 ,1,**, 胡书婷
,1,**, 胡书婷 ,2,**, 张凯2, 崔则瑾2, 李建生2, 杨小红2, 白光红
,2,**, 张凯2, 崔则瑾2, 李建生2, 杨小红2, 白光红 ,1,*
,1,*Phenotypic analysis and fine mapping of dek101 in maize
SONG Xin-Ran ,1,**, HU Shu-Ting
,1,**, HU Shu-Ting ,2,**, ZHANG Kai2, CUI Ze-Jin2, LI Jian-Sheng2, YANG Xiao-Hong2, BAI Guang-Hong
,2,**, ZHANG Kai2, CUI Ze-Jin2, LI Jian-Sheng2, YANG Xiao-Hong2, BAI Guang-Hong ,1,*
,1,*通讯作者:
收稿日期:2020-03-17接受日期:2020-07-2网络出版日期:2020-08-18
| 基金资助: |
Received:2020-03-17Accepted:2020-07-2Online:2020-08-18
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (2166KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
宋欣冉, 胡书婷, 张凯, 崔则瑾, 李建生, 杨小红, 白光红. 玉米籽粒突变体dek101的表型分析和精细定位[J]. 作物学报, 2020, 46(12): 1831-1838. doi:10.3724/SP.J.1006.2020.03017
SONG Xin-Ran, HU Shu-Ting, ZHANG Kai, CUI Ze-Jin, LI Jian-Sheng, YANG Xiao-Hong, BAI Guang-Hong.
胚乳作为玉米籽粒储藏物质的重要器官, 占籽粒干物质的70%~90% [1]。在玉米生殖生长发育过程中, 胚乳作为主要的库器官积累淀粉、蛋白质等营养物质直至生理成熟。同时, 在玉米种子萌发过程中为胚的生长发育提供营养。所以, 深入挖掘玉米籽粒形成和发育的关键基因, 解析籽粒发育规律和遗传调控机制, 对提高玉米产量和改良玉米品质具有重要意义。
籽粒突变体是作物种子发育基因克隆的重要遗传材料。目前为止, 以籽粒突变体为材料已经克隆了大量控制玉米籽粒发育的基因, 如参与碳水化合物代谢相关基因BT1、BT2、SH2、SU2、WAXY等, 这些基因的功能丧失降低了胚乳淀粉含量[2,3,4,5,6], 并在甜玉米、糯玉米等特用玉米品种选育中得到广泛的应用; 影响胚发育, 如EMP2、EMP4、DEK15、DEK38、DEK44、EMP12、EMP144等基因突变通过扰乱线粒体RNA编辑、叶绿体中核糖体亚基组装、减数分裂中核内复制等过程导致胚败育[7,8,9,10,11,12,13]。除了影响胚发育和胚乳淀粉累积的基因突变体外, 影响玉米籽粒蛋白品质的基因, 如O2、FL2、O7等突变改善了籽粒中赖氨酸等必需氨基酸比例, 使玉米蛋白品质大幅提高, 但同时也带来了许多不良性状, 如产量降低、籽粒变软、抗病性下降等, 难以达到育种推广的基本要求[14,15,16,17]。最近, 黄永财等[18]发现VKS1在籽粒发育早期特异性的高表达, 证明早期的胚乳细胞数目决定最终籽粒大小, 为玉米产量的遗传改良提供了候选基因。郑喜喜等[19]发现VP1直接调控玉米胚盾片的发育, 并参与控制胚乳和胚间营养重分配的过程。综上, 对玉米籽粒发育有关突变体的遗传研究, 对选育高产优质玉米新品种具有重要的指导意义。
本研究以玉米DH系选育过程中发现的玉米籽粒突变体dek101为研究材料, 通过对籽粒发育动态的观察、籽粒表型的测定和遗传分析与精细定位, 为玉米籽粒发育基因的克隆及籽粒发育机制的解析奠定一定的理论基础。
1 材料与方法
1.1 试验材料及田间试验
本研究所用的籽粒突变体dek101来源于美国玉米带种质的双单倍体选系(double haploid, DH), 即以PH4CV/PHB1M//PH4CV为材料, 用诱导系CM500诱导产生的单倍体加倍材料。由于dek101播种不能萌发, 故用杂合植株(+/dek101)与自交系B73杂交得到F1, 自交后, 获得F2和F3群体, 用于该突变基因的精细定位。种植方式为行长5 m, 每行21株, 行距0.5 m, 株距0.25 m, 按照当地的栽培方法管理。1.2 突变体的籽粒表型观察
2015年在中国农业大学北京上庄试验站种植杂合植株(+/dek101), 播种自交后, 分别取授粉后6、9、12、15、18、21、24、27、30、33和36 d的野生型和突变型籽粒各80粒, 观察授粉后不同发育时期表型变化。1.2.1 籽粒发育动态观察 挑取授粉后不同天数具代表性的野生型和突变型籽粒各1粒,照相记录, 观察籽粒发育动态过程。
1.2.2 籽粒鲜重和干重 挑选具有代表性的纯合野生型和杂合果穗(+/dek101)各3个, 取每个果穗中部具有代表性的野生型和突变型籽粒各50粒, 使用分析天平称量, 记录籽粒鲜重后, 保存于-20℃冰箱, 待全部取样结束后, 将各个时期的籽粒取出并于65℃条件下烘72 h, 记录烘干后的干重。
1.2.3 籽粒体积测量 采用排酒精法进行测量, 取野生型和突变型籽粒各20粒, 倒入滴定管, 读取加入籽粒前后液面刻度变化的差值, 即为20粒籽粒体积。
1.2.4 胚乳扫描电镜观察 分别取15、21和27 DAP (days after pollination)具有代表性的正常和突变型籽粒各6粒, 纵切后保存在FAA固定液中并抽真空, 通过缓冲液冲洗、乙醇梯度脱水、喷金等过程, 利用日立S-3400N扫描电镜进行胚乳淀粉粒观察。
1.3 突变体的遗传分析
对杂合植株(+/dek101)与B73杂交自交一代得到的4个结实较好的F2籽粒分离果穗进行表型鉴定, 计算每个果穗以及所有果穗中野生型籽粒和突变型籽粒的数目, 利用卡方测验进行3:1分离比检验。1.4 DNA提取和精细定位
种植F2定位群体, 采用CTAB法提取玉米叶片基因组DNA和44个突变籽粒的基因组DNA。从Maize GDB (利用组配的F2定位群体的441株个体及筛选得到的5个InDel标记, 将目的区段初步定位于标记IDP7291与IDP78之间。为验证该结果并进一步缩小定位区间, 对目的区间附近没有公共标记的区域, 利用NCBI数据库中的Primer-BLAST (https://www. ncbi.nlm.nih.gov/tools/primer-blast/)工具, 在有功能注释基因的5′-UTR、3′-UTR及跨内含子区域设计引物, 根据一代测序结果, 进一步开发InDel标记(表1)。再利用F2群体筛选到的重组类型播种所得1648株F3代个体, 以进一步缩小目的区间。
Table 1
表1
表1在亲本间有多态性InDel引物
Table 1
| 引物 Primer | 位置 Location (Mb) | 序列 Primer sequence (5′-3′) | 产物 Product (bp) |
|---|---|---|---|
| IDP112 | 42.1 | F: AGGAAGCTGTATCCCACACG; R: TCATGGGTTTCTTCTTTGCG | 624 |
| IDP8266 | 43.3 | F: GTTGTTGTGCTCCAGAGAAGG; R: TACGTTGCCTATCATTGCCC | 369 |
| IDP101 | 45.1 | F: CTATCCCGTTCGTGTTCA; R: CCCTGCGTTGTCTTTCTC | 261 |
| IDP643 | 46.2 | F: ACCCTCATCTTCAGCAGTCG; R: GGTGAAACGGCAGTACAAGG | 838 |
| IDP2182 | 47.1 | F: GGAATGTGTACACGGCAGGT; R: ACAGCAATCGGAGCAGTGTT | 225 |
| IDP4600 | 47.4 | F: CACTTCGACGAGGGGTTCAT; R: GTAACCACCTACCCACAA | 263 |
| IDP8401 | 49.3 | F: TAGGCACTGTTTGTTTCA; R: CTAGGGTTATGTGGCATT | 324 |
| IDP78 | 49.3 | F: CGGTTGTGACTGCTATGT; R: TGTGGCATTTATTGTTCAT | 342 |
新窗口打开|下载CSV
2 结果与分析
2.1 玉米籽粒突变体dek101的籽粒特征
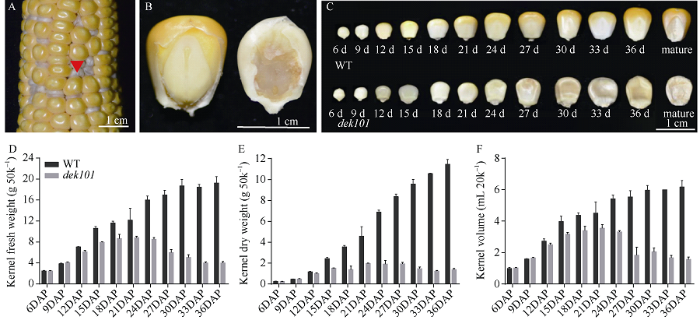
观察突变体dek101籽粒发现, 与野生型相比, 突变型籽粒严重干瘪, 粒重显著降低, 胚致死, 胚乳发育严重缺陷, 籽粒颜色灰白(图1-A, B)。对籽粒发育动态的观察发现, 在9 DAP时, 突变型籽粒与野生型籽粒相比发育状态基本一致; 12 DAP时, 野生型籽粒呈淡黄色, 突变型籽粒呈乳白色, 胚发育异常(图1-C), 表明该突变在籽粒发育早期阶段就能影响籽粒的发育。随着籽粒发育, 鲜重、干重和体积逐渐增大, 在21 DAP时达到最大, 之后突变体籽粒鲜重和体积逐渐减小, 干重不再增加, 表明突变籽粒无法进行正常的物质积累, 最终表现为胚败育的干瘪籽粒(图1-D~F)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1籽粒发育动态及授粉后不同时期野生型籽粒和突变型籽粒对比
A: 杂合果穗; B: 30 DAP时突变型籽粒和野生型籽粒对比; C: 授粉后不同时期野生型与突变型籽粒发育动态; D~E: 50颗野生型和突变型籽粒不同时期干重、鲜重的变化; F: 20颗野生型和突变型籽粒不同时期体积的变化。WT: 野生型籽粒; dek101: 突变体籽粒。A~C图标尺为1 cm。**, 突变型籽粒与野生型相比在统计学上有极显著差异 (P < 0.01)。DAP: 授粉后天数。
Fig. 1Overview of the kernel development dynamics and comparison between wild-type and mutant kernels in different days after pollination
A: Ear performance of heterozygous plants; B: Comparison of dek101 and wild-type kernels at 30 days after pollination; C: Developmental dynamics of wild-type and mutant kernels in different time series; D-E: Dynamics changes in dry weight, fresh weight of 50 wild-type and dek101 kernels at different stages. F: Dynamics changes in volume of 20 wild-type and dek101 kernels at different stages. WT: wild type; dek101: mutant. Bar in A-C: 1 cm. **, indicates extremely significant difference of expression in specific tissues between dek101 and WT (P < 0.01). DAP: days after pollination.
2.2 不同发育时期dek101胚乳细胞的扫描电镜观察
为了进一步观察突变型籽粒粒重降低原因, 我们使用扫描电镜分别观察15、21和27 DAP胚乳细胞中淀粉粒形态的变化。在相同时期, 野生型胚乳细胞中淀粉粒形态均匀, 排列有序; 突变体淀粉粒体积显著变小且排列疏松, 局部有明显空腔, 基质蛋白增多, 且有更多填充异常的小淀粉粒。随着授粉天数的增加, 野生型和突变体的淀粉粒不断增大, 发育过程中不断进行淀粉的积累, 且野生型淀粉粒物质填充更为饱满, 排列更为紧密, 证实了突变体在发育过程中籽粒重量的变化趋势(图2-A,B, D,E)。突变体籽粒的淀粉粒在发育过程中也有所增大, 但与野生型籽粒相比增长速率较为缓慢, 并在27 DAP籽粒胚乳细胞中发现较多小淀粉粒, 可能与突变基因参与调控胚乳细胞中淀粉粒的退化有关(图2-C, F)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2野生型与dek101籽粒淀粉粒扫描电镜观察
A~C: 授粉后15、21和27 d野生型籽粒扫描电镜观察; D~F: 授粉后15、21和27 d突变型籽粒扫描电镜观察。A~F图标尺: 5 μm。
Fig. 2SEM observation of the starch granule between WT and dek101 kernels
A-C: SEM observation of wild-type kernel of 15, 21, and 27 DAP; D-F: SEM observation of mutant type kernel of 15, 21, and 27 DAP. Bar = 5 μm.
2.3 dek101由隐性单基因控制
对4个F2分离果穗中的野生型和突变型籽粒粒数进行统计, 并对两者的分离比进行卡方测验, 结果表明野生型籽粒与突变型籽粒的分离比例符合3:1的孟德尔分离定律理论比例(表2), 表明该突变性状受隐性单基因控制。Table 2
表2
表2F2果穗突变型籽粒的分离比例
Table 2
| 果穗 Ear | 野生型籽粒数 Wild-type | 突变型籽粒数 Mutant | χ2 |
|---|---|---|---|
| 1 | 268 | 86 | 0.06 |
| 2 | 292 | 103 | 0.19 |
| 3 | 252 | 85 | 0.001 |
| 4 | 241 | 80 | 0.001 |
| 总计Total | 1053 | 354 | 0.019 |
新窗口打开|下载CSV
2.4 dek101被精细定位到300 kb区间
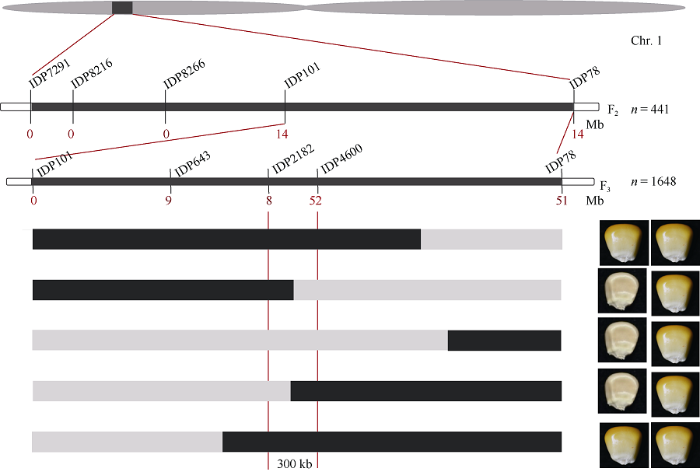
为了克隆dek101基因, 我们利用自交系B73和杂合植株(+/dek101)组配的F2群体进行初步定位。根据Maize GDB公共标记数据库, 选择均匀覆盖玉米10条染色体的271对InDel标记, 共筛选到103个在突变体dek101和自交系B73间具有多态性的标记。采用集团分离分析法(bulked segregation analysis, BSA), 对F2群体中野生型、dek101杂合型和突变型胚乳DNA的混池进行多态性筛选, 发现1号染色体上2个分子标记IDP7291和IDP78可能与目标性状连锁。随后, 在IDP7291和IDP78之间加密3个InDel标记(IDP8216、IDP8266和IDP101), 利用F2群体的441个个体将dek101初步定位在1号染色体短臂标记IDP101与IDP78之间, 物理区间为3.1 Mb。为进一步精细定位, 将检测到的重组单株于2019年夏季在内蒙播种, 获得1648个F3单株, 在IDP101与IDP78之间继续加密标记(IDP643、IDP2182和IDP4600) (表1), 筛选重组单株, 结合表型将dek101 精细定位在标记IDP2182和IDP4600之间, 物理距离约为300 kb (图3)。
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3dek101的精细定位
n: F2群体大小以及F3群体数目; 黑色竖线代表引物位置; 标记下方的红色数字代表重组单株数目; 矩形框代
Fig. 3Fine mapping of dek101
n: individuals of F2 and recombinant-derived F3 population; the black vertical lines represent primary physical distance; the red numbers under InDel markers represent recombination events; the rectangular boxes represent the genotypes and phenotypes of five recombinants; the black and gray rectangles represent B73 and heterozygous genotypes, respectively.
利用Gramene网站检索, 在该区间内共有5个注释基因, 包括1个WD40家族蛋白、3个病程相关蛋白基因和1个钙蛋白酶型半胱氨酸蛋白酶(表3)。为了预测候选基因, 利用Chen等[20]发表的转录组数据研究目标区段5个基因的组织表达特性, 发现3个病程相关蛋白基因在籽粒发育早期表达量都较低, 在发育晚期表达较高; WD家族蛋白和钙蛋白酶型半胱氨酸蛋白酶基因在玉米各个组织都有表达, 且在籽粒发育早期具有较高的表达量(图4)。这些结果表明Zm00001d028813和Zm00001d028818很有可能是dek101的候选基因。
Table 3
表3
表3dek101定位区间的候选基因注释
Table 3
| 基因位点 Gene locus | 基因注释 Gene annotation | 染色体 Chr. |
|---|---|---|
| Zm00001d028813 | Transducin family protein/WD-40 repeat family protein | Chr.1 |
| Zm00001d028814 | Pathogenesis-related protein 10 | Chr.1 |
| Zm00001d028815 | Pathogenesis-related protein 10 | Chr.1 |
| Zm00001d028816 | Pathogenesis-related protein 10 | Chr.1 |
| Zm00001d028818 | Calpain-type cysteine protease DEK1 | Chr.1 |
新窗口打开|下载CSV
图4
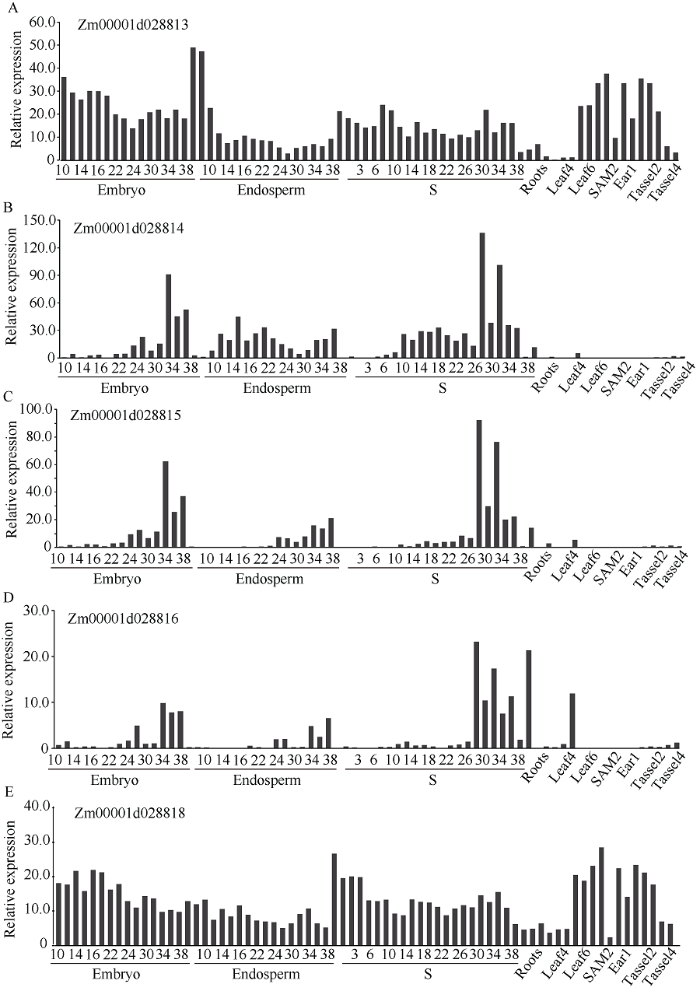 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4区段内5个预测基因在玉米自交系B73中不同组织的表达量
Fig. 4Relative expression levels of five predicted genes in different tissues of maize inbred line B73SAM: shoot apical meristem.
3 讨论
籽粒作为玉米光合产物的重要贮藏器官, 由胚、胚乳和种皮3个部分构成, 其中胚乳在玉米籽粒发育过程中积累淀粉、蛋白质等营养物质; 胚作为新生植株的幼体, 其萌发所需的营养物质来自于胚乳。因此, 以籽粒突变体为遗传材料, 挖掘调控其发育的相关基因, 并揭示籽粒发育的分子机制, 对玉米产量和品质的遗传改良具有重要意义。籽粒缺陷突变体dek101在12 DAP, 胚发育明显异常, 籽粒颜色发白; 完熟期的突变型籽粒, 胚败育, 胚乳发育异常, 籽粒严重皱缩。在目前已报道的籽粒突变体中, 如sh1、sh2、bt1、bt2、dul、waxy[2-3,6,21-23]籽粒胚发育正常, 胚乳呈现不同程度的皱缩或变小; zmprpl35-1、emp12、emp14[12-13,24]等突变籽粒胚败育, 但几乎不影响胚乳发育; dek10、dek35、dek36、dek37、dek39、dek40、dek42[25,26,27,28,29,30,31]有可以萌发的突变籽粒, 苗期致死; dek1、dek15、dek38、dek41、dek44[9-11,32-33]种子完全不能萌发, 为胚致死突变。通过表型比较, dek101突变籽粒则与dek1、dek15、dek38、dek41、dek44等类似, 胚致死, 突变籽粒完全不能萌发。除这些表型之外, 本研究还系统地观察了突变体和野生型籽粒鲜重和干重的动态变化, 均表现出先升高后降低的趋势(图1-D~E), 这与籽粒不同发育时期的含水量以及干物质积累有关。
在已报道的dek籽粒突变体中, dek1、dek15、dek38、dek41、dek44等[9-11,32-33]相关基因已被克隆。DEK15编码黏连蛋白亚基SCC4 (sister chromatid cohesion protein 4), 该基因突变后使细胞周期和核内复制受到扰乱, 导致种子完全不能萌发[9]; DEK38基因编码TTI2 (Tel2-interaction protein 2)分子伴侣蛋白, 影响雄性生殖细胞的发育[10]; DEK41编码三角状五肽重复蛋白, 该基因突变后导致线粒体复合体I亚基NAD4的第3个内含子剪切效率降低, 从而导致了烟酰胺腺嘌呤二核苷酸脱氢酶活性下降[33]; DEK44蛋白功能的丧失影响了线粒体和细胞核基因组中呼吸链相关蛋白编码基因的表达, 从而导致籽粒小、胚致死[11]。尽管这些籽粒发育相关基因已被克隆, 但是籽粒发育的调控机制有待于深入研究。本研究将DEK101基因定位在1号染色体47.1 Mb~47.4 Mb区间内, 包含已克隆基因DEK1 (Zm00001d028818)。DEK1参与玉米籽粒糊粉层细胞的分化和发育, 具有21个跨膜区域, 1个胞外环多肽和1个半胱氨酸蛋白激酶结构域。该基因突变之后导致胚致死, 影响糊粉层的发育以及胚乳醇溶蛋白含量的积累[34], 表明dek101的表型很有可能由dek1的等位突变导致。
除dek1之外, dek101的定位区间还包含4个候选基因。其中, Zm00001d028813编码WD40家族蛋白, 在细胞质和细胞核中表达, 参与植物生长发育、环境胁迫响应、信号转导调控基因表达等[35]。Zm00001d028814、Zm00001d028815和Zm00001d028816均编码病程相关蛋白基因, 是植物受到病原物侵染或非生物因子刺激后产生的一类蛋白, 参与植物的诱导抗病性[36]。结合这些基因的功能注释和表达谱, Zm00001d028813和Zm00001d028818均有可能是dek101的候选基因, 需要进一步确认。
4 结论
本研究以玉米DH系选育过程中发现的籽粒缺陷型突变体(dek101)为试验材料, 发现突变体dek101胚致死、胚乳发育缺陷, 淀粉粒填充异常。该突变体由隐性单基因控制, 被精细定位到物理距离约为300 kb的区间, 包括5个功能注释的基因, 其中Zm00001d028813和Zm00001d028818可能为候选基因。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
DOI:10.1104/pp.117.4.1235URLPMID:9701580 [本文引用: 2]

Amyloplasts of starchy tissues such as those of maize (Zea mays L.) function in the synthesis and accumulation of starch during kernel development. ADP-glucose pyrophosphorylase (AGPase) is known to be located in chloroplasts, and for many years it was generally accepted that AGPase was also localized in amyloplasts of starchy tissues. Recent aqueous fractionation of young maize endosperm led to the conclusion that 95% of the cellular AGPase was extraplastidial, but immunolocalization studies at the electron- and light-microscopic levels supported the conclusion that maize endosperm AGPase was localized in the amyloplasts. We report the results of two nonaqueous procedures that provide evidence that in maize endosperms in the linear phase of starch accumulation, 90% or more of the cellular AGPase is extraplastidial. We also provide evidence that the brittle-1 protein (BT1), an adenylate translocator with a KTGGL motif common to the ADP-glucose-binding site of starch synthases and bacterial glycogen synthases, functions in the transfer of ADP-glucose into the amyloplast stroma. The importance of the BT1 translocator in starch accumulation in maize endosperms is demonstrated by the severely reduced starch content in bt1 mutant kernels.
DOI:10.1104/pp.44.7.1058URLPMID:16657157 [本文引用: 2]

ADP-Glucose pyrophosphorylase activity has been detected in relatively low amounts in the embryos and endosperms of sh(2) and bt(2) mutant maize seeds. The total enzyme activities in sh(2) and bt(2) were about 12% and 17% respectively, of that found in starchy maize seeds (Dekalb 805). The ADP-glucose pyrophosphorylases from the starchy and mutant maize seeds were activated by 3-phosphoglycerate. However, the extent of the activation of the sh(2) enzyme was not as great as that observed with the bt(2) and Dekalb 805 enzymes. The low levels of ADP-glucose pyrophosphorylase activity in the maize mutants correlate well with the low levels of starch found in the endosperm of these mutants.
DOI:10.1007/s11103-004-0312-1URL [本文引用: 1]

Mutations in the maize gene sugary2 (su2) affect starch structure and its resultant physiochemical properties in useful ways, although the gene has not been characterized previously at the molecular level. This study tested the hypothesis that su2 codes for starch synthase IIa (SSIIa). Two independent mutations of the su2 locus, su2-2279 and su2-5178, were identified in a Mutator-active maize population. The nucleotide sequence of the genomic locus that codes for SSIIa was compared between wild type plants and those homozygous for either novel mutation. Plants bearing su2-2279 invariably contained a Mutator transposon in exon 3 of the SSIIa gene, and su2-5178 mutants always contained a small retrotransposon-like insertion in exon 10. Six allelic su2– mutations conditioned loss or reduction in abundance of the SSIIa protein detected by immunoblot. These data indicate that su2 codes for SSIIa and that deficiency in this isoform is ultimately responsible for the altered physiochemical properties of su2– mutant starches. A specific starch synthase isoform among several identified in soluble endosperm extracts was absent in su2-2279 or su2-5178 mutants, indicating that SSIIa is active in the soluble phase during kernel development. The immediate structural effect of the su2– mutations was shown to be increased abundance of short glucan chains in amylopectin and a proportional decrease in intermediate length chains, similar to the effects of SSII deficiency in other species.]]>
[本文引用: 1]
DOI:10.1016/0092-8674(83)90225-8URLPMID:6313224 [本文引用: 2]

The Waxy (Wx) locus in maize determines the amylose content of pollen and endosperm tissue. There are several mutant alleles of the locus caused by insertion of transposable controlling elements. In the present study, we have used the properties of controlling element alleles to identify the Wx locus and its gene product, with the subsequent objective of isolating the elements causing the mutations. We present evidence that the Wx locus encodes a starch granule-bound 58 kd polypeptide that is synthesized in vitro as a 65 kd precursor. We describe the isolation of recombinant plasmids containing cDNA inserts homologous to Wx mRNA and a recombinant lambda phage containing a genomic Eco RI fragment encompassing most or all of the Wx transcription unit. We show that a mutation caused by the controlling element Dissociation (Ds) is attributable to an insertion of approximately 2.4 kb at the Wx locus.
DOI:10.1105/tpc.006726URLPMID:12468731 [本文引用: 1]

The heat shock response (HSR) is an evolutionarily conserved molecular/biochemical reaction to thermal stress that is essential to the survival of eukaryotic organisms. Recessive Mutator transposon mutations at the maize empty pericarp2 (emp2) locus led to dramatically increased expression of heat shock genes, retarded embryo development, and early-stage abortion of embryogenesis. The developmental timing of emp2 mutant embryo lethality was correlated with the initial competence of maize kernels to invoke the HSR. Cloning and sequence analyses revealed that the emp2 gene encoded a predicted protein with high similarity to HEAT SHOCK BINDING PROTEIN1, which was first described in animals as a negative regulator of the HSR. emp2 is a loss-of-function mutation of an HSR-negative regulator in plants. Despite the recessive emp2 phenotype, steady state levels of emp2 transcripts were abundant in mutant kernels, and the predicted coding region was unaffected. These expression data suggest that emp2 transcription is feedback regulated, whereas S1 nuclease mapping suggests that emp2 mutant transcripts are 5' truncated and nontranslatable. In support of this model, immunoblot assays revealed that EMP2 protein did not accumulate in mutant kernels. These data support a model whereby an unattenuated HSR results in the early abortion of emp2 mutant embryos. Furthermore, the developmental retardation of emp2 mutant kernels before the HSR suggests an additional role for EMP2 during embryo development distinct from the HSR.
DOI:10.1105/tpc.105.039594URLPMID:17259266 [本文引用: 1]

The pentatricopeptide repeat (PPR) family represents one of the largest gene families in plants, with >440 members annotated in Arabidopsis thaliana. PPR proteins are thought to have a major role in the regulation of posttranscriptional processes in organelles. Recent studies have shown that Arabidopsis PPR proteins play an essential, nonredundant role during embryogenesis. Here, we demonstrate that mutations in empty pericarp4 (emp4), a maize (Zea mays) PPR-encoding gene, confer a seed-lethal phenotype. Mutant endosperms are severely impaired, with highly irregular differentiation of transfer cells in the nutrient-importing basal endosperm. Analysis of homozygous mutant plants generated from embryo-rescue experiments indicated that emp4 also affects general plant growth. The emp4-1 mutation was identified in an active Mutator (Mu) population, and cosegregation analysis revealed that it arose from a Mu3 element insertion. Evidence of emp4 molecular cloning was provided by the isolation of four additional emp4 alleles obtained by a reverse genetics approach. emp4 encodes a novel type of PPR protein of 614 amino acids. EMP4 contains nine 35-amino acid PPR motifs and an N-terminal mitochondrion-targeted sequence peptide, which was confirmed by a translational EMP4-green fluorescent protein fusion that localized to mitochondria. Molecular analyses further suggest that EMP4 is necessary to regulate the correct expression of a small subset of mitochondrial transcripts in the endosperm.
DOI:10.1105/tpc.18.00921URLPMID:30705131 [本文引用: 4]

Cohesin complexes maintain sister chromatid cohesion to ensure proper chromosome segregation during mitosis and meiosis. In plants, the exact components and functions of the cohesin complex remain poorly understood. Here, we positionally cloned the classic maize (Zea mays) mutant defective kernel 15 (dek15), revealing that it encodes a homolog of SISTER CHROMATID COHESION PROTEIN 4 (SCC4), a loader subunit of the cohesin ring. Developing dek15 kernels contained fewer cells than the wild type, but had a highly variable cell size. The dek15 mutation was found to disrupt the mitotic cell cycle and endoreduplication, resulting in a reduced endosperm and embryo lethality. The cells in the dek15 endosperm and embryo exhibited precocious sister chromatid separation and other chromosome segregation errors, including misaligned chromosomes, lagging chromosomes, and micronuclei, resulting in a high percentage of aneuploid cells. The loss of Dek15/Scc4 function upregulated the expression of genes involved in cell cycle progression and stress responses, and downregulated key genes involved in organic synthesis during maize endosperm development. Our yeast two-hybrid screen identified the chromatin remodeling proteins chromatin remodeling factor 4, chromatin remodeling complex subunit B (CHB)102, CHB105, and CHB106 as SCC4-interacting proteins, suggesting a possible mechanism by which the cohesin ring is loaded onto chromatin in plant cells. This study revealed biological functions for DEK15/SCC4 in mitotic chromosome segregation and kernel development in maize.
DOI:10.1073/pnas.1703498114URLPMID:28461460 [本文引用: 2]

We have used the newly engineered transposable element Dsg to tag a gene that gives rise to a defective kernel (dek) phenotype. Dsg requires the autonomous element Ac for transposition. Upon excision, it leaves a short DNA footprint that can create in-frame and frameshift insertions in coding sequences. Therefore, we could create alleles of the tagged gene that confirmed causation of the dek phenotype by the Dsg insertion. The mutation, designated dek38-Dsg, is embryonic lethal, has a defective basal endosperm transfer (BETL) layer, and results in a smaller seed with highly underdeveloped endosperm. The maize dek38 gene encodes a TTI2 (Tel2-interacting protein 2) molecular cochaperone. In yeast and mammals, TTI2 associates with two other cochaperones, TEL2 (Telomere maintenance 2) and TTI1 (Tel2-interacting protein 1), to form the triple T complex that regulates DNA damage response. Therefore, we cloned the maize Tel2 and Tti1 homologs and showed that TEL2 can interact with both TTI1 and TTI2 in yeast two-hybrid assays. The three proteins regulate the cellular levels of phosphatidylinositol 3-kinase-related kinases (PIKKs) and localize to the cytoplasm and the nucleus, consistent with known subcellular locations of PIKKs. dek38-Dsg displays reduced pollen transmission, indicating TTI2's importance in male reproductive cell development.
DOI:10.1104/pp.19.00546URLPMID:31182559 [本文引用: 4]

Mitochondrial respiration depends on proteins encoded by the nuclear and mitochondrial genomes. Many respiratory chain-related proteins are encoded by the mitochondrial genome and undergo translation by mitochondrial ribosomes. The newly identified maize (Zea mays) defective kernel44 (dek44) mutant produces small kernels showing embryo-lethal phenotypes. We cloned Dek44 by isolating the Mutator tag that produced the mutation and identified it as encoding a putative 50S ribosomal protein L9. Subcellular fractionation by ultracentrifugation confirmed that DEK44 is a mitochondrial ribosomal protein. DEK44 is highly conserved in monocots and only accumulates in kernels. Transcriptome and reverse transcription quantitative PCR analyses revealed that loss of DEK44 function affects the expression of genes encoding respiratory chain-related proteins from the mitochondrial and nuclear genomes. Blue native-PAGE revealed significantly reduced assembly of respiratory chain complexes in dek44 mutant kernels. Transmission electron microscopy indicated that the biogenesis and morphology of mitochondria were strongly affected in dek44 mutant kernels. Furthermore, DEK44 might regulate cell growth and kernel development via cyclin/cyclin-dependent kinase-mediated activities. This study provides insight into the regulation of kernel development based on mitochondrial ribosomal protein function.
DOI:10.1111/tpj.12161URL [本文引用: 2]

Embryo-specific mutants in maize define a unique class of genetic loci that affect embryogenesis without a significant deleterious impact on endosperm development. Here we report the characterization of an embryo specific12 (emb12) mutant in maize. Embryogenesis in the emb12 mutants is arrested at or before transition stage. The mutant embryo at an early stage exhibits abnormal cell structure with increased vacuoles and dramatically reduced internal membrane organelles. In contrast, the mutant endosperm appears normal in morphology, cell structure, starch, lipid and protein accumulation. The Emb12 locus was cloned by transposon tagging and predicts a protein with a high similarity to prokaryotic translation initiation factor 3 (IF3). EMB12GFP fusion analysis indicates that EMB12 is localized in plastids. The RNA in situ hybridization and protein immunohistochemical analyses indicate that a high level of Emb12 expression localizes in the embryo proper at early developmental stages and in the embryo axis at later stages. Western analysis indicates that plastid protein synthesis is impaired. These results indicate that Emb12 encodes the plastid IF3 which is essential for embryogenesis but not for endosperm development in maize.
DOI:10.1111/tpj.13045URLPMID:26771182 [本文引用: 2]

The embryo defective (emb) mutants in maize genetically define a unique class of loci that is required for embryogenesis but not endosperm development, allowing dissection of two developmental processes of seed formation. Through characterization of the emb14 mutant, we report here that Emb14 gene encodes a circular permuted, YqeH class GTPase protein that likely functions in 30S ribosome formation in plastids. Loss of Emb14 function in the null mutant arrests embryogenesis at the early transition stage. Emb14 was cloned by transposon tagging and was confirmed by analysis of four alleles. Subcellular localization indicated that the EMB14 is targeted to chloroplasts. Recombinant EMB14 is shown to hydrolyze GTP in vitro (Km = 2.42 +/- 0.3 mum). Emb14 was constitutively expressed in all tissues examined and high level of expression was found in transition stage embryos. Comparison of emb14 and WT indicated that loss of EMB14 function severely impairs accumulation of 16S rRNA and several plastid encoded ribosomal genes. We show that an EMB14 transgene complements the pale green, slow growth phenotype conditioned by mutations in AtNOA1, a closely related YqeH GTPase of Arabidopsis. Taken together, we propose that the EMB14/AtNOA1/YqeH class GTPases function in assembly of the 30S subunit of the chloroplast ribosome, and that this function is essential to embryogenesis in plants.
DOI:10.1126/science.145.3629.279URLPMID:14171571 [本文引用: 1]

Preliminary tests have shown that the endosperms of maize seeds homozygous for the opaque-2 mutant gene have a higher lysine content than normal kernels. As a critical test, a backcross progeny was divided into opaque-2 and normal kernels, the endosperms separated, and the amino acids determined. The opaque-2 endosperms had a different amino acid pattern and 69 percent more lysine than the normal seeds. The major reason for these changes is the synthesis of proteins with a greater content of basic amino acids in the acid-soluble fraction of the mutant endosperm. This is accompanied by a reduction in the ratio of zein to glutelin.
DOI:10.1105/tpc.4.6.701URLPMID:1392591 [本文引用: 1]

By utilizing a homologous transient expression system, we have demonstrated that the Opaque-2 (O2) gene product O2 confers positive trans-regulation on a 22-kD zein promoter. This trans-acting function of the O2 protein is mediated by its sequence-specific binding to a cis element (the O2 target site) present in the 22-kD zein promoter. A multimer of a 32-bp promoter fragment containing this O2 target site confers transactivation by O2. A single nucleotide substitution in the O2 target sequence not only abolishes O2 binding in vitro, but also its response to transactivation by O2 in vivo. We have also demonstrated that an amino acid domain including the contiguous basic region and the heptameric leucine repeat is essential for the trans-acting function of the O2 protein. Similar but not identical O2 target sequence motifs can be found in the promoters of zein genes of different molecular weight classes. Conversion of such a motif in the 27-kD zein promoter to an exact O2 target sequence by site-directed mutagenesis was sufficient to increase the binding affinity of the O2 protein in vitro and to confer transactivation by O2 in vivo.
[本文引用: 1]
[本文引用: 1]
DOI:10.1105/tpc.18.00966URLPMID:30962394 [本文引用: 1]

Cell number is a critical factor that determines kernel size in maize (Zea mays). Rapid mitotic divisions in early endosperm development produce most of the cells that make up the starchy endosperm; however, the mechanisms underlying early endosperm development remain largely unknown. We isolated a maize mutant that shows a varied-kernel-size phenotype (vks1). Vks1 encodes ZmKIN11, which belongs to the kinesin-14 subfamily and is predominantly expressed in early endosperm development. VKS1 dynamically localizes to the nucleus and microtubules and plays key roles in the migration of free nuclei in the coenocyte as well as in mitosis and cytokinesis in early mitotic divisions. Absence of VKS1 has relatively minor effects on plants but causes deformities in spindle assembly, sister chromatid separation, and phragmoplast formation in early endosperm development, thereby resulting in reduced cell proliferation. Severities of aberrant mitosis and cytokinesis within individual vks1 endosperms differ, thereby resulting in varied kernel sizes. Our discovery highlights VKS1 as a central regulator of mitosis in early maize endosperm development and provides a potential approach for future yield improvement.
DOI:10.1105/tpc.19.00444URLPMID:31530735 [本文引用: 1]

During maize (Zea mays) seed development, the endosperm functions as the major organ for storage of photoassimilate, serving to nourish the embryo. alpha-Zeins and globulins (GLBs) predominantly accumulate in the maize endosperm and embryo, respectively. Here, we show that suppression of alpha-zeins by RNA interference (alphaRNAi) in the endosperm results in more GLB1 being synthesized in the embryo, thereby markedly increasing the size and number of protein storage vacuoles. Glb genes are strongly expressed in the middle-to-upper section of the scutellum, cells of which are significantly enlarged by alphaRNAi induction. Elimination of GLBs caused an apparent reduction in embryo protein level, regardless of whether alpha-zeins were expressed or suppressed in the endosperm, indicating that GLBs represent the dominant capacity for storage of amino acids allocated from the endosperm. It appears that protein reallocation is mostly regulated at the transcriptional level. Genes differentially expressed between wild-type and alphaRNAi kernels are mainly involved in sulfur assimilation and nutrient metabolism, and many are transactivated by VIVIPAROUS1 (VP1). In vp1 embryos, misshapen scutellum cells contain notably less cellular content and are unable to respond to alphaRNAi induction. Our results demonstrate that VP1 is essential for scutellum development and protein reallocation from the endosperm to embryo.
DOI:10.1104/pp.114.240689URL [本文引用: 1]

Heterosis is important for agriculture; however, little is known about the mechanisms driving hybrid vigor. Ultimately, heterosis depends on the interactions of specific alleles and epialleles provided by the parents, which is why hybrids can exhibit different levels of heterosis, even within the same species. We characterize the development of several intraspecific Arabidopsis (Arabidopsis thaliana) F1 hybrids that show different levels of heterosis at maturity. We identify several phases of heterosis beginning during embryogenesis and culminating in a final phase of vegetative maturity and seed production. During each phase, the hybrids show different levels and patterns of growth, despite the close relatedness of the parents. For instance, during the vegetative phases, the hybrids develop larger leaves than the parents to varied extents, and they do so by exploiting increases in cell size and cell numbers in different ratios. Consistent with this finding, we observed changes in the expression of genes known to regulate leaf size in developing rosettes of the hybrids, with the patterns of altered expression differing between combinations. The data show that heterosis is dependent on changes in development throughout the growth cycle of the hybrid, with the traits of mature vegetative biomass and reproductive yield as cumulative outcomes of heterosis at different levels, tissues, and times of development.
DOI:10.1007/BF00485135URLPMID:1016220 [本文引用: 1]

Evidence is presented to show that the Sh locus specifies sucrose synthetase in the developing endosperm of maize. The sh/sh/sh endosperm possesses less than 10% sucrose synthetase activity as compared to the normal Sh/sh/sh endosperm. The residual enzyme activity in five independently derived mutant genotypes is attributable to a protein molecule of different electrophoretic and immunochemical specificities that is presumably independent of the sh locus. Sucrose synthetase activity in the embryo in both the genotypes is electrophoretically indistinguishable from the one present in the mutant endosperm. Mutant endosperm has a reduced starch content as compared to the normal. This observation constitutes genetic evidence supporting a critical role for sucrose synthetase in starch biosynthesis.
DOI:10.1007/BF00485834URL
DOI:10.1105/tpc.10.3.399URLPMID:9501113 [本文引用: 1]

The maize dull1 (du1) gene is a determinant of the structure of endosperm starch, and du1- mutations affect the activity of two enzymes involved in starch biosynthesis, starch synthase II (SSII) and starch branching enzyme IIa (SBEIIa). Six novel du1- mutations generated in Mutator-active plants were identified. A portion of the du1 locus was cloned by transposon tagging, and a nearly full-length Du1 cDNA sequence was determined. Du1 codes for a predicted 1674-residue protein, comprising one portion that is similar to SSIII of potato, as well as a large unique region. Du1 transcripts are present in the endosperm during the time of starch biosynthesis, but the mRNA was undetectable in leaf or root tissue. The predicted size of the Du1 gene product and its expression pattern are consistent with those of maize SSII. The Du1 gene product contains two repeated regions in its unique N terminus. One of these contains a sequence identical to a conserved segment of SBEs. We conclude that Du1 codes for a starch synthase, most likely SSII, and that secondary effects of du1- mutations, such as reduction of SBEIIa, result from the primary deficiency in this starch synthase.
DOI:10.1104/pp.103.030767URLPMID:14730079 [本文引用: 1]

In emb (embryo specific) mutants of maize (Zea mays), the two fertilization products have opposite fates: Although the endosperm develops normally, the embryo shows more or less severe aberrations in its development, resulting in nonviable seed. We show here that in mutant emb8516, the development of mutant embryos deviates as soon as the transition stage from that of wild-type siblings. The basic events of pattern formation take place because mutant embryos display an apical-basal polarity and differentiate a protoderm. However, morphogenesis is strongly aberrant. Young mutant embryos are characterized by protuberances at their suspensor-like extremity, leading eventually to structures of irregular shape and variable size. The lack of a scutellum or coleoptile attest to the virtual absence of morphogenesis at the embryo proper-like extremity. Molecular cloning of the mutation was achieved based on cosegregation between the mutant phenotype and the insertion of a MuDR element. The Mu insertion is located in gene ZmPRPL35-1, likely coding for protein L35 of the large subunit of plastid ribosomes. The isolation of a second allele g2422 and the complementation of mutant emb8516 with a genomic clone of ZmPRPL35-1 confirm that a lesion in ZmPRPL35-1 causes the emb phenotype. ZmPRPL35-1 is a low-copy gene present at two loci on chromosome arms 6L and 9L. The gene is constitutively expressed in all major tissues of wild-type maize plants. Lack of expression in emb/emb endosperm shows that endosperm development does not require a functional copy of ZmPRPL35-1 and suggests a link between plastids and embryo-specific signaling events.
DOI:10.1534/genetics.116.199331URLPMID:28213476 [本文引用: 1]

Respiration, the core of mitochondrial metabolism, depends on the function of five respiratory complexes. Many respiratory chain-related proteins are encoded by the mitochondrial genome and their RNAs undergo post-transcriptional modifications by nuclear genome-expressed factors, including pentatricopeptide repeat (PPR) proteins. Maize defective kernel 10 (dek10) is a classic mutant with small kernels and delayed development. Through positional cloning, we found that Dek10 encodes an E-subgroup PPR protein localized in mitochondria. Sequencing analysis indicated that Dek10 is responsible for the C-to-U editing at nad3-61, nad3-62, and cox2-550 sites, which are specific editing sites in monocots. The defects of these editing sites result in significant reduction of Nad3 and the loss of Cox2. Interestingly, the assembly of complex I was not reduced, but its NADH dehydrogenase activity was greatly decreased. The assembly of complex IV was significantly reduced. Transcriptome and transmission electron microscopy (TEM) analysis revealed that proper editing of nad3 and cox2 is critical for mitochondrial functions, biogenesis, and morphology. These results indicate that the E-subgroup PPR protein Dek10 is responsible for multiple editing sites in nad3 and cox2, which are essential for mitochondrial functions and plant development in maize.
DOI:10.1016/j.molp.2016.08.008URLPMID:27596292 [本文引用: 1]

In higher plants, the splicing of organelle-encoded mRNA involves a complex collaboration with nuclear-encoded proteins. Pentatricopeptide repeat (PPR) proteins have been implicated in these RNA-protein interactions. In this study, we performed the cloning and functional characterization of maize Defective kernel 35 (Dek35). The dek35-ref mutant is a lethal-seed mutant with developmental deficiency. Dek35 was cloned through Mutator tag isolation and further confirmed by four additional independent mutant alleles. Dek35 encodes an P-type PPR protein that targets the mitochondria. The dek35 mutation causes significant reduction in the accumulation of DEK35 proteins and reduced splicing efficiency of mitochondrial nad4 intron 1. Analysis of mitochondrial complex in dek35 immature seeds indicated severe deficiency in the complex I assembly and NADH dehydrogenase activity. Transcriptome analysis of dek35 endosperm revealed enhanced expression of genes involved in the alternative respiratory pathway and extensive differentially expressed genes related to mitochondrial function and activity. Collectively, these results indicate that Dek35 encodes an PPR protein that affects the cis-splicing of mitochondrial nad4 intron 1 and is required for mitochondrial function and seed development.
DOI:10.1111/nph.14507URLPMID:28277611 [本文引用: 1]

Mitochondria are semi-autonomous organelles that are the powerhouse of the cells. Plant mitochondrial RNA editing guided by pentatricopeptide repeat (PPR) proteins is essential for energy production. We identify a maize defective kernel mutant dek36, which produces small and collapsed kernels, leading to embryos and/or seedlings lethality. Seed filling in dek36 is drastically impaired, in line with the defects observed in the organization of endosperm transfer tissue. Positional cloning reveals that DEK36, encoding a mitochondria-targeted E+ subgroup PPR protein, is required for mitochondrial RNA editing at atp4-59, nad7-383 and ccmFN -302, thus resulting in decreased activities of mitochondrial complex I, complex III and complex IV in dek36. Loss-of-function of its Arabidopsis ortholog At DEK36 causes arrested embryo and endosperm development, leading to embryo lethality. At_dek36 also has RNA editing defects in atp4, nad7, ccmFN1 and ccmFN2 , but at the nonconserved sites. Importantly, efficiency of all editing sites in ccmFN1 , ccmFN2 and rps12 is severely decreased in At_dek36, probably caused by the impairment of their RNA stabilization. These results suggest that the DEK36 orthologue pair are essential for embryo and endosperm development in both maize and Arabidopsis, but through divergent function in regulating RNA metabolism of their mitochondrial targets.
DOI:10.1534/genetics.117.300602URLPMID:29301905 [本文引用: 1]

Mitochondrial group II introns require the participation of numerous nucleus-encoded general and specific factors to achieve efficient splicing in vivo Pentatricopeptide repeat (PPR) proteins have been implicated in assisting group II intron splicing. Here, we identified and characterized a new maize seed mutant, defective kernel 37 (dek37), which has significantly delayed endosperm and embryo development. Dek37 encodes a classic P-type PPR protein that targets mitochondria. The dek37 mutation causes no detectable DEK37 protein in mutant seeds. Mitochondrial transcripts analysis indicated that dek37 mutation decreases splicing efficiency of mitochondrial nad2 intron 1, leading to reduced assembly and NADH dehydrogenase activity of complex I. Transmission Electron Microscopy (TEM) revealed severe morphological defects of mitochondria in dek37 Transcriptome analysis of dek37 endosperm indicated enhanced expression in the alternative respiratory pathway and extensive differentially expressed genes related to mitochondrial function. These results indicated that Dek37 is involved in cis-splicing of mitochondrial nad2 intron 1 and is required for complex I assembly, mitochondrial function, and seed development in maize.
DOI:10.1111/jipb.12602URL [本文引用: 1]

dek6 in maize. Loss of Dek6 function leads to delayed embryogenesis and endosperm development, reduced kernel size, and seedling lethality. Dek6 encodes an E sub-class PPR protein that targets to both mitochondria and chloroplasts, and is involved in RNA editing in mitochondrial NADH dehydrogenase3 (nad3) at nad3-247 and nad3-275. C to U editing of nad3-275 is not conserved and even lost in Arabidopsis, consisting with the idea that no close DEK6 homologs present in Arabidopsis. However, the amino acids generated by editing nad3-247 and nad3-275 are highly conserved in many other plant species, and the reductions of editing at these two sites decreased the activity of mitochondria NADH dehydrogenase complex I, indicating that the alteration of amino acid sequence is necessary for Nad3 function. Our results indicate that Dek6 encodes an E sub-class PPR protein that is involved in RNA editing of multiple sites and is necessary for seed development of maize.]]>
DOI:10.1093/jxb/erz391URLPMID:31598687 [本文引用: 1]

Pentatricopeptide repeat (PPR) proteins are one of the largest protein families, which consists of >400 members in most species. However, the molecular functions of many PPR proteins are still uncharacterized. Here, we isolated a maize mutant, defective kernel 40 (dek40). Positional cloning, and genetic and molecular analyses revealed that DEK40 encodes a new E+ subgroup PPR protein that is localized in the mitochondrion. DEK40 recognizes and directly binds to cox3, nad2, and nad5 transcripts and functions in their processing. In the dek40 mutant, abolishment of the C-to-U editing of cox3-314, nad2-26, and nad5-1916 leads to accumulated reactive oxygen species and promoted programmed cell death in endosperm cells due to the dysfunction of mitochondrial complexes I and IV. Furthermore, RNA sequencing analysis showed that gene expression in some pathways, such as glutathione metabolism and starch biosynthesis, was altered in the dek40 mutant compared with the wild-type control, which might be involved in abnormal development of the maize mutant kernels. Thus, our results provide solid evidence on the molecular mechanism underlying RNA editing by DEK40, and extend our understanding of PPR-E+ type protein in editing functions and kernel development in maize.
DOI:10.1111/jipb.12798URL [本文引用: 1]

dek42 has small defective kernels and lethal seedlings. Dek42 was cloned by Mutator tag isolation and further confirmed by an independent mutant allele and clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 materials. Dek42 encodes an RRM_RBM48 type RNA-binding protein that localizes to the nucleus. Dek42 is constitutively expressed in various maize tissues. The dek42 mutation caused a significant reduction in the accumulation of DEK42 protein in mutant kernels. RNA-seq analysis showed that the dek42 mutation significantly disturbed the expression of thousands of genes during maize kernel development. Sequence analysis also showed that the dek42 mutation significantly changed alternative splicing in expressed genes, which were especially enriched for the U12-type intron-retained type. Yeast two-hybrid screening identified SF3a1 as a DEK42-interacting protein. DEK42 also interacts with the spliceosome component U1-70K. These results suggested that DEK42 participates in the regulation of pre-messenger RNA splicing through its interaction with other spliceosome components. This study showed the function of a newly identified RBP and provided insights into alternative splicing regulation during maize kernel development.]]>
DOI:10.1073/pnas.042098799URLPMID:11929961 [本文引用: 2]

Endosperm of cereal grains is one of the most important renewable resources for food, feed, and industrial raw material. It consists of four triploid cell types, i.e., aleurone, starchy endosperm, transfer cells, and cells of the embryo surrounding region. In maize, the aleurone layer is one cell layer thick and covers most of the perimeter of the endosperm. Specification of maize aleurone cell fate is proposed to occur through activation of the tumor necrosis factor receptor-like receptor kinase CRINKLY4. A second maize gene essential for aleurone cell development is defective kernel 1 (dek1). Here we show that DEK1 shares high homology with animal calpains. The predicted 2,159-aa DEK1 protein has 21 transmembrane regions, an extracellular loop, and a cysteine proteinase domain that shares high homology with domain II of m-calpain from animals. We propose that DEK1 functions to maintain and restrict the aleurone cell fate imposed by CR4 through activation of its cysteine proteinase by contact with the outer endosperm surface. DEK1 seems to be the only member of the calpain superfamily in plants, Arabidopsis DEK1 sharing 70% overall identity with maize DEK1. The expression of dek1 in most plant tissues in maize and Arabidopsis, as well as its presence in a variety of higher plants, including angiosperms and gymnosperms, suggests that DEK1 plays a conserved role in plant signal transduction.
DOI:10.1093/jxb/erz193URLPMID:31020318 [本文引用: 3]

The splicing of organelle-encoded mRNA in plants requires proteins encoded in the nucleus. The mechanism of splicing and the factors involved are not well understood. Pentatricopeptide repeat (PPR) proteins are known to participate in such RNA-protein interactions. Maize defective kernel 41 (dek41) is a seedling-lethal mutant that causes developmental defects. In this study, the Dek41 gene was cloned by Mutator tag isolation and allelic confirmation, and was found to encode a P-type PPR protein that targets mitochondria. Analysis of the mitochondrial RNA transcript profile revealed that dek41 mutations cause reduced splicing efficiency of mitochondrial nad4 intron 3. Immature dek41 kernels exhibited severe reductions in complex I assembly and NADH dehydrogenase activity. Up-regulated expression of alternative oxidase genes and deformed inner cristae of mitochondria in dek41, as revealed by TEM, indicated that proper splicing of nad4 is essential for correct mitochondrial functioning and morphology. Consistent with this finding, differentially expressed genes in the dek41 endosperm included those related to mitochondrial function and activity. Our results indicate that DEK41 is a PPR protein that affects cis-splicing of mitochondrial nad4 intron 3 and is required for correct mitochondrial functioning and maize kernel development.
DOI:10.1104/pp.111.177725URL [本文引用: 1]

The maize (Zea mays) aleurone layer occupies the single outermost layer of the endosperm. The defective kernel1 (dek1) gene is a central regulator required for aleurone cell fate specification. dek1 mutants have pleiotropic phenotypes including lack of aleurone cells, aborted embryos, carotenoid deficiency, and a soft, floury endosperm deficient in zeins. Here we describe the thick aleurone1 (thk1) mutant that defines a novel negative function in the regulation of aleurone differentiation. Mutants possess multiple layers of aleurone cells as well as aborted embryos. Clonal sectors of thk1 mutant tissue in otherwise normal endosperm showed localized expression of the phenotype with sharp boundaries, indicating a localized cellular function for the gene. Sectors in leaves showed expanded epidermal cell morphology but the mutant epidermis generally remained in a single cell layer. Double mutant analysis indicated that the thk1 mutant is epistatic to dek1 for several aspects of the pleiotropic dek1 phenotype. dek1 mutant endosperm that was mosaic for thk1 mutant sectors showed localized patches of multilayered aleurone. Localized sectors were surrounded by halos of carotenoid pigments and double mutant kernels had restored zein profiles. In sum, loss of thk1 function restored the ability of dek1 mutant endosperm to accumulate carotenoids and zeins and to differentiate aleurone. Therefore the thk1 mutation defines a negative regulator that functions downstream of dek1 in the signaling system that controls aleurone specification and other aspects of endosperm development. The thk1 mutation was found to be caused by a deletion of approximately 2 megabases.
DOI:10.1007/s13238-011-1018-1URL [本文引用: 1]

The WD40 domain exhibits a beta-propeller architecture, often comprising seven blades. The WD40 domain is one of the most abundant domains and also among the top interacting domains in eukaryotic genomes. In this review, we will discuss the identification, definition and architecture of the WD40 domains. WD40 domain proteins are involved in a large variety of cellular processes, in which WD40 domains function as a protein-protein or protein-DNA interaction platform. WD40 domain mediates molecular recognition events mainly through the smaller top surface, but also through the bottom surface and sides. So far, no WD40 domain has been found to display enzymatic activity. We will also discuss the different binding modes exhibited by the large versatile family of WD40 domain proteins. In the last part of this review, we will discuss how post-translational modifications are recognized by WD40 domain proteins.
DOI:10.1016/j.jplph.2009.07.004URLPMID:19682768 [本文引用: 1]

A novel PR10 gene (ZmPR10.1) was isolated from maize and its expression and function were compared with the previous ZmPR10. ZmPR10.1 shares 89.8% and 85.7% identity to ZmPR10 at the nucleotide and amino acid sequence level, respectively. ZmPR10 and ZmPR10.1 were mainly expressed in root tissue with low expression in other tissues. ZmPR10.1 had significantly lower expression than ZmPR10 in all tissues examined. The expression of both ZmPR10 and ZmPR10.1 was induced by most abiotic stresses including SA, CuCl(2), H(2)O(2), coldness, darkness and wounding during the 16-h treatments, and biotic stresses such as Erwinia stewartii and Aspergillus flavus infection. However, ZmPR10.1 was induced only 2 HAT and down-regulated thereafter, whereas ZmPR10 remained induced during the 16-h NAA treatment. Also, inoculation with Erwinia chrysanthemi caused about 2-fold induction in ZmPR10.1 expression 60 HAT but not significant changes for ZmPR10. Both ZmPR10.1 and ZmPR10 showed RNase activity in vitro with an optimal pH and temperature of 6.5 and 55 degrees C. Their RNase activities were significantly inhibited by low concentrations (1.0mM) of Cu(2+), Ag(+), Co(2+), SDS, EDTA or DTT. However, ZmPR10.1 possessed significantly higher (8-fold) specific RNase activity than ZmPR10. Also, ZmPR10.1 showed a stronger inhibition against bacterium Pseudomonas syringae pv. tomato DC3000 in vivo and fungus A. flavus in vitro than ZmPR10, indicating that ZmPR10.1 may also play an important role in host plant defense.
