 ,, 罗艳君, 赵潘婷, 贾海燕
,, 罗艳君, 赵潘婷, 贾海燕 ,*, 马正强南京农业大学农学院/作物遗传与种质创新国家重点实验室/江苏省现代作物生产协同创新中心, 江苏南京 210095
,*, 马正强南京农业大学农学院/作物遗传与种质创新国家重点实验室/江苏省现代作物生产协同创新中心, 江苏南京 210095Overexpression of TaJRL53 enhances the Fusarium head blight resistance in wheat
CHEN Tong-Rui ,, LUO Yan-Jun, ZHAO Pan-Ting, JIA Hai-Yan
,, LUO Yan-Jun, ZHAO Pan-Ting, JIA Hai-Yan ,*, MA Zheng-QiangCollege of Agriculture, Nanjing Agricultural University / State Key Laboratory for Crop Genetics and Germplasm Enhancement / Jiangsu Collaborative Innovation Center for Modern Crop Production, Nanjing 210095, Jiangsu, China
,*, MA Zheng-QiangCollege of Agriculture, Nanjing Agricultural University / State Key Laboratory for Crop Genetics and Germplasm Enhancement / Jiangsu Collaborative Innovation Center for Modern Crop Production, Nanjing 210095, Jiangsu, China通讯作者:
收稿日期:2020-06-17接受日期:2020-09-13网络出版日期:2021-01-12
| 基金资助: |
Received:2020-06-17Accepted:2020-09-13Online:2021-01-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3076KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陈同睿, 罗艳君, 赵潘婷, 贾海燕, 马正强. 过表达TaJRL53基因提高了小麦赤霉病抗性[J]. 作物学报, 2021, 47(1): 19-29. doi:10.3724/SP.J.1006.2021.01050
CHEN Tong-Rui, LUO Yan-Jun, ZHAO Pan-Ting, JIA Hai-Yan, MA Zheng-Qiang.
植物在自然界生长过程中不断受到各种病原物的侵袭。在长期的进化过程中, 植物与病原物之间互相适应、协同进化。植物通过一系列信号传递激发自身的防御体系, 从而产生抗病反应, 例如水杨酸、茉莉酸、乙烯、钙离子等在抗病信号转导途径中扮演着至关重要的作用[1,2]。小麦, 作为世界三大粮食作物之一, 产量仅次于玉米和水稻。在过去的几十年间, 在小麦种植面积没有增加的前提下, 截至2017年全球小麦总产量增长超过50% [3]。然而, 它的生长过程受各种病原菌的侵害, 包括锈病、白粉病、赤霉病、纹枯病等。近年来, 随着全球气候变化、小麦耕作制度及农业生产技术变化的影响, 小麦赤霉病的发生频率越来越高, 病害发生的面积逐渐增加。在我国, 赤霉病主要发生在长江中下游麦区、华南冬麦区和川滇冬麦区等。小麦赤霉病的发生不仅仅降低小麦的产量和品质, 更为严重的是感赤霉病的籽粒含有赤霉菌分泌的多种毒素如脱氧雪腐镰刀菌烯醇, 严重影响人畜健康[4,5]。鉴于赤霉病对小麦生产和粮食安全造成的严重威胁, 该病害引起了人们越来越多的关注。
凝集素是一类广泛存在于生物体内的糖蛋白或糖特异结合蛋白, 与机体的多种生理功能密切相关[6]。植物凝集素按照同源性、种属来源及进化关系的差异可分为12个不同家族[7,8], Jacalin-related lectins (JRLs)是植物凝集素超家族中的一个新家族, 因被发现于木菠萝而被命名[9]。根据其糖结合特性被划分为半乳糖特异性JRL (gJRLs)和甘露糖或葡萄糖特异性JRL (mJRLs), 前者是桑科植物特异的, 主要储存于液泡中[10]; 后者广泛分布于单子叶植物、双子叶植物、苏铁植物和蕨类植物, 大多数定位于细胞质和细胞核中。JRLs蛋白结构具有多样性, 可包含一个或多个Jacalin结构域[8,11], 也可与其他结构域嵌合。其中与疾病响应元件Dirigent嵌合的JRL是单子叶植物特有的类型, 在单子叶植物中广泛存在, 又称为单子叶嵌合凝集素[12]。
迄今为止, 在植物中已有功能研究报道的JRLs超过26个[13], 它们大多数都与抗病、抗逆及胁迫压力的响应相关, 但是它们抗病/逆的机制却各不相同。比如, 从红芸豆中提取的一种同源二聚凝集素, 通过其自身的有毒肽对真菌Fusarium oxysporum、Coprinus comatus和Rhizoctonia solani的生长产生抑制作用[14]。LecRK-V.5通过负调控拟南芥的气孔免疫, 从而对丁香假单胞菌和胡萝卜软腐菌的生长产生影响[15,16]。JAX1介导的免疫抗性不同于其他病毒抗性机制, 它独立于传统抗性(R)蛋白介导的抗性和植物防御激素信号传导[17,18]。辣椒中的甘露糖结合凝集素基因CaMBL1通过识别高甘露糖型N-聚糖调控PCD, 进而对微生物病原体的防御反应[19]。凝集素受体激酶ERK1在LysM区域抗真菌MAMP信号感知中发挥重要作用[20,21]。RTM1通过与自身或RTM3相互作用来限制植物病毒的长距离移动[22,23,24]。PYK10通过与不同的JRLs (JAL31和JAL23)互作, 控制PYK10聚合物的尺寸, 从而在防御反应中发挥作用[25]。OsJAC1具有对多种病原菌的广谱抗性, 这种抗性是由自身的Jacalin和Dirigent两个结构域相互作用提供[26], OsMBL1可增强对稻瘟病菌的抗性, 其抗性的产生是由于与稻瘟病基因MoChi1竞争几丁质, 从而触发水稻细胞中的ROS反应, 并且通过积极调控SA和ABA响应基因, 进而通过参与PTI途径防御稻瘟病菌的侵染[27]; LEM2参与了大麦系统获得性抗性[20]。
在小麦中, 目前为止仅有以下4个JRL基因被克隆并进行了功能及机制方面的探索: TaJRLL1与小麦白粉菌和赤霉菌抗性有关, 被认为是水杨酸和茉莉酸植物防御信号机制的组成部分[28]; TaWC1-1基因响应白粉菌的侵染[29], TaHfr-1对黑森麦秆虫幼虫的发育有抑制作用[30]; TaJA1是OsJAC1在小麦中的同源基因, 过量表达该基因可提高烟草对多种病害的抗性[31]。此外, 也有研究者在大麦、水稻、高粱、小麦和一些草属的NBS-LRR区域发现了整合的Jacalin结构域, 这类整合结构域, 有些可以诱捕入侵的病原体, 启动相应的防御响应[32,33]。我们前期研究从小麦基因组中鉴定获得了67个JRL基因, 其中TaJRL53是一个单拷贝基因, 位于小麦4A染色体长臂上, 属于与Dirigent结构域嵌合的mJRLs, 通过电子表达及RT-PCR检测到TaJRL53可对赤霉菌的侵染产生响应, 并在MeJA和SA激素处理后上调表达[6]。本研究拟通过VIGS技术和基因枪介导的遗传转化技术进一步探究TaJRL53的生物学功能以及抗性机制。
1 材料与方法
1.1 植物材料
普通小麦中国春和望水白用于该基因的克隆, 地方品种望水白用于基因的组织特异性表达分析; 中抗赤霉病材料SSD006用于VIGS实验; 感赤霉病材料Bobwhite和PH691用于稳定遗传转化。组织特异性表达分析的材料: 取望水白正常生长季节苗期根、茎、叶和开花后5 d的根、茎、叶、穗于液氮冻存。转基因植株中TaJRL53的表达所用的材料: T0代转基因植株成株期的叶片。亚细胞定位材料用新鲜的洋葱。1.2 基因的克隆
参照文献[31]获得的TaJRL53基因序列设计特异引物(F引物: 5'-ATGGCCACAACCACCG-3'; R引物: 5'-GATGGTATAAACACCAATCGAAG-3'), 从中国春的cDNA中扩增TaJRL53的开放读码框。1.3 总RNA的提取、cDNA的合成、半定量PCR和实时定量PCR
利用TRIzol试剂(Invotrogen, Carlsbad, CA)按照操作说明提取总RNA, 所有的RNA材料均用DNaseI (Fermentas, Canada)消化以确保完全去除DNA污染。随后用M-MLV反转录酶(Promega, USA)按照操作说明合成cDNA。半定量RT-PCR扩增体系为25 μL, 反应程序为94℃预变性3 min, 94℃变性30 s, 退火30 s (退火温度视具体引物而定), 72oC延伸(延伸时间视扩增片段长度而定), 25~30个循环(因基因的表达丰度不同而异), 最后72oC延伸5 min。扩增产物在2%的琼脂糖凝胶中电泳检测。以小麦组成型表达基因TaActin作为内参。
参照THUNDERBIRD SYBR qPCR Mix (Toyoba, Japan)使用说明, 实时定量RT-PCR反应体系为20 μL, 包括约5 ng模板、10 μL SYBR-Green PCR Mastermix (Toyoba, Japan)和各8 pmol的正反向引物。以TaActin作为参照, 每个目标基因3个重复, 相对表达量2-??CT方法计算[32], 用StepOnePlus Real- time PCR Systems (Applied Biosystems)进行反应。按[33]中所用引物对信号通路中的标志基因的表达进行检测, 检测TaJRL53表达的正向引物为5'-GTTGC CAAAACGCACGGTA-3', 反向引物为5'-CCCAGTC ATTATCTGCTTCATCCTC-3'。
1.4 载体构建
1.4.1 瞬时表达载体的构建 用KOD FX DNA聚合酶从望水白穗部cDNA中扩增TaJRL53的编码区(不含终止子), 正向引物为5'-CTAGTCTAGAAT GGCCACAACCAC-3' (下画线表示Xba I酶切位点), 反向引物为5'-CGCGGATCCGATGGTATAAACA-3' (下画线表示BamH I酶切位点)。扩增产物在1%琼脂糖凝胶上电泳分离检测后, 用DNA凝胶回收试剂盒回收, 将回收到的扩增产物用Xba I和Bam H I酶切, 回收定量后与经过Xba I和Bam H I酶切过的pEGFP表达载体按3∶1摩尔比混合, 在4°C条件下用T4-DNA连接酶(Promega, USA)连接过夜, 连接产物用热击法转入大肠杆菌感受态细胞DH5α。阳性克隆送上海华大公司测序验证。1.4.2 BSMV沉默载体的构建 本实验室内野生型BSMV ND18 α、β及γ载体引自美国堪萨斯州立大学Scofield实验室。利用DNA聚合酶从中国春的cDNA中扩增TaJRL53, 上游引物 5'-CCCGTTAATTAA GCCACAACCACCGAAGAACC-3' (下画线表示Pac I酶切位点), 下游引物为5'-TATAGCGGCCGCCAG CACCGTCATAGACACCCC-3' (下画线表示Not I的酶切位点), 将PCR产物和野生型BSMV ND18 γ用Pac I和Not I (Promega, USA)双酶切, 将载体去磷酸化, 分别回收酶切后的PCR产物和质粒, 并按3∶1的摩尔比混合, 在4°C条件下用T4-DNA连接酶(Promega, USA)连接过夜。用热击转化法将连接产物转入大肠杆菌感受态细胞DH5α。挑取阳性克隆送上海华大基因科技股份有限公司测序, 测序结果正确, 获得可用于VIGS转化的载体。
1.4.3 稳定表达载体的构建 用TaJRL53的特异引物从中国春的cDNA中扩增TaJRL53的开放读码框, 正向引物为5'-TACGCTGCAGATGGCCACAAC CACCG-3', 反向引物为5'-ATGCCTGCAGGATGGTA TAAACACCAATCGAAG-3' (下画线为Pst I的酶切位点)。将扩增片段插入到中间载体pUT (pTA2-Ubi- Ter)中构建Ubi驱动的表达盒, 然后将Ubi::TaJRL53- Ter表达盒用Hind III切下, 再插入到表达载体pUCBS中, 构成用于基因枪转化的TaJRL53稳定表达载体(图1)。
图1
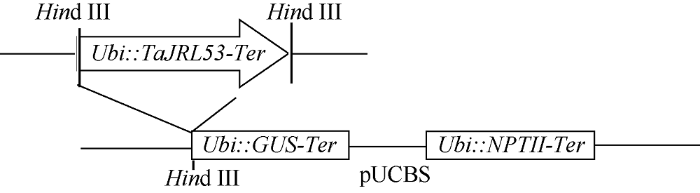 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1TaJRL53过量表达载体结构图
Fig. 1Scheme of the TaJRL53 overexpression vector
1.5 亚细胞定位
剥去新鲜洋葱最外的两层鳞茎, 用尖嘴镊子小心撕取完好鳞茎的内表皮, 平铺于1/2 MS固体培养基上, 注意要将内表面朝上, 且要平铺于培养皿中心位置。盖上培养皿, 黑暗中25oC恒温预培养2~4 h。参照文献[34]进行质粒包裹和基因枪轰击。将洋葱表皮细胞置于25°C恒温培养箱中暗培养16~18 h, 在激光共聚焦显微镜下观察荧光并照相记录。1.6 VIGS
1.6.1 体外转录物的合成 提取野生型BSMV ND18 α、β、γ及BSMV:TaJRL53的质粒, 分别用Mlu I线性化BSMV ND18 α、γ及BSMV:TaJRL53质粒DNA, Spe I线性化BSMV ND18 β质粒DNA, 用1%的凝胶电泳检测线性化水平。待线性化完全后用酚、氯仿抽提、纯化, 用RNase-free水溶解, 调整浓度为0.2~1.0 μg μL-1。以抽提纯化的质粒为模板, 用T7-DNA依赖的RNA聚合酶(mMessage mMachine T7 Kit; Ambion) 进行体外转录。转录产物用1% RNA-free的凝胶电泳跑胶, EB染色后在紫外灯下拍照检测RNA质量。野生型BSMV ND18 α、β及γ为模板的体外转录物与FES按1∶1∶1∶25的比例混合为空BMSV病毒对照, 同样以野生型BSMV ND18 α、β及BSMV:TaJRL53为模板的体外转录物与FES也按1∶1∶1∶25的比例混合, 取8 μL上述两种混合液均匀液涂于穗部, 同时单独涂抹FES缓冲液做空白对照。
1.6.2 体外涂抹 在抽穗期选取长势良好且大小一致的穗子, 分别涂抹FES缓冲液、FES缓冲液和BSMV病毒混合液, FES缓冲液和BSMV:TaJRL53病毒混合液, 以只涂抹FES缓冲液和涂抹FES缓冲液、BSMV病毒混合液的植株作为对照组, 涂抹FES缓冲液和BSMV:TaJRL53病毒混合液的植株作为实验组, 每种病毒混合液至少接种10株, 3次重复。
1.7 小麦的遗传转化及转基因植株的检测
取开花后14 d左右的小麦种子脱粒。在无菌条件下将脱粒的种子置于灭菌小罐中, 用70%乙醇浸泡5 min, 无菌水冲洗2~3次; 2%的NaClO浸泡8 min, 无菌水冲洗3~4次。于2 mg L-1 2,4-D的MS培养基上, 25℃暗培养3~5 d后, 幼胚经过脱分化形成愈伤组织。参照文献[34]的方法基因枪轰击, 轰击后的小麦愈伤继续在25oC黑暗培养箱培养16~18 h, 然后转移至诱导培养基上进行暗培养14 d。将愈伤组织转移到1 mg L-1 KT和20 mg L-1筛选剂G418的分化筛选培养基上, 在25oC、12 h光照条件下培养, 待愈伤分化出绿点后进行继代培养, 筛选4周后将绿苗进行生根培养, 2周后移栽到土壤中。采用SDS法提取受体材料和小麦再生植株的DNA[35], DNA稀释后跑电泳检测质量和浓度, 用Ubiquitin的正向引物5'-ATCTCCCCCAAATCC ACCCG-3'和TaJRL53基因的反向引物5'-ATGCCTG CAGGATGGTATAAACACCAATCGAAG-3'进行PCR检测, 同时以含有TaJRL53 ORF的载体质粒为阳性对照, 未转化的野生型植株DNA为非转基因对照, 水为空白对照。PCR总体积为25 μL, 包含模板、正反引物、r-Taq DNA聚合酶和10×PCR缓冲液。PCR程序包括: 94oC预变性5 min, 94oC变性40 s, 60oC退火30 s, 72oC延伸2 min, 36个循环, 最后72oC延伸5 min。PCR产物在1%的琼脂糖凝胶电泳分离检测, 用EB染色后在紫外灯下观察结果并照相。
1.8 赤霉病抗性鉴定
1.8.1 小麦叶片的赤霉病抗性鉴定 取T1代转基因株系返青至拔节期的小麦叶片接种赤霉菌孢子液, 孢子液为F15、F609、F7136及F. grminearum tri5-GFP菌株的分生孢子混合液, 接种时在叶片表面用枪头轻轻划伤, 悬空放置在培养基中间, 然后接种6 μL浓度为1×106个 mL-1赤霉菌分生孢子的菌液。接种36 h后观察赤霉菌菌丝的侵染情况。实验设置3个重复, 每个重复中各接种5个叶片, 差异显著性用t-test进行检验。1.8.2 小麦穗部的赤霉病抗性鉴定 采用单花滴注法对实验组和对照组长势一致的穗子接种赤霉菌, 具体方法参照文献[36], 每个处理至少接种15个穗子, 接种后7 d和14 d调查并记录病小穗数和病轴长, 每个转基因株系至少接种10个穗子, 差异显著性用t-test进行检验。21 d时对接种的穗子进行拍照。
2 结果与分析
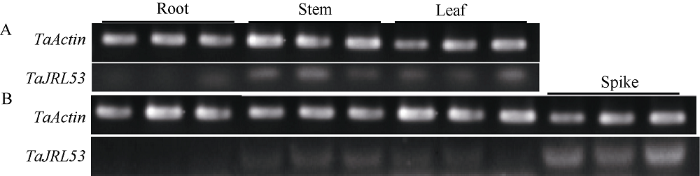
2.1 TaJRL53的克隆和组织表达特异性
利用TaJRL53特异引物从普通小麦中国春穗部cDNA中克隆获得了1056 bp的全长序列,该序列与抗赤霉病种质望水白中的序列信息一致, 说明该基因序列在抗感材料中没有差异。对TaJRL53基因在苗期和开花期各组织中的表达进行分析, 以TaActin作为参照, 结果如图2所示, 苗期主要在茎和叶片中表达(图2-A), 开花期的根茎叶穗4个组织中, 穗部表达量最高(图2-B)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2TaJRL53组织表达特异性分析
A: 苗期; B: 开花期。标尺为50 μm。
Fig. 2Tissue-specific expression of TaJRL53
A: seedling stage; B: flowering stage. Bar=50 μm.
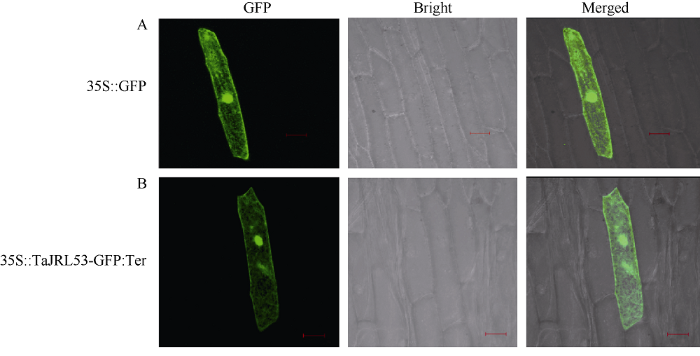
2.2 TaJRL53的亚细胞定位
为了研究TaJRL53蛋白在细胞中的表达部位, 以PMD19-EGFP (35S::GFP)作为对照, 将TaJRL53的GFP瞬时表达载体(35S::TaJRL53-GFP)转入洋葱表皮细胞后, 在激光共聚焦显微镜下观察, TaJRL53与GFP均在整个细胞中发出绿色荧光, 尤其在细胞核上的荧光更为明显, 表明TaJRL53分布于细胞质及细胞核上(图3), 是一个核-质蛋白, 与mJRLs的亚细胞定位特点一致。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3TaJRL53-GFP融合蛋白在洋葱表皮细胞中的表达
Fig. 3Expression of TaJRL53-GFP fusion protein in onion epidermal cells
2.3 VIGS介导TaJRL53沉默后降低了植株赤霉病的抗性
接种10 d后, 涂抹BSMV病毒和BSMV: TaJRL53病毒的麦穗出现病毒侵染后的表型。采用RT-PCR法检测TaJRL53在籽粒中的表达水平, 与接种FES缓冲液和BSMV病毒的对照植株相比, 接种BSMV:TaJRL53病毒的植株中的TaJRL53的表达量显著降低, 表明TaJRL53的表达被成功抑制(图4-A)。各挑选长势一致的15个植株用单花滴注法接种赤霉菌。接种3 d后, 所有接种小穗可见明显的接种点。第7 d, 涂抹BSMV:TaJRL53病毒的植株, 其接种小穗上的赤霉菌由接种点扩展到邻近小穗, 穗轴呈褐色。至接种15 d, 只涂抹FES缓冲液、涂抹FES缓冲液和BSMV空载病毒混合液及FES缓冲液和BSMV:TaJRL53病毒混合液的植株病小穗数的平均值分别为1.3个、1.3个、2.4个(图4-B), 差异达显著水平(P < 0.05); 病轴长分别为0.2、0.3、1.3 cm (图4-C), 差异达极显著水平。接种21 d后, 涂抹FES缓冲液、FES缓冲液和BSMV病毒的混合液及FES缓冲液和BSMV:TaJRL53病毒混合液的植株病小穗数和病轴长差异最为显著(图4-D), 平均病小穗数分别为1.6个、1.5个和4.6个(图4-B); 平均病轴长分别为0.3、0.3和2.1 cm (图4-C)。图4
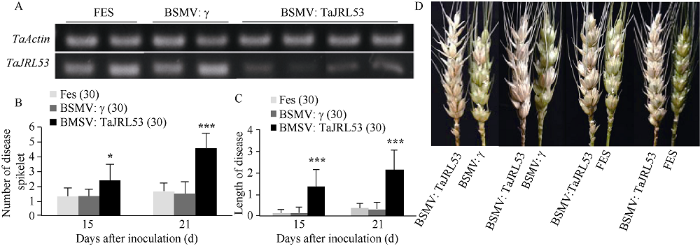 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4TaJRL53沉默后穗部的赤霉菌抗性
A: 涂抹病毒后TaJRL53在穗部的表达; B病小穗数; C: 病轴长; D: 穗部病症。图B和C中括号里的数值表示调查的样本数。*, ***分别表示在P < 0.05和P < 0.001水平的差异。
Fig. 4The FHB resistance of spikes after TaJRL53 silenced
A: Expression level of TaJRL53 in spike after inoculation with virus; B: Number of disease spikelet; C: Length of diseased rachides; D: Disease symptoms of spikes; The numbers in brackets show the number of samples. * and *** represent significant difference at level P < 0.05 and P < 0.001 (Student’s t-test).
综上所述, VIGS介导中抗赤霉病小麦SSD006中的TaJRL53沉默后, 小麦植株对赤霉病的抗性明显降低, 认为 TaJRL53对小麦赤霉病抗性有重要影响。
2.4 小麦稳定转化及阳性植株的基因型和表型鉴定
采用基因枪法将TaJRL53过量表达载体导入到感赤霉病小麦品种Bobwhite和PH691中, 经愈伤诱导和连续的G418筛选后, 对T0代抗性植株进行分子检测, 结果共获得6株以Bobwhite为受体的转基因植株, 1株以PH691为受体的转基因植株(图5-A)。利用RT-PCR分析TaJRL53在各转基因植株中的表达水平, 结果表明7个转基因植株的表达都明显高于受体, 以Bobwhite为受体的转基因植株中编号为1和2的转基因植株表达量最高, 6最低, 以PH691为受体的转基因植株7中的表达水平低于以Bobwhite为受体的1~5号植株), 略高于6号植株(图5-B)。图5
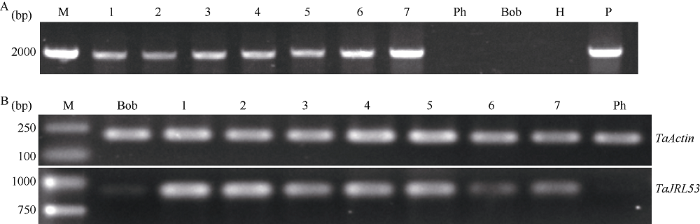 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5T0代转基因植株的PCR鉴定(A)和TaJRL53的表达水平(B)
M: 分子量marker; 1~6: 以Bobwhite为受体的转基因植株; 7: 以PH691为受体的转基因植株; Ph: PH691; Bob: Bobwhite; H: 水对照; P: 阳性对照;
Fig. 5PCR test and expression level of TaJRL53 in T0 transgenic plants with TaJRL53 overexpression
M: marker; 1-6: transgenic plants from Bobwhite; 7: transgenic plant from PH691. PH: PH691; Bob: Bobwhite; H: H2O; P: positive control.
为探究TaJRL53过量表达植株对赤霉病的抗性, 取纯合转基因T1代株系六叶一心期的叶片在离体条件下接种含GFP 基因的赤霉菌, 36 h后在荧光显微镜下观察发现, 非转基因对照Bobwhite与以其为受体的转基因植株叶片均能观察到绿色荧光, 但转基因植株叶片上的荧光与对照相比明显弱(图6-A)。与对照菌丝完全遮盖接种点相比, 转基因株系仅能看见接种点, 其中Bobwhite为受体的1、2和4号株系病斑面积显著减小, 差异在P<0.01水平显著; 编号为3和5的株系病斑面积在P<0.05水平显著(图6-B)。阴性对照PH691和以其为受体的7号转基因植株在接菌之后菌丝均沿接种点向四周扩散, 但转基因植株菌丝扩散程度与对照相比较轻, 其病斑面积的平均值与对照相比小20%, 差异在P < 0.01水平显著(图6-A, B)。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6T1代转TaJRL53基因植株的抗病表现
A: 接种赤霉菌的叶片; B: 病斑面积; C: 接种赤霉菌的穗部; D: 病小穗数; E: 病轴长。1~5: 以Bobwhite为受体的转基因植株; 7: 以Ph691为受体的转基因植株。图B、D和E中括号里的数值表示统计的样本数。*、**和***分别表示在P < 0.05、P < 0.01和P < 0.001水平差异显著。
Fig. 6FHB resistance of TaJRL53 overexpression transgenic plants
A: inoculated leaf; B: lesion area; C: inoculated spike; D: number of diseases spikelets; E: length of diseased rachide. 1-5: transgenic plants from Bobwhite; 6: transgenic plant from Ph691. The numbers in brackets of B, D, and E show the number of samples. *, **, and ***: significant difference at P < 0.05, P < 0.01, and P < 0.001 (Student’s t-test).
对纯合T1代转基因株系穗部采用单花滴注法接种赤霉菌, 接种10 d后, 未转基因对照穗部的赤霉菌由接种点开始向临近小穗蔓延, 但转基因株系并未出现明显的赤霉菌扩展现象。接种14 d后, 转基因株系与非转基因对照的病小穗数和病轴长差异显著(图6-C, D, E), 其中非转基因的Bobwhite病小穗数平均为4.2个, 病轴长平均为3.0 cm, 转基因株系的病小穗数平均值在1.7~2.0个之间, 病轴长在1.4~2.5 cm之间; 对照PH691的病小穗数与病轴长的平均值分别为5.9个和4.7 cm, 但以PH691为受体的转基因植株的病小穗数和病轴长的平均值分别为1.0个和1.6 cm, 与对照相比, 转基因植株的病小穗数与病轴长差异均在P < 0.001水平极显著(图6-D, E)。
2.5 TaJRL53过量表达的转基因植株中抗病信号途径相关基因的表达
为探究TaJRL53过量表达的转基因植株提高赤霉病抗性的分子机制, 对主要抗病信号途径SA、JA、ROS、Ca2+转运信号通路和木质素合成途径的标志基因进行了赤霉菌诱导后的表达分析。如图7所示, 当赤霉菌侵染TaJRL53过表达的转基因植株24 h时, JA信号转导途径中的基因12-OPR3、ACO以及效应基因ERF1、病程相关蛋白基因PR-3的表达均显著提高(图7-A~D)。对ROS生成途径中的两个关键酶SAMDC、PAO及与活性氧运输相关的Ca2+转运信号通路相关基因calcium-transporting ase1的表达进行检测, 发现这3个基因在转基因株系中的表达量都显著高于非转基因对照植株(图7-E~G)。在SA信号转导途径中除了病程相关蛋白基因PR1、PR2表达量显著提高外(图7-H, I), 效应基因Glu2、NPR1、EDS1 (图7-J~L), SA合成相关基因ICS1和PAL等的表达都无显著差异(图7-M, N); 转基因植株与非转基因植株的木质素合成基因CCOMT表达水平无显著差异(图7-O)。由此初步推测TaJRL53提高小麦赤霉病抗性与JA和ROS信号途径以及病程相关蛋白有关。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7抗病信号途径中标志基因的表达
A~C: JA信号途径的标志基因; D, H, I: 病程相关基因; E, F: ROS合成途径相关基因; G: 活性氧运输相关基因; J~L: SA信号途径相关基因; M, N: SA合成相关基因; O: 木质素合成基因; Bob: Bobwhite; 1~4: Bobwhite为受体的转基因植株; *和**分别表示在P < 0.05和P < 0.01水平差异显著。
Fig. 7Expression of marker genes in resistance signaling pathways
A-C: marker genes in JA singnal pathway; D, H, I: pathogenesis-related genes; E, F: ROS biosynthesis-related genes; G: ROS transport-related genes; J-L: SA singnal pathway-related genes; M, N: SA biosynthesis-related genes; O: lignin biosynthesis genes; Bob: Bobwhite; 1-4: transgenic plants from Bobwhite; * and **: significant difference at P < 0.05 and P < 0.01 (Student’s t-test).
3 讨论
尽管目前发现的JRL凝集素存在于植物的多种组织, 但对于某一个特定的JRL凝集素而言, 其在植物体内的表达往往仅限于某一个或少数几个组织, 具有一定的组织特异性。Song等[6]和Jiang等[7]对JRL基因在不同组织中的表达谱进行统计分析后发现, 21.4%的JRL基因都具有组织表达特异性。例如Lem2在内外稃及胚芽中表达[40], MRL-1和MRL-2都在根中表达[41], TaJA1在叶鞘中表达[31]。本研究对TaJRL53的组织特异性表达进行分析, 发现TaJRL53苗期主要在茎和叶片中表达, 开花期在茎、叶、穗部都有表达, 但主要在穗部表达。根据糖基结合的特异性可以将JRLs划分为两类: 一类是半乳糖特异的jacalin凝集素(galactose- specific jacalin-related lectins, gJRLs), 另一类是甘露糖或葡萄糖特异的jacalin凝集素(mannose-specific jacalin- related lectins, mJRLs)。现阶段所鉴定的JRLs大多数属于mJRLs, 少数属于gJRLs。对水稻、拟南芥和小麦中的mJRLs亚细胞定位结果显示大多数位于核/质间[39,40,41]。本研究通过洋葱表皮细胞亚细胞定位, 发现TaJRL53蛋白分布在细胞核/质中, 表明TaJRL53具有mJRLs亚家族成员典型的蛋白定位特征, 初步推测TaJRL53属于mJRLs亚家族成员。特异糖基结合试验有助于进一步确定该凝集素本身的性质。
Dirigent蛋白通常被认为在木质素和木脂素的生物合成中起重要作用, 与Dirigent结构域嵌合的JRLs基因在植物的应激反应和发育中扮演着重要角色[12], 例如TaJA1, 及其在水稻和大麦中的同源基因OsJAC1、HvJAC1均具有广谱抗性[31,45]。TaJRL53的蛋白编码结构与TaJA1相似, 都具有N端的Dirigent结构域和C端的Jacalin结构域[6]。本研究对TaJRL53的功能进行探究, 认为该基因参与对赤霉菌的防御过程, 对赤霉菌的侵染具有抗性。本研究首先采用BSMV的方法降低TaJRL53在中抗赤霉病品种SSD006中的表达, 发现植株对赤霉病的抗性降低。然后采用基因枪介导的遗传转化技术, 将TaJRL53过量表达载体转入感赤霉病品种Bobwhite和PH691中, 分别获得6株和1株TaJRL53的过量表达植株。对T1代转基因植株采用离体叶片法和穗部单花滴注法接种赤霉菌鉴定转基因株系对赤霉菌的抗性, 离体叶片法接菌结果显示: 以Bobwhite为受体的转基因株系其叶片上的荧光与对照相比明显减弱, 菌斑面积显著减小。阴性对照PH691和以其为受体的转基因植株在接菌之后菌丝均沿接种点向四周扩散, 但转基因植株菌丝扩散程度与对照相比较轻。单花滴注法接种T1代小穗后, 结果显示转基因株系与对照均出现赤霉病的典型症状, 但转基因株系与受体亲本相比病害较轻。对T2代转基因株系的穗部接菌进行抗性鉴定, 各转基因株系与T1代的抗病趋势一致, 表明TaJRL53的转基因株系可以减轻由禾谷镰刀菌引起的病害。同时, 本研究发现两个不同背景的转基因株系抗病性存在差异, 这可能是因为两个受体材料对赤霉病的抗性和自身的组培性能存在差异, 转基因植株中TaJRL53的拷贝数不同造成的。
SA和JA介导的信号传导途径是植物在应对病原菌侵染时被激活的防卫途径, JA在死体寄生和兼性寄生型病原菌侵染过程中起重要作用, SA在活体寄生型病原菌的侵染过程中起重要作用, SA和JA途径均参与兼性寄生型真菌赤霉菌的侵染反应[46,47]。TaJRLL1是mJRLs亚家族中的一员, 该基因通过参与SA和JA的植物防御信号机制的组成部分, 实现小麦植株对死体寄生的灰霉菌, 活体寄生的白粉菌以及兼性寄生的赤霉菌的抗性[28]。本研究对接种赤霉菌后的TaJRL53转基因植株的SA和JA信号转导途径相关基因的表达进行检测, 发现JA信号转导途径主要标志基因的表达量与对照相比有所提高, 但SA信号转导途径相关基因的表达并无明显变化。ROS的产生是植物最早期的防御反应途径, ROS的积累一方面可诱导防御基因的表达, 另一方面通过胼胝质沉积增加植物细胞壁的厚度[48]。在TaJRL53过量表达的转基因植株中ROS生成途径的关键基因SAMDC和PAO的表达上调, 但是细胞壁合成相关的酶基因CCOMT并无表达变化, 说明TaJRL53过量表达只是改变了植株体内ROS的产生水平, 并不影响细胞壁的合成。
综上所述, TaJRL53可能通过调控植物体内活性氧产生、JA信号途径、诱导病程相关蛋白基因的表达水平, 从而提高植株对赤霉菌的抗性。但该基因的赤霉病抗病机制还不清楚, 其发挥作用的结构域是Dirigent还是Jacalin或是共同作用还不得而知, 碳水化合物结合蛋白作为mJRLs细胞活动中的关键物质, 其结合特异性在植物防御机制中的作用今后还有待阐明。
4 结论
通过VIGS技术和基因枪介导的遗传转化技术证明TaJRL53与赤霉病的抗性呈正相关, 赤霉菌侵染转基因株系后, ROS和JA信号途径相关基因表现出上调趋势, 说明TaJRL53可能是通过参与ROS合成途径和JA信号转导途径来提高转基因植株对赤霉病的抗性。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.cell.2006.02.008URLPMID:16497589 [本文引用: 1]

The evolution of the plant immune response has culminated in a highly effective defense system that is able to resist potential attack by microbial pathogens. The primary immune response is referred to as PAMP-triggered immunity (PTI) and has evolved to recognize common features of microbial pathogens. In the coevolution of host-microbe interactions, pathogens acquired the ability to deliver effector proteins to the plant cell to suppress PTI, allowing pathogen growth and disease. In response to the delivery of pathogen effector proteins, plants acquired surveillance proteins (R proteins) to either directly or indirectly monitor the presence of the pathogen effector proteins. In this review, taking an evolutionary perspective, we highlight important discoveries over the last decade about the plant immune response.
URLPMID:17108957 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1080/07060660009501155URL [本文引用: 1]
URLPMID:20565626 [本文引用: 1]
DOI:10.1007/s11103-013-0121-5URL [本文引用: 4]

Jacalin-related lectins (JRLs) are carbohydrate-binding proteins widely present in plants and have one or more jacalin domains in common. However, JRLs' structural types and functions are still poorly understood. In the present study, a total of 67 wheat (Triticum aestivum) JRL genes were identified through an exhausted search of EST database coupling with genome walking using published 454 sequence reads of Chinese Spring. A comparison of the translated wheat JRL proteins with those from other plants showed plant JRLs generally had low sequence similarity within and between species but exhibited conserved modular domain structures. More JRL genes encoded multiple jacalin domains in Arabidopsis thaliana, whereas more genes encoded chimeric JRLs in cereal plants. Dirigent domain-containing JRL genes were Poaceae-specific and accounted for nearly half of the identified wheat JRL genes. The dirigent domains were evolutionarily significantly correlated with the covalently linked jacalin domains. A phylogenetic analysis showed JRL proteins have experienced a substantial diversification after speciation. Moreover, new structural features conserved across the taxa were identified. Digital expression analysis and RT-PCR assays showed the expression of wheat JRL genes was largely tissue specific, typically low, and mostly inducible by biotic and abiotic stresses and stress hormones. These results suggest plant JRLs are critical for plant adaptation to stressful environments.
DOI:10.1186/1471-2148-10-79URLPMID:20236552 [本文引用: 2]

BACKGROUND: Lectins are a class of carbohydrate-binding proteins. They play roles in various biological processes. However, little is known about their evolutionary history and their functions in plant stress regulation. The availability of full genome sequences from various plant species makes it possible to perform a whole-genome exploration for further understanding their biological functions. RESULTS: Higher plant genomes encode large numbers of lectin proteins. Based on their domain structures and phylogenetic analyses, a new classification system has been proposed. In this system, 12 different families have been classified and four of them consist of recently identified plant lectin members. Further analyses show that some of lectin families exhibit species-specific expansion and rapid birth-and-death evolution. Tandem and segmental duplications have been regarded as the major mechanisms to drive lectin expansion although retrogenes also significantly contributed to the birth of new lectin genes in soybean and rice. Evidence shows that lectin genes have been involved in biotic/abiotic stress regulations and tandem/segmental duplications may be regarded as drivers for plants to adapt various environmental stresses through duplication followed by expression divergence. Each member of this gene superfamily may play specialized roles in a specific stress condition and function as a regulator of various environmental factors such as cold, drought and high salinity as well as biotic stresses. CONCLUSIONS: Our studies provide a new outline of the plant lectin gene superfamily and advance the understanding of plant lectin genes in lineage-specific expansion and their functions in biotic/abiotic stress-related developmental processes.
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URLPMID:30065388 [本文引用: 1]
DOI:10.1093/glycob/cwh140URLPMID:15329359 [本文引用: 1]

Lectins are known to be important for many biological processes, due to their ability to recognize cell surface carbohydrates with high specificity. Plant lectins have been model systems to study protein-carbohydrate recognition, because individually they exhibit high sensitivity and as a group large diversity in recognizing carbohydrate structures. Although extensive studies have been carried out for legume lectins that have led to interesting insights into the sequence determinants of sugar recognition in them, frameworks with such specific correlations are not available for other plant lectin families. This study reports a large-scale data acquisition and extensive analysis of sequences and structures of beta-prism-I or jacalin-related lectins (JRLs) and shows that hypervariability in the binding site loops generates carbohydrate recognition diversity, a strategy analogous to that in legume lectins. Analyses of the size, conformation, and sequence variability in key regions reveal the existence of a common theme, encoded as a set of structural features over a common scaffold, in defining specificity. This study also points to the remarkable range of domain architectures, often arising out of gene duplication events in lectins of this family. The data analyzed here also indicate a spectacular variety of quaternary associations possible in this family of lectins that have implications for glycan recognition. These results thus provide sequence-structure-function correlations, an understanding of the molecular basis of carbohydrate recognition by beta-prism-I lectins, and also a rationale for engineering specific recognition capabilities in relevant molecules.
DOI:10.3109/07388551.2013.793650URLPMID:23886351 [本文引用: 2]

Monocot chimeric jacalins are a small group of lectins (currently with nine members), each typically consisting of a dirigent domain and a jacalin-related lectin domain. This unique module structure, along with their limited taxonomic distribution and short time window in molecular evolution, makes them a novel family of lectins. Recent studies have shown that these proteins play important roles in plant stress responses and development. Our knowledge of these proteins in functional domain and evolution has also made significant progress.
DOI:10.3390/ijms18071592URL [本文引用: 1]
DOI:10.1023/a:1012276619686URLPMID:11732688 [本文引用: 1]

A homodimeric lectin adsorbed on Affi-gel blue gel and CM-Sepharose and possessing a molecular weight of 67 kDa was isolated from red kidney beans. The hemagglutinating activity of this lectin was inhibited by glycoproteins but not by simple sugars. The lectin manifested inhibitory activity on human immunodeficiency virus-1 reverse transcriptase and alpha-glucosidase. The N-terminal sequence of the lectin exhibited some differences from previously reported lectins from Phaseolus vulgaris but showed some similarity to chitinases. It exerted a suppressive effect on growth of the fungal species Fusarium oxysporum, Coprinus comatus, and Rhizoctonia solani. The lectin had low ribonuclease and negligible translation-inhibitory activities.
DOI:10.1371/journal.ppat.1002513URLPMID:22346749 [本文引用: 1]

Stomata play an important role in plant innate immunity by limiting pathogen entry into leaves but molecular mechanisms regulating stomatal closure upon pathogen perception are not well understood. Here we show that the Arabidopsis thaliana L-type lectin receptor kinase-V.5 (LecRK-V.5) negatively regulates stomatal immunity. Loss of LecRK-V.5 function increased resistance to surface inoculation with virulent bacteria Pseudomonas syringae pv tomato DC3000. Levels of resistance were not affected after infiltration-inoculation, suggesting that LecRK-V.5 functions at an early defense stage. By contrast, lines overexpressing LecRK-V.5 were more susceptible to Pst DC3000. Enhanced resistance in lecrk-V.5 mutants was correlated with constitutive stomatal closure, while increased susceptibility phenotypes in overexpression lines were associated with early stomatal reopening. Lines overexpressing LecRK-V.5 also demonstrated a defective stomatal closure after pathogen-associated molecular pattern (PAMP) treatments. LecRK-V.5 is rapidly expressed in stomatal guard cells after bacterial inoculation or treatment with the bacterial PAMP flagellin. In addition, lecrk-V.5 mutants guard cells exhibited constitutive accumulation of reactive oxygen species (ROS) and inhibition of ROS production opened stomata of lecrk-V.5. LecRK-V.5 is also shown to interfere with abscisic acid-mediated stomatal closure signaling upstream of ROS production. These results provide genetic evidences that LecRK-V.5 negatively regulates stomatal immunity upstream of ROS biosynthesis. Our data reveal that plants have evolved mechanisms to reverse bacteria-mediated stomatal closure to prevent long-term effect on CO(2) uptake and photosynthesis.
DOI:10.1105/tpc.112.095778URL [本文引用: 1]
URLPMID:22307853 [本文引用: 1]
DOI:10.1094/MPMI-04-13-0094-RURL [本文引用: 1]
URLPMID:21205632 [本文引用: 1]
[本文引用: 2]
[本文引用: 1]
URLPMID:10618445 [本文引用: 1]
URLPMID:11743111 [本文引用: 1]
[本文引用: 1]
URLPMID:18467340 [本文引用: 1]
DOI:10.1016/j.molp.2015.12.009URLPMID:26708413 [本文引用: 1]

Modular proteins are an evolutionary answer to optimize performance of proteins that physically interact with each other for functionality. Using a combination of genetic and biochemical experiments, we characterized the rice protein OsJAC1, which consists of a jacalin-related lectin (JRL) domain predicted to bind mannose-containing oligosaccharides, and a dirigent domain which might function in stereoselective coupling of monolignols. Transgenic overexpression of OsJAC1 in rice resulted in quantitative broad-spectrum resistance against different pathogens including bacteria, oomycetes, and fungi. Overexpression of this gene or its wheat ortholog TAJA1 in barley enhanced resistance against the powdery mildew fungus. Both protein domains of OsJAC1 are required to establish resistance as indicated by single or combined transient expression of individual domains. Expression of artificially separated and fluorescence-tagged protein domains showed that the JRL domain is sufficient for targeting the powdery mildew penetration site. Nevertheless, co-localization of the lectin and the dirigent domain occurred. Phylogenetic analyses revealed orthologs of OsJAC1 exclusively within the Poaceae plant family. Dicots, by contrast, only contain proteins with either JRL or dirigent domain(s). Altogether, our results identify OsJAC1 as a representative of a novel type of resistance protein derived from a plant lineage-specific gene fusion event for better function in local pathogen defense.
DOI:10.1104/pp.18.01594URLPMID:30696749 [本文引用: 1]

The genome of rice blast fungus (Magnaporthe oryzae) encodes 15 glycoside hydrolase 18 family chitinases. In this study, we characterized the function of an M. oryzae extracellular chitinase, MoChi1, and its interaction with a host protein, OsMBL1, a jacalin-related Mannose-Binding Lectin (MBL) in rice (Oryza sativa). Deletion of MoChi1 resulted in reduced aerial hyphal formation and reduced virulence in rice by activating the expression of defense-responsive genes. We confirmed MoChi1 interaction with rice OsMBL1 in vitro and in vivo. OsMBL1 was induced by pathogen-associated molecular patterns and M. oryzae infection. Overexpression of OsMBL1 led to activation of rice defense-responsive genes and a chitin-induced reactive oxygen species burst, thereby enhancing resistance to M. oryzae Knockdown of OsMBL1 enhances susceptibility of rice plants to M. oryzae Furthermore, MoChi1 suppressed chitin-induced reactive oxygen species in rice cells and competed with OsMBL1 for chitin binding. Taken together, our study reveals a mechanism in which MoChi1 targets a host lectin to suppress rice immunity.
DOI:10.1093/jxb/err226URLPMID:21862481 [本文引用: 2]

Jacalin-related lectins (JRLs) are a subgroup of proteins with one or more jacalin-like lectin domains. Although JRLs are often associated with biotic or abiotic stimuli, their biological functions in plants, as well as their relationships to plant disease resistance, are poorly understood. A mannose-specific JRL (mJRL)-like gene (TaJRLL1) that is mainly expressed in stem and spike and encodes a protein with two jacalin-like lectin domains was identified in wheat. Pathogen infection and phytohormone treatments induced its expression; while application of the salicylic acid (SA) biosynthesis inhibitor paclobutrazol and the jasmonic acid (JA) biosynthesis inhibitor diethyldithiocarbamic acid, respectively, substantially inhibited its expression. Attenuating TaJRLL1 through virus-induced gene silencing increased susceptibility to the facultative fungal pathogen Fusarium graminearum and the biotrophic fungal pathogen Blumeria graminis. Arabidopsis thaliana transformed with TaJRLL1 displayed increased resistance to F. graminearum and Botrytis cinerea. JA and SA levels in transgenic Arabidopsis increased significantly. A loss or increase of disease resistance due to an alteration in TaJRLL1 function was correlated with attenuation or enhancement of the SA- and JA-dependent defence signalling pathways. These results suggest that TaJRLL1 could be a component of the SA- and JA-dependent defence signalling pathways.
DOI:10.1105/tpc.8.4.629URLPMID:8624439 [本文引用: 1]

Systemic acquired resistance is an important component of the disease resistance repertoire of plants. In this study, a novel synthetic chemical, benzo(1,2,3)thiadiazole-7-carbothioic acid S-methyl ester (BTH), was shown to induce acquired resistance in wheat. BTH protected wheat systemically against powdery mildew infection by affecting multiple steps in the life cycle of the pathogen. The onset of resistance was accompanied by the induction of a number of newly described wheat chemically induced (WCI) genes, including genes encoding a lipoxygenase and a sulfur-rich protein. With respect to both timing and effectiveness, a tight correlation existed between the onset of resistance and the induction of the WCI genes. Compared with other plant activators, such as 2,6-dichloroisonicotinic acid and salicylic acid, BTH was the most potent inducer of both resistance and gene induction. BTH is being developed commercially as a novel type of plant protection compound that works by inducing the plant's inherent disease resistance mechanisms.
DOI:10.1104/pp.108.116145URLPMID:18467454 [本文引用: 1]

We previously cloned and characterized a novel jacalin-like lectin gene from wheat (Triticum aestivum) plants that responds to infestation by Hessian fly (Mayetiola destructor) larvae, a major dipteran pest of this crop. The infested resistant plants accumulated higher levels of Hfr-1 (for Hessian fly-responsive gene 1) transcripts compared with uninfested or susceptible plants. Here, we characterize the soluble and active recombinant His(6)-HFR1 protein isolated from Escherichia coli. Functional characterization of the protein using hemagglutination assays revealed lectin activity. Glycan microarray-binding assays indicated strong affinity of His(6)-HFR1 to Manalpha1-6(Manalpha1-3)Man trisaccharide structures. Resistant wheat plants accumulated high levels of HFR1 at the larval feeding sites, as revealed by immunodetection, but the avirulent larvae were deterred from feeding and consumed only small amounts of the lectin. Behavioral studies revealed that avirulent Hessian fly larvae on resistant plants exhibited prolonged searching and writhing behaviors as they unsuccessfully attempted to establish feeding sites. During His(6)-HFR1 feeding bioassays, Drosophila melanogaster larvae experienced significant delays in growth and pupation, while percentage mortality increased with progressively higher concentrations of His(6)-HFR1 in the diet. Thus, HFR1 is an antinutrient to dipteran larvae and may play a significant role in deterring Hessian fly larvae from feeding on resistant wheat plants.
DOI:10.1016/j.biochi.2009.11.008URLPMID:19958808 [本文引用: 4]

Jasmonates are known to induce the transcriptional activation of plant defense genes, which leads to the production of jasmonate-regulated proteins (JRP). We previously cloned and characterized a novel jacalin-like lectin gene (Ta-JA1) from wheat (Triticum aestivum L.), which codes a modular JRP with disease response and jacalin-related lectin (JRL) domains and is present only in the Gramineae family. The function of this protein is still unclear. Phylogenetic analysis indicated that Ta-JA1 and related proteins from cereals grouped together, which diverged from JRL with an additional N-terminal disease response domain. The recombinant Ta-JA1 proteins agglutinated rabbit erythrocytes, and this hemagglutination activity was preferentially inhibited by mannose. The Ta-JA1 protein was able to inhibit E. coli cell growth. Overexpression of Ta-JA1 in transgenic tobacco plants increased their resistance to infection by tobacco bacterial, fungal and viral pathogens. Our results suggest that Ta-JA1 belongs to a mannose-specific lectin, which may confer a basal but broad-spectrum resistance to plant pathogens. Ta-JA1 and its homologues in maize, rice, sorghum and creeping bentgrass may represent a new type of monocot lectin with a modular structure and diversity of physiological functions in biotic and abiotic stress responses.
URLPMID:27427289 [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
URLPMID:11846609 [本文引用: 1]
URLPMID:21533105 [本文引用: 1]
DOI:10.1038/s41588-019-0426-7URLPMID:31182810 [本文引用: 1]

Head or ear blight, mainly caused by Fusarium species, can devastate almost all staple cereal crops (particularly wheat), resulting in great economic loss and imposing health threats on both human beings and livestock(1-3). However, achievement in breeding for highly resistant cultivars is still not satisfactory. Here, we isolated the major-effect wheat quantitative trait locus, Qfhs.njau-3B, which confers head blight resistance, and showed that it is the same as the previously designated Fhb1. Fhb1 results from a rare deletion involving the 3' exon of the histidine-rich calcium-binding-protein gene on chromosome 3BS. Both wheat and Arabidopsis transformed with the Fhb1 sequence showed enhanced resistance to Fusarium graminearum spread. The translation products of this gene's homologs among plants are well conserved and might be essential for plant growth and development. Fhb1 could be useful not only for curbing Fusarium head blight in grain crops but also for improving other plants vulnerable to Fusarium species.
[本文引用: 2]
DOI:10.4161/psb.24563URLPMID:23603955 [本文引用: 2]

Plant microRNAs (miRNAs) are important regulators of development and stress responses and are oftentimes under transcriptional regulation by stresses and plant hormones. We recently showed that polycistronic MIR842 and MIR846 are expressed from the same primary transcript which is subject to alternative splicing. ABA treatment affects the alternative splicing of the primary cistronic transcript which results in differential expression of the two miRNAs that are predicted to target the same family of jacalin lectin genes. One variant of miR846 in roots can direct the cleavage of AT5G28520, which is also highly upregulated by ABA in roots. In this addendum, we present additional results further supporting the regulation of AT5G28520 by MIR846 using a T-DNA insertion line mapping upstream of MIR842 and MIR846. We also show that AT5G28520 is transcriptionally induced by ABA and this induction is subject to ABA signaling effectors in seedlings. Based on previous results and data presented in this paper, we propose an interaction loop between MIR846, AT5G28520 and ABA in roots.
DOI:10.1371/journal.pone.0004854URLPMID:19287503

BACKGROUND: O-linked beta-N-acetylglucosamine (O-GlcNAc) modification of proteins mediates stress response and cellular motility in animal cells. The plant lectin concanavalin A can increase nuclear O-GlcNAc levels and decrease cytoplasmic O-GlcNAc levels in T lymphocytes. However, the functions of O-GlcNAc signaling in plants, as well as the relation between plant lectins and O-GlcNAc in response to environmental stimuli are largely undefined. METHODOLOGY/PRINCIPAL FINDINGS: We describe a jacalin-like lectin VER2 in wheat that shows N-acetylglucosamine and galactose specificity. Immunocytochemical localization showed VER2 expression induced predominantly at potential nuclear structures in shoot tips and young leaves and weakly in cytoplasm in response to vernalization. In contrast, under devernalization (continuous stimulation with a higher temperature after vernalization), VER2 signals appeared predominantly in cytoplasm. 2-D electrophoresis, together with western blot analysis, showed phosphorylation modification of VER2 under vernalization. Immunoblot assay with O-GlcNAc-specific antibody revealed that vernalization increased O-GlcNAc modification of proteins at the global level. An O-GlcNAc-modified protein co-immunoprecipitated with VER2 in vernalized wheat plants but not in devernalized materials. The dynamic of VER2 was observed in transgenic Arabidopsis overexpressing the VER2-GFP fusion protein. Overexpressed VER2 accelerated nuclear migration. Immunogold labeling and indirect immunofluoresence colocalization assay indicated that VER2-GFP was targeted to the secretory pathway. CONCLUSIONS/SIGNIFICANCE: O-GlcNAc signaling is involved in the vernalization response in wheat, and phosphorylation is necessary for the lectin VER2 involving O-GlcNAc signaling during vernalization. Our findings open the way to studies of O-GlcNAc protein modification in response to environmental signals in plants.
URLPMID:27718311
DOI:10.3390/ijms20040815URL
DOI:10.1016/j.molp.2015.12.009URLPMID:26708413 [本文引用: 1]

Modular proteins are an evolutionary answer to optimize performance of proteins that physically interact with each other for functionality. Using a combination of genetic and biochemical experiments, we characterized the rice protein OsJAC1, which consists of a jacalin-related lectin (JRL) domain predicted to bind mannose-containing oligosaccharides, and a dirigent domain which might function in stereoselective coupling of monolignols. Transgenic overexpression of OsJAC1 in rice resulted in quantitative broad-spectrum resistance against different pathogens including bacteria, oomycetes, and fungi. Overexpression of this gene or its wheat ortholog TAJA1 in barley enhanced resistance against the powdery mildew fungus. Both protein domains of OsJAC1 are required to establish resistance as indicated by single or combined transient expression of individual domains. Expression of artificially separated and fluorescence-tagged protein domains showed that the JRL domain is sufficient for targeting the powdery mildew penetration site. Nevertheless, co-localization of the lectin and the dirigent domain occurred. Phylogenetic analyses revealed orthologs of OsJAC1 exclusively within the Poaceae plant family. Dicots, by contrast, only contain proteins with either JRL or dirigent domain(s). Altogether, our results identify OsJAC1 as a representative of a novel type of resistance protein derived from a plant lineage-specific gene fusion event for better function in local pathogen defense.
DOI:10.1055/s-2005-872705URLPMID:16435264 [本文引用: 1]

Plant defences against pathogens and herbivorous insects form a comprehensive network of interacting signal transduction pathways. The signalling molecules salicylic acid (SA) and jasmonic acid (JA) play important roles in this network. SA is involved in signalling processes providing systemic acquired resistance (SAR), protecting the plant from further infection after an initial pathogen attack. SAR is long-lasting and provides broad spectrum resistance to biotrophic pathogens that feed on a living host cell. The regulatory protein NPR1 is a central positive regulator of SAR. SA-activated NPR1 localizes to the nucleus where it interacts with TGA transcription factors to induce the expression of a large set of pathogenesis-related proteins that contribute to the enhanced state of resistance. In a distinct signalling process, JA protects the plant from insect infestation and necrotrophic pathogens that kill the host cell before feeding. JA activates the regulatory protein COI1 that is part of the E3 ubiquitin ligase-containing complex SCFCOI1, which is thought to derepress JA-responsive genes involved in plant defence. Both synergistic and antagonistic interactions have been observed between SA- and JA-dependent defences. NPR1 has emerged as a critical modulator of cross-talk between the SA and JA signal and is thought to aid in fine tuning defence responses specific to the encountered attacker. Here we review SA- and JA-dependent signal transduction and summarize our current understanding of the molecular mechanisms of cross-talk between these defences.
DOI:10.1046/j.1365-313x.2003.01717.xURLPMID:12694596 [本文引用: 1]

The signal transduction network controlling plant responses to pathogens includes pathways requiring the signal molecules salicylic acid (SA), jasmonic acid (JA), and ethylene (ET). The network topology was explored using global expression phenotyping of wild-type and signaling-defective mutant plants, including eds3, eds4, eds5, eds8, pad1, pad2, pad4, NahG, npr1, sid2, ein2, and coi1. Hierarchical clustering was used to define groups of mutations with similar effects on gene expression and groups of similarly regulated genes. Mutations affecting SA signaling formed two groups: one comprised of eds4, eds5, sid2, and npr1-3 affecting only SA signaling; and the other comprised of pad2, eds3, npr1-1, pad4, and NahG affecting SA signaling as well as another unknown process. Major differences between the expression patterns in NahG and the SA biosynthetic mutant sid2 suggest that NahG has pleiotropic effects beyond elimination of SA. A third group of mutants comprised of eds8, pad1, ein2, and coi1 affected ethylene and jasmonate signaling. Expression patterns of some genes revealed mutual inhibition between SA- and JA-dependent signaling, while other genes required JA and ET signaling as well as the unknown signaling process for full expression. Global expression phenotype similarities among mutants suggested, and experiments confirmed, that EDS3 affects SA signaling while EDS8 and PAD1 affect JA signaling. This work allowed modeling of network topology, definition of co-regulated genes, and placement of previously uncharacterized regulatory genes in the network.
DOI:10.1007/s13313-016-0455-yURL [本文引用: 1]
