results provide valuable information for studying the genetic control of salt tolerance in B. napus seedlings and may facilitate marker-based breeding for rapeseed salt tolerance.
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/jxb/eru423URLPMID:25371500 [本文引用: 1]

Drought escape and dehydration avoidance represent alternative strategies for drought adaptation in annual crops. The mechanisms underlying these two strategies are reported to have a negative correlation, suggesting a trade-off. We conducted a quantitative trait locus (QTL) analysis of flowering time and root mass, traits representing each strategy, in Brassica napus to understand if a trade-off exists and what the genetic basis might be. Our field experiment used a genotyped population of doubled haploid lines and included both irrigated and rainfed treatments, allowing analysis of plasticity in each trait. We found strong genetic correlations among all traits, suggesting a trade-off among traits may exist. Summing across traits and treatments we found 20 QTLs, but many of these co-localized to two major QTLs, providing evidence that the trade-off is genetically constrained. To understand the mechanistic relationship between root mass, flowering time, and QTLs, we analysed the data by conditioning upon correlated traits. Our results suggest a causal model where such QTLs affect root mass directly as well as through their impacts on flowering time. Additionally, we used draft Brassica genomes to identify orthologues of well characterized Arabidopsis thaliana flowering time genes as candidate genes. This research provides valuable clues to breeding for drought adaptation as it is the first to analyse the inheritance of the root system in B. napus in relation to drought.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1080/07352680490433286URL
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:9254694 [本文引用: 1]
URLPMID:25146293 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1365-313X.2005.02593.xURLPMID:16441347 [本文引用: 1]

The abiotic stresses of drought, salinity and freezing are linked by the fact that they all decrease the availability of water to plant cells. This decreased availability of water is quantified as a decrease in water potential. Plants resist low water potential and related stresses by modifying water uptake and loss to avoid low water potential, accumulating solutes and modifying the properties of cell walls to avoid the dehydration induced by low water potential and using protective proteins and mechanisms to tolerate reduced water content by preventing or repairing cell damage. Salt stress also alters plant ion homeostasis, and under many conditions this may be the predominant factor affecting plant performance. Our emphasis is on experiments that quantify resistance to realistic and reproducible low water potential (drought), salt and freezing stresses while being suitable for genetic studies where a large number of lines must be analyzed. Detailed protocols for the use of polyethylene glycol-infused agar plates to impose low water potential stress, assay of salt tolerance based on root elongation, quantification of freezing tolerance and the use of electrolyte leakage experiments to quantify cellular damage induced by freezing and low water potential are also presented.
