摘要/Abstract
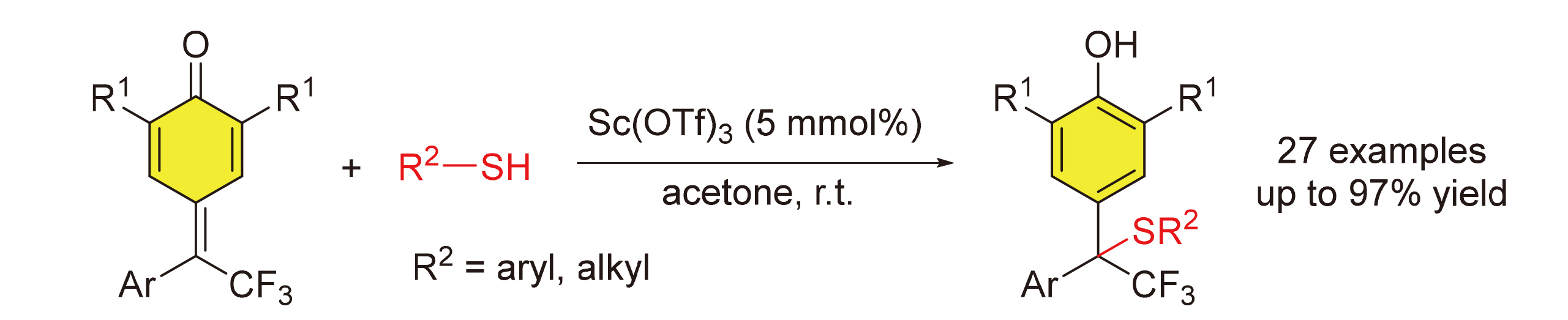
报道了一种δ-三氟甲基-δ-芳基-二取代的对亚甲基苯醌和硫醇参与的1,6-共轭加成反应. 在5 mol%三氟甲基磺酸钪催化的条件下, 得到了结构多样化的含有三氟甲基四级碳中心的二芳基甲烷硫醚. 反应具有良好的官能团兼容性和底物范围. 此外, 烷基硫醇和苄硫醇也适用于该反应. 鉴于二芳基甲烷硫醚骨架以及三氟甲基在生物活性分子中的重要性, 发展的含有三氟甲基取代四级碳中心的二芳基甲烷硫醚的高效合成方法将为生物活性分子的发现提供一条简洁、高效的策略.
关键词: δ-三氟甲基-δ-芳基-二取代, 对亚甲基苯醌, 二芳基甲烷, 硫醚, 1,6-共轭加成
An efficient and practical 1,6-conjuate addition reaction of δ-CF3-δ-aryl-disubstituted para-quinone methides with thiols has been described. This approach provides a straightforward access to structurally diverse diarylmethane thioethers bearing CF3-substituted quaternary stereocenters using 5 mol% Sc(OTf)3 as catalyst. The reaction has an excellent functional-group tolerance, and displays a broad scope with respect to both δ-CF3-δ-aryl-disubstituted para-quinone methides and thiophenols. Moreover, alkyl thioalcohols and benzylmercaptan have proven to be suitable substrates. Diarylmethane thioethers belong to an important structural scaffold that widely exists in a number of bioactive molecules and trifluoromethyl group has a profound effect on physiological properties of organic molecules. Therefore, the efficient method for the synthesis of diarylmethane thioethers bearing CF3-substituted quaternary stereocenters might provide a powerful strategy for the discovery of biologically interesting agents.
Key words: δ-CF3-δ-aryl-disubstituted, para-quinone methides, diarylmethane, thioethers, 1,6-conjuate addition
PDF全文下载地址:
点我下载PDF
