摘要/Abstract
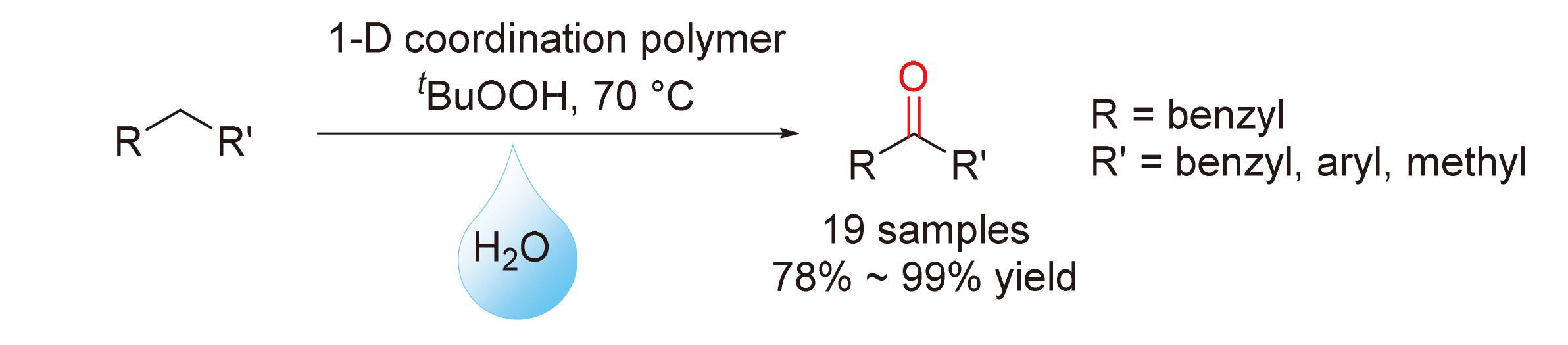
通过苄位C—H直接氧化制备了一系列芳香酮类化合物. 该反应体系采用钴(II)-三联吡啶配位聚合物作为催化剂, 叔丁基过氧化氢(TBHP)作为氧化剂, Na2CO3作为助催化剂, 水作为溶剂, 以较高的收率(78%~99%)制得了19种芳香酮类化合物. 该方法底物适用范围广, 具有较高的化学选择性和官能团耐受性. 通过2,2,6,6-四甲基哌啶-1-氧(TEMPO)的自由基捕获实验及对反应过程中中间体的监测, 提出了一种合理的自由基反应机理.
关键词: 苄位直接氧化, 配位聚合物, 叔丁基过氧化氢, 酮类
Direct benzylic C—H oxidation for the synthesis of aromatic ketones was developed. Employing cobalt(II)-terpyridine coordination polymers as catalyst, tert-butyl hydroperoxide (TBHP) as oxidant and Na2CO3 as activator, 19 aromatic ketones were prepared with good to excellent yields (78%~99%) in water. The reaction showed a broad range of substrates with good functional group tolerance and chemical selectivity. By control experiments, a trapping experiment using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and detection of intermediates during reaction, a reasonable radical mechanism for this type of reaction was also demonstrated.
Key words: direct benzylic oxidation, coordination polymer, tert-butyl hydroperoxide, ketone
PDF全文下载地址:
点我下载PDF
