摘要/Abstract
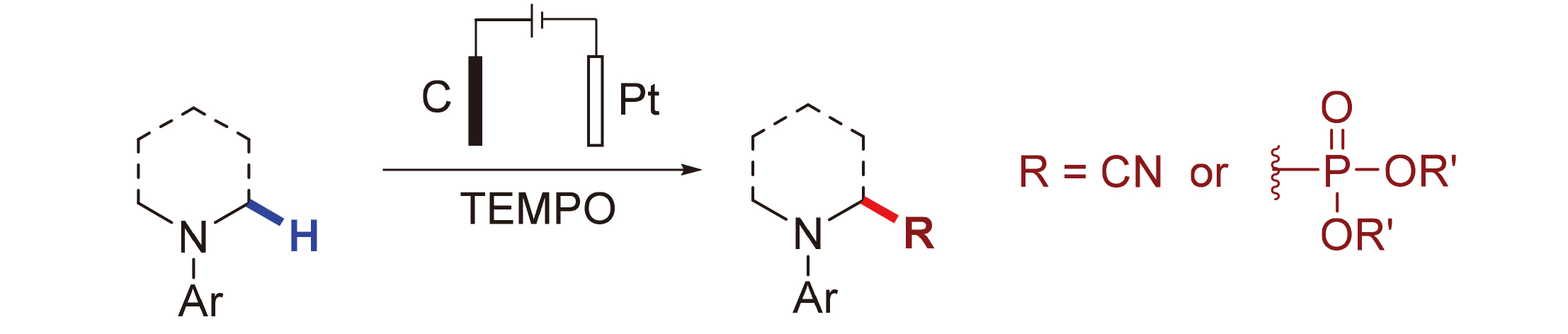
探究了无金属电化学氧化氰化和膦酸酯基化反应, 其中2,2,6,6-四甲基哌啶-氮-氧化物(TEMPO)降低了底物的电极电位, 避免了某些富电子芳香胺在电化学条件下的过氧化反应. 该方法具有良好的官能团兼容性, 是在温和条件下合成α-氨基腈和α-氨基膦酸酯的一种有效且实用的方法, 通过研究表明产物通过Shono氧化形成亚胺物种来实现的.
关键词: 电化学, Shono氧化, 氰基化, 膦酸酯基化
Metal-free electrochemical oxidation cyanation and phosphonylation reactions had been developed, in which 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO) reduced the electrode potential of substrate and avoided over oxidation of some electron rich aromatic amines under electrochemical conditions. This protocol had good functional group compatibility, which made it to be a practical and efficient method to synthesize α-aminonitriles and α-amino phosphonates under mild conditions. Preliminary study indicated that the formation of the product was through the Shono oxidation of imine species.
Key words: electrochemistry, Shono oxidation, cyanation, phosphonylation
PDF全文下载地址:
点我下载PDF
