 ,1,2,*
,1,2,*Cloning and expression analysis of sugarcane lipoxygenase gene ScLOX1
SUN Ting-Ting1, WANG Wen-Ju1, LOU Wen-Yue1, LIU Feng1, ZHANG Xu1, WANG Ling1, CHEN Yu-Feng1, QUE You-Xiong1,2, XU Li-Ping1,2, LI Da-Mei1,2, SU Ya-Chun ,1,2,*
,1,2,*通讯作者:
收稿日期:2018-11-5接受日期:2019-01-29网络出版日期:2019-03-22
| 基金资助: |
Received:2018-11-5Accepted:2019-01-29Online:2019-03-22
| Fund supported: |
作者简介 About authors
E-mail:sunting3221@163.com。

摘要
关键词:
Abstract
Keywords:
PDF (5142KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
孙婷婷, 王文举, 娄文月, 刘峰, 张旭, 王玲, 陈玉凤, 阙友雄, 许莉萍, 李大妹, 苏亚春. 甘蔗脂氧合酶基因ScLOX1的克隆与表达分析[J]. 作物学报, 2019, 45(7): 1002-1016. doi:10.3724/SP.J.1006.2019.84143
SUN Ting-Ting, WANG Wen-Ju, LOU Wen-Yue, LIU Feng, ZHANG Xu, WANG Ling, CHEN Yu-Feng, QUE You-Xiong, XU Li-Ping, LI Da-Mei, SU Ya-Chun.
脂氧合酶(Lipoxygenase, EC1.13.11.12, LOX)是一种非血红素、无硫铁双加氧酶, 每个多肽中含1个铁原子, 是脂肪氧化途径的重要因子, 广泛存在于动植物和真菌中[1,2]。LOX催化亚麻酸(linolenic acid, LNA)和亚油酸(leinoleic acid, LA)形成氢过氧化物(hydroperoxides, HPOs), 随后经过氢过氧化物裂解酶(hydroperoxide lyase, HPL)途径、二乙烯醚合酶(divinyl ether synthase, DES)途径、氢过氧化物异构酶(peroxygenase, POX)途径和丙二烯氧合酶(allene oxide synthase, AOS)途径等至少7条支路形成一系列活性物质参与防御外界刺激或者调控植物生长发育[3,4]。根据加氧位置的不同, LOX在植物中分为传统9-LOX、13-LOX和非传统9/13-LOX三种类型, 根据氨基酸氨基末端质体转运肽的有无分为type I (包括传统9-LOX、13-LOX和非传统9/13-LOX)和type II (几乎全部为13-LOX) 2种[5,6,7]。亚细胞定位研究表明, LOX蛋白主要存在于细胞质、叶绿体、油体膜、微体膜和液泡等部位[8,9]。目前, 有关LOX基因的分离和鉴定在大豆(Glycine max)[10]、拟南芥(Arabidopsis thaliana)[11]、水稻(Oryza sativa)[12]、番茄(Lycopersicon esculentum)[13]、葡萄(Vitis vinifera)[14]和黄瓜(Cucumis sativus)[15]等植物中被广泛开展。
大量研究表明, LOX基因不仅参与调控植物生长发育, 也参与植物对各种生物和非生物胁迫的防御[16,17,18,19,20,21,22,23,24,25]。Grechkin[17]发现大麦(Hordeum vulgare)、黄瓜和大豆在种子萌发早期会诱导特异的LOX基因表达。刘南南等[19]将OsLOX3的反义植物表达载体转入水稻植株中, 发现转基因植株对水分胁迫、白叶枯病和稻瘟病均比非转基因对照表现敏感, 表明OsLOX3可能参与水稻对上述逆境胁迫的防御作用。Burow等[20]从花生(Arachis hypogaea)种子中分离出1个LOX基因(PnLOX1), 其在不成熟子叶中为组成型表达, 在成熟子叶中能被茉莉酸甲酯(methyl jasmonate, MeJA)、伤害和曲霉属(Aspergillus)真菌高度诱导。邵琪等[21]对甜瓜(Cucumis melo)接种枯萎病菌(Fusarium oxysporum f. sp. melonis)后, 甜瓜叶片和根部分别有13个和5个CmLOXs基因上调表达, 表明CmLOXs基因可能参与甜瓜对该病原菌的防御反应。此外, 研究表明LOX基因与果实成熟过程中的乙烯(ethylene, ET)合成相关[22,23]。干旱胁迫后, 在5个人参(Panax ginseng) PgLOXs基因中只有PgLOX3有响应[24]。在辣椒(Capsicum annuum)中, CaLOX2的表达能被MeJA、水杨酸(salicylic acid, SA)、过氧化氢(hydrogen peroxide, H2O2)、机械伤害和高盐胁迫诱导上调, 但在低温条件下被抑制[25]。
甘蔗(Saccharum spp.)是主要的糖料作物和生物能源作物之一, 具有重要的生物学与经济价值。由于甘蔗遗传背景复杂、生育周期长等特点, 使得在培育高产和抗逆优良新品种时, 基因工程育种比传统育种方法凸显优势[26,27]。因此, 挖掘优良抗性基因, 为甘蔗抗逆基因工程育种提供可靠的基因资源, 有助于促进甘蔗产业发展。前人研究表明植物LOX基因在多种逆境胁迫中扮演重要的角色, 属于较为重要的抗性基因[16,17,18,19,20,21,22,23,24,25], 但至今尚未从甘蔗中获得, 鉴于此, 本研究从甘蔗中分离获得了LOX同源基因ScLOX1, 并对其开展了生物信息学、组织特异性以及不同外源胁迫下的表达模式分析。此外, 本研究还在本氏烟(Nicotiana benthamiana)叶片中瞬时表达该基因, 并进行烟草(Nicotiana tabacum)不同病原菌接种后的表型观察、3,3’-二氨基联苯胺(3,3’- diaminobenzidine, DAB)染色和烟草免疫相关标记基因的检测。该研究为进一步了解ScLOX1基因的功能和发掘甘蔗抗逆基因资源奠定了基础。
1 材料与方法
1.1 材料处理
甘蔗抗黑穗病品种崖城05-179、感黑穗病品种ROC22和甘蔗黑穗病菌(Sporisorium scitamineum)混合冬孢子均来源于农业部福建甘蔗生物学与遗传育种重点实验室基地。在37℃条件下将采集自田间的甘蔗黑穗病鞭子烘干, 置4℃冰箱保存备用。田间随机选取6株长势一致的ROC22成熟甘蔗植株, 连根挖起, 清水洗净后, 分别取乳白色幼嫩蔗根、+1叶(最高可见肥厚带下第1片叶)、蔗芽、蔗皮和蔗肉后, 立即液氮速冻和-80℃保存, 用于甘蔗组织特异性表达分析。
选取4个月大且长势一致的ROC22组培苗, 置清水中, 在28℃下光照16 h/黑暗8 h复性培养1周后进行处理[28]。组培苗叶面分别喷施100 μmol L-1 MeJA (含0.1%乙醇和0.05%吐温-20, v/v)、5 mmol L-1水杨酸(含0.01%吐温-20, v/v)和100 μmol L-1脱落酸(abscisic acid, ABA)。MeJA和SA处理0、3、12和24 h后及ABA处理0、0.5、3、6和24 h后立即将整株组培苗用液氮速冻, 用于分析目的基因在不同植物激素胁迫下的表达模式。
挑选生长健壮且长势一致的崖城05-179和ROC22成熟蔗茎, 切成双芽茎段, 用稀释1200倍的80%多菌灵可湿性粉剂浸泡3 h, 再于流动的清水中浸泡24 h, 然后摆盘并用湿纱布覆盖置培养箱(32℃, 光照16 h/黑暗8 h)催芽培养。待蔗芽长至2 cm左右, 一部分材料用5×106个 mL-1 (含0.01%吐温-20, v/v)黑穗病菌孢子悬浮液针刺接种蔗芽, 以无菌水(含0.01%吐温-20, v/v)接种蔗芽作为对照组, 所有处理材料在28℃下光照16 h/黑暗8 h培养[29], 分别于接种后0、24、48和72 h立即取5个单芽于液氮中速冻和-80℃保存。另一部分未接种的ROC22蔗芽材料继续水培至4~5叶龄, 然后挑选长势一致的种茎苗分别置250 mmol L-1氯化钠(sodium chloride, NaCl)和25.0%聚乙二醇8000 (polyethylene glycol 8000, PEG-8000)水溶液中, 胁迫处理0、0.5、3、6和24 h后立即取+1叶于液氮中速冻, 取3株为1个重复, 共3次生物学重复, 于-80℃保存备用。
1.2 总RNA的提取及cDNA第1链合成
所有植物样品总RNA的提取及cDNA第1链的合成参考Liu等[30]的方法, 其中, 基因克隆和表达分析用的模板分别按照RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Vilnius, Lithuania)和PrimerScript RT-PCR Kit (Takara, 中国大连)试剂盒说明书合成。1.3 ScLOX1基因克隆
依据课题组已获得的转录组注释文库[31], 筛选到1条与LOX同源的unigene序列(unigene ID: c151059.graph_c1), 并根据其序列设计基因特异引物ScLOX1-cDNAF/ScLOX1-cDNAR (表1), 以ROC22蔗芽cDNA为模板进行PCR扩增。PCR程序为94℃预变性4 min; 94℃变性30 s, 55℃退火30 s, 72℃延伸3 min, 共35个循环; 72℃延伸10 min。随后对PCR产物进行回收纯化、克隆与测序鉴定[32]。Table 1
表1
表1本研究所用引物序列及用途
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') | 用途 Purpose |
|---|---|---|
| ScLOX1-cDNAF | CCATCCATCCACCAACCA | RT-PCR |
| ScLOX1-cDNAR | ACGGCACAGCACAACATTTA | RT-PCR |
| GAPDH-QF | CACGGCCACTGGAAGCA | qRT-PCR |
| GAPDH-QR | TCCTCAGGGTTCCTGATGCC | qRT-PCR |
| ScLOX1-QF | ATCATTGAGGCTGTTCTT | qRT-PCR |
| ScLOX1-QR | TCTGCTATTAGTGGATTGT | qRT-PCR |
| ScLOX1-Gate-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGTTCTGGCACGGGGTCGC | Transient overexpression vector construction |
| ScLOX1-Gate-R | GGGGACCACTTTGTACAAGAAAGCTGGGTCTATGGAGATGCTGTTGGGAA | Transient overexpression vector construction |
| M13-F | TGTAAAACGACGGCCAGT | Transient overexpression vector construction |
| M13-R | CAGGAAACAGCTATGACC | Transient overexpression vector construction |
| NtHSR201-F | CAGCAGTCCTTTGGCGTTGTC | qRT-PCR after transient overexpression |
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') | 用途 Purpose |
| NtHSR201-R | GCTCAGTTTAGCCGCAGTTGTG | qRT-PCR after transient overexpression |
| NtHSR203-F | TGGCTCAACGATTACGCA | qRT-PCR after transient overexpression |
| NtHSR203-R | GCACGAAACCTGGATGG | qRT-PCR after transient overexpression |
| NtHSR515-F | TTGGGCAGAATAGATGGGTA | qRT-PCR after transient overexpression |
| NtHSR515-R | TTTGGTGAAAGTCTTGGCTC | qRT-PCR after transient overexpression |
| NtPR-1a/c-F | AACCTTTGACCTGGGACGAC | qRT-PCR after transient overexpression |
| NtPR-1a/c-R | GCACATCCAACACGAACCGA | qRT-PCR after transient overexpression |
| NtPR2-F | TGATGCCCTTTTGGATTCTATG | qRT-PCR after transient overexpression |
| NtPR2-R | AGTTCCTGCCCCGCTTT | qRT-PCR after transient overexpression |
| NtPR3-F | CAGGAGGGTATTGCTTTGTTAGG | qRT-PCR after transient overexpression |
| NtPR3-R | CGTGGGAAGATGGCTTGTTGTC | qRT-PCR after transient overexpression |
| NtEFE26-F | CGGACGCTGGTGGCATAAT | qRT-PCR after transient overexpression |
| NtEFE26-R | CAACAAGAGCTGGTGCTGGATA | qRT-PCR after transient overexpression |
| NtAccdeaminase-F | TCTGAGGTTACTGATTTGGATTGG | qRT-PCR after transient overexpression |
| NtAccdeaminase-R | TGGACATGGTGGATAGTTGCT | qRT-PCR after transient overexpression |
| NtEF-1α-F | TGCTGCTGTAACAAGATGGATGC | qRT-PCR after transient overexpression |
| NtEF-1α-R | GAGATGGGGACAAAGGGGATT | qRT-PCR after transient overexpression |
新窗口打开|下载CSV
1.4 ScLOX1基因生物信息学分析
分别利用ORF (open reading frame) Finder、NCBI conserved domains、ProtParam、SWISSMODEL、Rastop、SignalP 4.0 Server、TMHMM Server v. 2.0和ProtScale软件分析ScLOX1基因序列的理化特性、保守结构域、一级结构、三级结构、信号肽、跨膜结构和亲疏水性。参考Wang等[5]的方法, 利用DNAMAN 6.0软件将ScLOX1基因编码的氨基酸序列与NCBI下载的其他同源植物LOX氨基酸序列进行多序列比对。参考曹嵩晓等[4]的方法, 利用MEGA 6.06软件, 选取包括5个玉米(Zea mays)、2个水稻、6个番茄、4个拟南芥、3个烟草等在内的48个LOX蛋白与ScLOX1基因编码的氨基酸构建系统进化树。1.5 实时荧光定量PCR (quantitative real-time PCR, qRT-PCR)分析
根据ScLOX1基因序列设计特异性定量PCR引物, 命名为ScLOX1-QF和ScLOX1-QR (表1), 选用甘油醛-3-磷酸脱氢酶基因(glyceraldehyde-3- phosphate dehydrogenase, GAPDH)作为内参基因, 利用SYBR Green染料法[30], 定量检测ScLOX1基因在不同甘蔗组织(根、芽、叶、蔗肉和蔗皮)以及在甘蔗黑穗病菌、植物激素(SA、MeJA、ABA)、模拟盐(NaCl)和干旱(PEG)等胁迫下的表达特性。每个样品设3次生物学重复和3次技术重复, 参照2-ΔΔCT法[33]计算基因相对表达量。采用DPS 7.05对数据进行显著性分析, Origin 8.0软件作柱状图。1.6 瞬时表达分析
根据ScLOX1基因序列设计gateway入门载体引物ScLOX1-Gate-F/ScLOX1-Gate-R (表1), 以质粒pMD 19-T-ScLOX1为模板进行RT-PCR扩增和胶回收纯化。利用gateway克隆方法构建入门载体(pDONR-221- ScLOX1)。以获得的pDONR-221-ScLOX1质粒为模板, 利用M13-F/M13-R引物(表1), 进行PCR扩增、胶回收纯化、重组过表达载体(pEarleyGate 203-ScLOX1)构建, 具体参考Liu等[30]的方法。将pEarleyGate 203-ScLOX1转入农杆菌Gv3101, 参考Choi等[34]方法, 收集和重悬菌体后, 注射5~8叶龄且长势一致的本氏烟叶片, 每3株一组作为生物学重复。将本氏烟植株置24℃培养, 1 d后取注射叶进行目的基因检测和8个烟草免疫相关标记基因[包括过敏反应(Hypersensitive reaction, HR)标记基因NtHSR201、NtHSR203和NtHSR515; 水杨酸途径相关基因NtPR-1a/c; 茉莉酸(jasmonate, JA)途径相关基因NtPR2和NtPR3; 乙烯合成依赖基因NtEFE26和NtAccdeaminase] (表1)表达模式分析。培养2 d时, 取注射叶片进行DAB染色[35]。另外, 将瞬时过表达目的基因的本氏烟培养1 d后, 分别接种烟草病原真菌茄病镰刀菌蓝色变种(Fusarium solani var. coeruleum)和烟草病原细菌青枯菌(Ralstonia solanacearum), 培养1 d和7 d后分别进行表型观察、DAB染色和烟草免疫相关标记基因表达模式分析[30,36]。2 结果与分析
2.1 ScLOX1基因克隆及理化性质分析
从甘蔗转录组数据库中挖掘并克隆获得了ScLOX1基因序列(GenBank登录号为MK106188), 该基因cDNA全长2813 bp, ORF长度为2664 bp, 编码887个氨基酸(图1)。ScLOX1基因编码蛋白的分子式为C4532H7006N1238O1314S16, 理论相对分子量为100.37 kDa, 理论等电点为6.23, 不稳定系数为39.77, 亲水性平均值为-0.437, 无信号肽和跨膜结构。由图2可知,ScLOX1的三级结构主要由α-螺旋、β-折叠和无规则卷曲组成, 且含有1个铁原子。保守结构域预测显示, ScLOX1蛋白含有PLN02337型结构域, 具有LOX相关蛋白的活性位点PLAT_LH2和Lipoxygenase (图3)。由此推测, ScLOX1为酸性稳定亲水性非分泌LOX蛋白。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1ScLOX1基因核苷酸序列及其推导的氨基酸序列
下画线部分为克隆引物; *表示终止密码子。
Fig. 1Nucleotide acid sequence and deduced amino acid sequence of ScLOX1 gene
The sequences underlined are cloning primers; * indicates stop codon.
图2
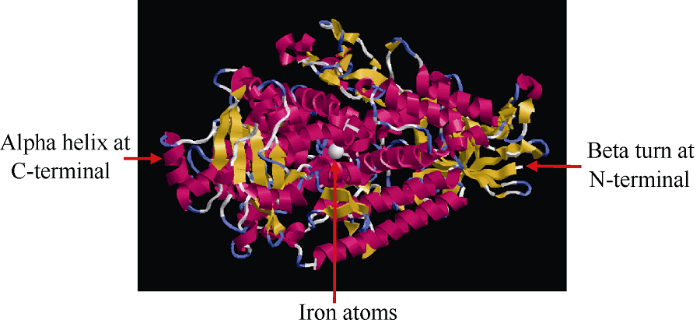 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2ScLOX1蛋白三级结构预测
Fig. 2Third structure prediction of ScLOX1 protein
图3
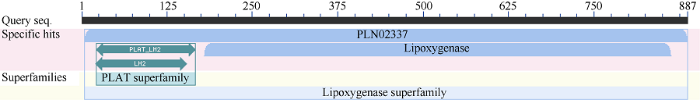 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3ScLOX1氨基酸序列的保守结构域
Fig. 3Conserved domains of amino acid sequences of ScLOX1
2.2 系统进化树分析
参考曹嵩晓等[4]的方法, 将ScLOX1与48个其他物种LOX蛋白的氨基酸序列构建系统进化树(图4)。结果显示, ScLOX1与OsLOX1 (DQ389164)、r9- LOX1 (AB099850)、ZmLOX1 (AF271894)和ZmLOX3 (AF329371)聚合在type I单子叶植物分支上, 此分支为非传统的LOX。利用DNAMAN软件将ScLOX1氨基酸序列与NCBI查找的5个单子叶植物LOX氨基酸序列进行同源序列比对(图5)显示, ScLOX1与水稻(O. sativa, DQ389164)、玉米(Z. mays, AF271894)、大麦(H. vulgare, L37358)、高粱(S. bicolor, XP_002466613)和谷子(Setaria italica, XP_ 004982082) LOX氨基酸序列相似性分别为77.25%、63.00%、61.05%、95.96%和90.65%。上述6个LOX序列均含有LOX蛋白保守区域, 包括底物结合位点(Domain I)、氧结合位点(Domain II)和铁结合有关的保守氨基酸残基。此外, ScLOX1蛋白含有苏氨酸/缬氨酸(Thr/Val, 594/595)和精氨酸(Arg, 745), 具有9-LOX特性。分析表明, ScLOX1属于type I类非传统9-LOX。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4ScLOX1与其他植物LOX蛋白构建的系统发育树
ScLOX1用▲标识。玉米LOXs: ZmLOX1 (AF271894)、ZmLOX3 (AF329371)、ZmLOX6 (DQ335764)、ZmLOX10 (DQ335768)、ZmLOX11 (DQ335769); 水稻LOXs: OsLOX1 (DQ389164)、r9-LOX1 (AB099850); 番茄LOXs: TomLOXA (U09026)、TomLOXB (U09025)、TomLOXC (U37839)、TomLOXD (U37840)、TomLOXE (AY008278)、TomLOXF (FJ617476); 拟南芥LOXs: AtLOX1 (NM_104376)、AtLOX2 (AY062611)、AtLOX3 (AJ249794)、AtLOX4 (NM_105911); 烟草LOXs: NaLOX1 (X84040)、NaLOX2 (AY254348)、NaLOX3 (AY254349); 马铃薯LOXs: StLOX1 (X95513)、StLOX2 (X96405)、StLOX3 (X96406); 大豆LOXs: GmLOX9 (EU003576)、GmLOX10 (EU003577); 花生LOX: PnLOX2 (DQ068249); 豌豆LOX: LOXN2 (AJ749702); 桃LOXs: PpLOX1 (EU883638)、PpLOX2 (FJ029110)、PpLOX3 (FJ032015)、PpLOX4 (EF568783); 葡萄LOXs: VvLOXA (FJ858255)、VvLOXC (FJ858256)、VvLOXO (FJ858257); 橄榄LOXs: OeLOX (EU678670)、Oep1LOX2 (EU513352)、Oep2LOX2 (EU513353); 辣椒LOXs: CaLOX1 (FJ377708)、CaLOX2 (JQ219046); 杨树LOXs: PdLOX1 (DQ131178)、PdLOX2 (DQ131179); 杏LOXs: LOX1:Pd:1 (AJ404331)、LOX1:Pd:2 (AJ418043); 菜豆LOX: PvLOX6 (EF196866); 甘蓝LOX: BoLOX (EF123056); 鬼箭锦鸡儿LOX: CjLOX (EF530043); 茶树LOX: CasLOX1 (EU195885); 榛子LOX: CaLOX (AJ417975)。
Fig. 4Phylogenetic tree estimated by the ScLOX1 and those proteins from other plants
ScLOX1 is marked with a black triangle. Zea mays LOXs: ZmLOX1 (AF271894), ZmLOX3 (AF329371), ZmLOX6 (DQ335764), ZmLOX10 (DQ335768), ZmLOX11 (DQ335769); Oryza sativa LOXs: OsLOX1 (DQ389164), r9-LOX1 (AB099850); Lycopersicum esculentum LOXs: TomLOXA (U09026), TomLOXB (U09025), TomLOXC (U37839), TomLOXD (U37840), TomLOXE (AY008278), TomLOXF (FJ617476); Arabidopsis thaliana LOXs: AtLOX1 (NM_104376), AtLOX2 (AY062611), AtLOX3 (AJ249794), AtLOX4 (NM_105911); Nicotiana tabacum LOXs: NaLOX1 (X84040), NaLOX2 (AY254348), NaLOX3 (AY254349); Solanum tuberosum LOXs: StLOX1 (X95513), StLOX2 (X96405), StLOX3 (X96406); Glycine max LOXs: GmLOX9 (EU003576), GmLOX10 (EU003577); Arachis hypogaea LOX: PnLOX2 (DQ068249); Pisum sativum LOX: LOXN2 (AJ749702); Prunus persica LOXs: PpLOX1 (EU883638), PpLOX2 (FJ029110), PpLOX3 (FJ032015), PpLOX4 (EF568783); Vitis vinifera LOXs: VvLOXA (FJ858255), VvLOXC (FJ858256), VvLOXO (FJ858257); Olea europaea LOXs: OeLOX (EU678670), Oep1LOX2 (EU513352), Oep2LOX2 (EU513353); Capsicum annuum LOXs: CaLOX1 (FJ377708), CaLOX2 (JQ219046); Populus deltoids LOXs: PdLOX1 (DQ131178), PdLOX2 (DQ131179); Prunus dulcis LOXs: LOX1:Pd:1 (AJ404331), LOX1:Pd:2 (AJ418043); Phaseolus vulgaris LOX: PvLOX6 (EF196866); Brassica oleracea LOX: BoLOX (EF123056); Caragana jubata LOX: CjLOX (EF530043); Camellia sinensis LOX: CasLOX1 (EU195885); Corylus avellana LOX: CaLOX (AJ417975).
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5甘蔗ScLOX1与其他植物LOX氨基酸序列比对
文本框表示底物结合结构域(Domain I和II)和C末端保守区域。箭头表示与铁结合有关的保守氨基酸残基。加号为TH(V)/R(K), 是一个对确定加氧位置特异性至关重要的基序。OsLOX1: 水稻(DQ389164); ZmLOX1: 玉米(AF271894); HvLOXC: 大麦(L37358); SbLOX4: 高粱(XP_002466613); SiLOX4: 谷子(XP_ 004982082)。
Fig. 5Amino acids sequence aligenment of ScLOX1 and other plant LOXs
The substrate-binding domain (Domains I and II) and C-terminus conserved regions are shown by text boxes. An arrow indicates a conserved amino acid residue related to iron binding. The plus sign indicates TH(V)/R(K), a motif proposed to be essential for determining oxygen-adding positional specificity. OsLOX1: Oryza sativa (DQ389164); ZmLOX1: Zea mays (AF271894); HvLOXC: Hordeum vulgare (L37358); SbLOX4: Sorghum bicolor (XP_ 002466613); SiLOX4: Setaria italica (XP_004982082).
2.3 ScLOX1基因在甘蔗各组织中的表达
利用qRT-PCR技术分析ScLOX1基因在成熟甘蔗植株ROC22不同组织中的相对表达情况(图6), 结果显示, ScLOX1基因只在蔗芽组织中特异性表达。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6ScLOX1基因在甘蔗ROC22不同组织中的表达情况
以甘油醛-3-磷酸脱氢酶基因(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)为内参基因。数据点为平均值±标准误(n = 3)。Leaf: 叶; Stem pith: 蔗肉; Bud: 蔗芽; Epidermis: 蔗皮; Root: 根。
Fig. 6Expression pattern of ScLOX1 gene in different sugarcane tissues of ROC22
Using GAPDH as an internal reference gene. Data points are means ± SE (n = 3).
2.4 ScLOX1基因在黑穗病菌胁迫下的表达分析
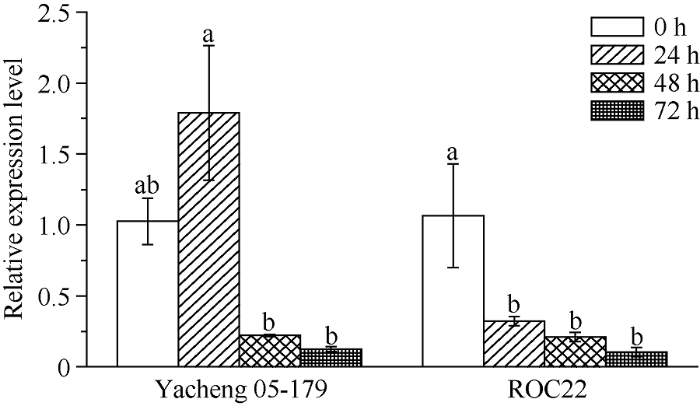
由图7可知, ScLOX1基因的表达量在甘蔗抗病品种崖城05-179受黑穗病菌侵染24 h时有所上升, 在48 h和72 h时下降。在感病品种ROC22中, 随着黑穗病菌侵染时间的延长, ScLOX1基因的表达量在24 h时显著下降, 且在24~72 h基本保持不变。相比而言, ScLOX1基因的表达量在抗病品种接种黑穗病孢子第1天略有上升, 而在感病品种中已显著下降, 同时两者的表达量相差约456.29%。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7不同基因型甘蔗与黑穗病菌互作过程中ScLOX1基因的表达量
以甘油醛-3-磷酸脱氢酶基因(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)作为内参。柱上不同的字母代表在P < 0.05时显著性的差异。数据点为平均值±标准误(n = 3)。Yacheng 05-179代表甘蔗抗黑穗病品种; ROC22代表甘蔗感黑穗病品种。
Fig. 7Gene expression level of ScLOX1 in the interactions of different sugarcane genotypes and smut pathogen
Using GAPDH as an internal reference gene. Bars superscripted by different lowercase letters are significantly different at P < 0.05. Data points are means ± SE (n = 3). Yacheng05-179 is a smut-resistant sugarcane variety. ROC22 is a smut-susceptible sugarcane variety.
2.5 本氏烟叶片瞬时过表达ScLOX1基因对不同病原菌胁迫的响应情况
分别将对照空载pEarleyGate 203 (35S::00)和重组载体pEarleyGate 203-ScLOX1 (35S::ScLOX1)转入农杆菌Gv3101, 然后注射本氏烟叶片。在注射1 d时, RT-PCR分析显示, ScLOX1基因在叶片中有表达(图8-A)。DAB染色结果显示, 瞬时过表达ScLOX1基因的叶片颜色与对照相比无明显差异(图8-B)。通过qRT-PCR检测瞬时过表达ScLOX1基因1 d后叶片中8个烟草免疫相关基因的表达情况(图8-C)显示, HR标记基因NtHSR201、JA途径相关基因NtPR2和NtPR3以及ET合成依赖基因NtEFE26的表达量显著上调, 分别为对照的2.62、2.31、3.70和5.46倍, 其余基因表达量与对照相比无显著差异, 推测ScLOX1基因在本氏烟叶片中的瞬时过表达可能诱导植物免疫反应。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8ScLOX1基因在本氏烟叶片中瞬时表达
A: RT-PCR检测ScLOX1基因在接种携带35S::00和35S::ScLOX1载体的农杆菌菌株GV3101的本氏烟叶片1 d的表达情况。B: 对接种携带35S::00和35S::ScLOX1载体的农杆菌2 d的本氏烟叶片进行DAB染色。(1)和(2)分别代表索尼相机和显微镜拍摄的图像。C: 烟草免疫相关标记基因在接种携带35S::00和35S::ScLOX1载体的农杆菌1 d的本氏烟叶片中的相对表达水平。D和F: 瞬时过表达ScLOX1基因1 d后接种烟草青枯菌和茄病镰刀菌蓝色变种1 d和7 d时的本氏烟叶片发病情况和DAB染色结果。(1)和(2)分别代表索尼相机和显微镜拍摄的图像。E和G: 烟草免疫相关标记基因在瞬时过表达ScLOX1基因的本氏烟叶片接种青枯菌和茄病镰刀菌蓝色变种1 d和7 d时的表达情况分析。烟草免疫相关标记基因包括HR标记基因NtHSR201、NtHSR203和NtHSR515, SA途径相关基因NtPR-1a/c, JA途径相关基因NtPR2和NtPR3, ET合成依赖基因NtEFE26和NtAccdeaminase。内参基因为NtEF-1α基因。柱上不同的字母代表在P < 0.05时显著性的差异。所有的点均为平均值±标准误 (n = 3)。a和b分别表示载体35S::00和35S::ScLOX1。
Fig. 8Transient overexpression of ScLOX1 in Nicotiana benthamiana leaves
A: RT-PCR analysis of ScLOX1 gene in the N. benthamiana leaves after infiltration by Agrobacterium strain GV3101 carrying vector 35S::00 or 35S::ScLOX1 for 1 d. B: DAB staining with N. benthamiana leaves 2 d after 35S::ScLOX1-containing Agrobacterium strain infiltration. (1) and (2) represent images taken by SONY camera and microscope, respectively. C: Relative expression level of the tobacco immunity-associated marker genes in 35S::ScLOX1-transiently expressing leaves after infiltration for 1 d. D and F: The results of disease symptoms and DAB staining of N. benthamiana leaves after inoculation with 35S::ScLOX1 1 d and then infection with Ralstonia solanacearum and Fusarium solani var. coeruleum for 1 d and 7 d, respectively. (1) and (2) represent images taken by SONY camera and microscope, respectively. E and G: Analysis of the immunity related marker genes in the N. benthamiana leaves by R. solanacearum and F. solani var. coeruleum for 1 d and 7 d post inoculation. The tobacco immunity-associated marker genes including the HR marker genes NtHSR201, NtHSR203 and NtHSR515, the SA pathway related genes NtPR-1a/c, the JA pathway-associated genes NtPR2 and NtPR3, and the ET synthesis depended genes NtEFE26 and NtAccdeaminase. Using NtEF-1α as an internal reference gene. Bars superscripted by different lowercase letters are significantly different at P < 0.05. Data points are means ± SE (n = 3). The vectors of 35S::00 and 35S::ScLOX1 are indicated by a and b, respectively.
对已注射携带空载35S::00和重组载体35S::ScLOX1农杆菌1 d的本氏烟叶片分别接种了烟草青枯菌和茄病镰刀菌蓝色变种。在接种青枯菌1 d时, 35S::ScLOX1本氏烟叶片和35S::00本氏烟叶片均未观察到明显的病症(图8-D)。35S::ScLOX1叶片中的NtHSR201、NtHSR515、NtPR2和NtPR3的表达量与对照相比无显著差异, NtPR-1a/c和NtEFE26的表达量显著低于对照, NtHSR203和NtAccdeaminase的表达量显著高于对照(图8-E)。接种青枯菌7 d时, 35S::ScLOX1和35S::00的本氏烟叶片均出现病斑, 但两者的病症差异不大(图8-D)。35S::ScLOX1叶片中的NtHSR201、NtHSR515和NtAccdeaminase的表达量与对照相比无显著差异, NtPR2、NtPR3和NtEFE26的表达量显著低于对照, NtHSR203和NtPR-1a/c的表达量显著高于对照(图8-E)。此外, 处理组与对照组接种1 d和7 d的本氏烟叶片DAB染色结果无明显差异(图8-D)。
注射35S::ScLOX1和35S::00的本氏烟叶片在接种茄病镰刀菌蓝色变种1 d后均无明显的病症出现(图8-F), 此时, 瞬时表达ScLOX1的叶片中NtAccdeaminase的表达量显著高于对照, NtHSR201、NtHSR515、NtPR-1a/c、NtPR2和NtPR3的表达量显著低于对照, NtHSR203和NtEFE26的表达量与对照相比无显著差异(图8-G)。接种茄病镰刀菌蓝色变种7 d时, 35S::ScLOX1和35S::00叶片均出现大片病斑, 但对照发病相对较严重(图8-F)。此外, 瞬时表达ScLOX1基因的本氏烟叶片中NtHSR515、NtPR3、NtEFE26和NtAccdeaminase的表达量显著低于对照, 但NtHSR201、NtHSR203、NtPR-1a/c和NtPR2的表达量均显著高于对照(图8-G)。接种1 d和7 d的叶片经DAB染色后均有深棕色斑点出现, 但处理组与对照组差异不明显(图8-F)。
2.6 ScLOX1基因在不同植物激素胁迫下的表达分析
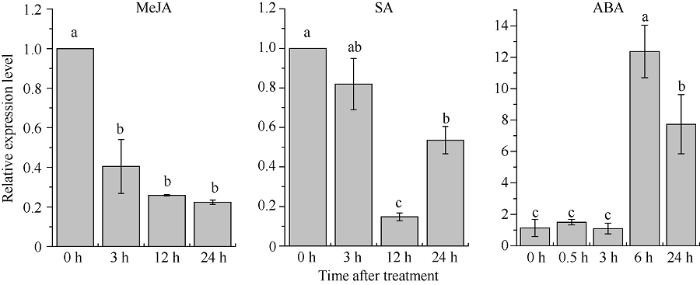
利用qRT-PCR分析ROC22组培苗中ScLOX1基因在不同植物激素胁迫下的表达情况(图9)显示, MeJA处理后, ScLOX1的转录本积累量显著下降, 且在3~24 h基本不变。SA处理后, ScLOX1基因表达量在3 h时维持在对照水平, 12 h时显著下降, 为对照的0.15倍, 24 h时有所上升但仍显著低于对照水平。受ABA胁迫后, ScLOX1基因表达量在0.5~3.0 h时基本保持不变, 在6 h显著上升并呈现表达高峰, 为对照的10.88倍, 24 h时有所下降但仍显著高于对照水平。图9
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9ScLOX1基因在不同植物激素胁迫下的相对表达量
内参基因为甘油醛-3-磷酸脱氢酶基因(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)。柱上不同的字母代表在P < 0.05时显著性的差异。数据点为平均值±标准误(n = 3)。
Fig. 9Relative expression level of ScLOX1 gene under the stress of different plant hormones
Using GAPDH as an internal reference gene. Bars superscripted by different lowercase letters are significantly different at P < 0.05. Data points are means ± SE (n = 3).
2.7 ScLOX1基因在干旱和盐胁迫下的表达分析
由图10可知, ScLOX1基因在正常培养条件下的ROC22种茎苗叶片(0 h)中不表达, 但在NaCl和PEG胁迫下被诱导表达。随着NaCl处理(模拟盐胁迫)时间的延长, ScLOX1基因的表达量在0.5 h显著上升, 在3 h时达到峰值, 其后显著下降且与0.5 h的基因表达量无显著差异。利用PEG模拟干旱胁迫后, ScLOX1基因在0.5~6.0 h被诱导上调表达且维持在一定的水平, 并在24 h时显著上调表达且达到峰值。图10
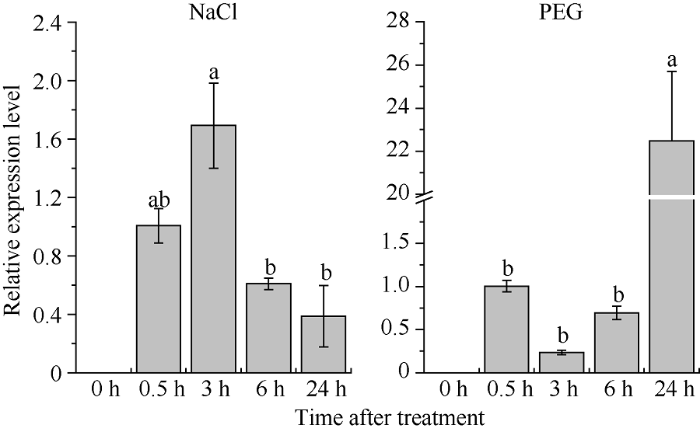 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10ScLOX1基因在NaCl和PEG胁迫下的表达情况
内参基因为甘油醛-3-磷酸脱氢酶基因(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)。柱上不同小写字母代表在P < 0.05时显著性的差异。数据点为平均值±标准误(n = 3)。
Fig. 10Expression of ScLOX1 gene under the stresses of PEG and NaCl
Using GAPDH as an internal reference gene. Bars superscripted by different lowercase letters are significantly different at P < 0.05. Data points are means ± SE (n = 3).
3 讨论
LOX属于脂氧合酶超家族(lipoxygenase superfamily), 在植物中以多基因家族形式存在, 参与植物生长发育的调节和对逆境胁迫的抵御[7]。本研究从甘蔗中首次分离鉴定出1个ScLOX1基因, 该基因推导的编码蛋白属于type I类非传统9-LOX。前人研究报道, 单子叶植物type I类LOXs可能参与植物对害虫和病原菌的防御[4]。例如, 水稻OsLOX1参与植物对机械损伤和稻褐飞虱(Nilaparvata lugens)攻击的响应[5]。在玉米中, ZmLOX1基因在玉米籽苗中能被机械损伤和MeJA诱导[37], ZmLOX3基因能够响应曲霉菌(Aspergillus spp.)对种子的入侵[38]。水稻OsLOX1的转录本可以在未成熟的种子和新发芽的籽苗中低量表达[5]。AmLOX1基因在金鱼草(Antirrhinum majus)的根、茎、叶和花中差异表达, 其在花中的表达量最高[39]。在马铃薯(Solanum tuberosum)中, LOX1在根和块茎中表达并参与块茎的生长发育; LOX2在叶片中表达并积极响应损伤胁迫; LOX3在叶片和根中表达, 其在植株受到损伤时转录本增加[40,41]。在菜豆(Phaseolus vulgaris)胚轴中, PvLOX2在生长区大量表达, 而在成熟区则几乎不存在[42]。在本研究中, ScLOX1只在成熟ROC22植株的蔗芽中表达。上述结果表明, LOX基因在植物不同组织器官和不同发育时期的表达存在差异。
LOX基因能参与调控植物对生物胁迫的防御作用。棉花(Gossypium hirsutum) LOX基因(GhLOX1)在黄单胞菌(Xanthomonas campestris)诱导的HR反应高度表达[43]。在根瘤菌(Rhizobium tropici)胁迫下, PvLOX3的mRNA在14~16 d时有最大表达量[42]。辛翠花等[44]对抗晚疫病马铃薯转基因株系DR1、DR3和晚疫病敏感型野生株DG接种晚疫病原菌(Phytophthora infestans)后, 马铃薯JA合成途径关键酶基因poaos和polox在叶片中的表达量均有不同程度的提高, 但转基因株系叶片内目标基因的表达量高于野生型, 表明polox基因可能参与了马铃薯抗晚疫病反应。在转基因番茄植株中, TomloxD的过表达增加了内源JA的产生, 并且增强了植株对番茄叶霉菌(Cladosporium fulvum)和高温胁迫的抗性[45]。也有研究指出, 拟南芥和小麦(Triticum aestivum) 9-LOXs的表达能够促进禾谷镰孢菌(Fusarium graminearum)的感染[46]。在本研究中, 不同甘蔗基因型的蔗芽接种甘蔗黑穗病菌后, ScLOX1基因的表达量在抗病品种崖城05-179中短暂上升, 而在感病品种ROC22中显著下降。由于蔗芽是甘蔗黑穗病菌侵染的唯一通道[47], ScLOX1基因在蔗芽中的特异性表达暗示其可能在该甘蔗组织中积极参与对黑穗病菌的防御作用。据前人报道, 植物在防御病原菌侵染的过程中会由于细胞凋亡产生包括免疫相关基因表达、防御相关植物激素的累积、ROS和离子通量的增多等在内的一系列生物反应[34,48]。在瞬时表达ScLOX1基因的本氏烟叶片上接种烟草病原菌显示, ScLOX1基因瞬时过表达后对烟草病原细菌青枯菌的防御作用相较于对照无明显差异, 但能够增强本氏烟对烟草病原真菌茄病镰刀菌蓝色变种的防御, 且接种7 d的本氏烟叶片的HR标记基因NtHSR201和NtHSR203、SA途径相关基因NtPR-1α/c、JA途径相关基因NtPR2的表达量显著上调, 推测ScLOX1可能通过参与多个植物免疫基因的转录调控和过敏性坏死反应来调节植物对病原菌的防御, 但针对不同类型的病原菌其发挥的作用可能不同, 该研究结果有待稳定遗传转化后作进一步抗病验证。
近年来, 植物LOX基因在各种诱导因子(JA、SA和ABA)和非生物胁迫(盐和干旱)下的表达模式被广泛报道[11,15,43,49-50]。Lim等[49]在拟南芥中过表达了CaLOX1基因(CaLOX1-overexpressing, CaLOX1-OX), 发现与野生型相比, CaLOX1-OX植株在种子萌发和幼苗发育过程中对ABA、甘露醇和高盐胁迫表现出更强的耐受性; 用高盐和干旱条件胁迫处理生长过程中的拟南芥, CaLOX1-OX植株同样比野生型表现出更强的耐受性。在杨树(Populus deltoides)中, PdLOX1和PdLOX2在MeJA和机械损伤诱导下上调, 而受SA诱导均下调[50]。Porta等[43]用损伤、MeJA和ABA处理菜豆成熟叶片能诱导PvLOX2的累积; 在水分胁迫和ABA胁迫下, PvLOX2的表达量在菜豆胚轴生长区减少, 而在成熟区增加。此外, 对黄瓜中的12个LOX基因研究发现, 10个LOX基因(CsLOX1、CsLOX2、CsLOX4、CsLOX8、CsLOX16、CsLOX17、CsLOX19、CsLOX20、CsLOX22和CsLOX23)在MeJA诱导下上调; 在ABA、冷害和盐胁迫下, 8个LOX基因(CsLOX1、CsLOX2、CsLOX8、CsLOX17、CsLOX19、CsLOX20、CsLOX22和CsLOX23)表达上调[15]。上述研究结果表明, 不同物种或同一物种的LOX基因家族的不同成员之间存在功能分化。在本研究中, ScLOX1的表达量被JA和SA抑制下调, 但在ABA、NaCl和PEG胁迫条件下上调表达。有研究报道, ABA是植物响应非生物胁迫(如盐胁迫)的关键调控因子[51]。SA、JA和乙烯则被认为是植物系统获得性抗性(systemic acquired resistance, SAR)和诱导系统性抗性(induced systemic resistance, ISR)的重要信号分子[52,53]。由此推测, ScLOX1基因可能通过ABA介导的信号通路来积极响应NaCl和PEG的胁迫。
4 结论
从甘蔗中挖掘与鉴定了1个ScLOX1基因。该基因cDNA序列全长2813 bp, ORF全长2664 bp, 编码887个氨基酸, 预测其编码蛋白为酸性稳定亲水性非分泌蛋白, 属于type I类非传统9-LOX。ScLOX1在蔗芽中特异性表达。受黑穗病菌侵染后, ScLOX1基因的表达量在甘蔗抗病品种中短时间略微上调, 但在感病品种中显著下调。在本氏烟叶片中瞬时表达ScLOX1, 发现ScLOX1基因能够增强本氏烟对烟草病原真菌茄病镰刀菌蓝色变种的抵抗, 但对烟草病原细菌青枯菌的作用相较于对照无明显差异。此外, 在外源胁迫下, ScLOX1基因的表达量被JA和SA抑制下调, 但在ABA、NaCl和PEG胁迫条件下上调表达, 推测ScLOX1基因可能通过ABA介导的信号通路来提高甘蔗对盐和干旱胁迫的耐受性。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1074/jbc.274.34.23679URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
[本文引用: 4]
DOI:10.1007/s11103-007-9278-0URL [本文引用: 4]
DOI:10.1146/annurev.arplant.53.100301.135248URL [本文引用: 1]
DOI:10.1016/j.phytochem.2009.05.008URL [本文引用: 2]
DOI:10.1104/pp.123.4.1281URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/0076-6879(81)71055-3URL [本文引用: 1]
[本文引用: 2]
[本文引用: 1]
DOI:10.1104/pp.106.1.109URL [本文引用: 1]
[本文引用: 1]
[本文引用: 3]
DOI:10.1016/j.jplph.2005.11.006URL [本文引用: 2]
DOI:10.1016/S0163-7827(98)00014-9URL [本文引用: 3]
[本文引用: 2]
[本文引用: 2]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
URL [本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1006/meth.2001.1262URL [本文引用: 1]
DOI:10.1105/tpc.112.095869URL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1023/B:PLAN.0000004331.94803.b0URL [本文引用: 1]
DOI:10.1094/MPMI.2001.14.8.980URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1074/jbc.271.35.21012URL [本文引用: 1]
DOI:10.1105/tpc.13.3.613URL [本文引用: 1]
[本文引用: 2]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/tpj.2010.64.issue-3URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.4161/psb.2.3.4156URL [本文引用: 1]
DOI:10.1126/science.261.5122.754URL [本文引用: 1]
[本文引用: 1]
