摘要/Abstract
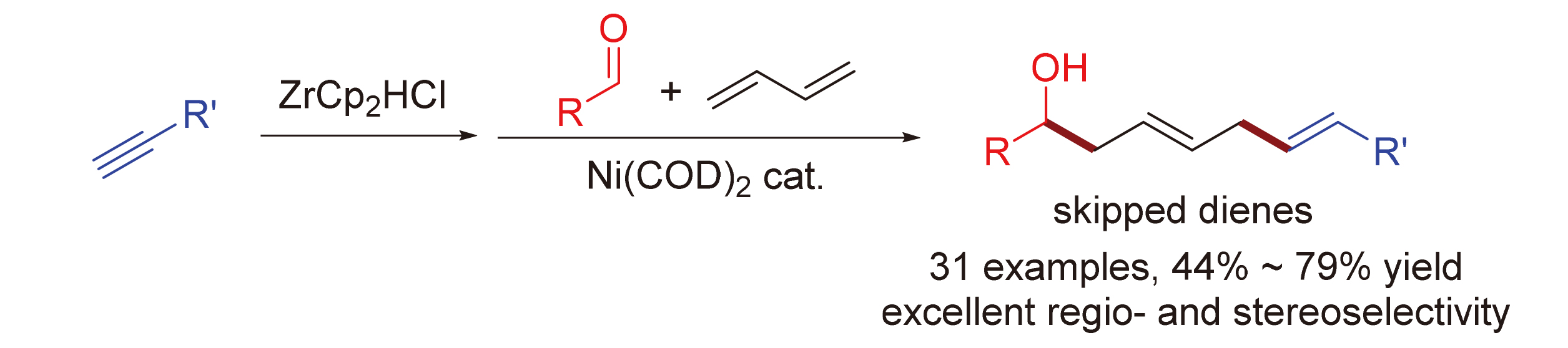
1,4-二烯结构广泛存在于一系列具有生物活性的化合物分子中, 其构建是有机合成中的重要研究领域之一. 使用简单易得的原料合成1,4-二烯具有重要的研究意义. 发展了镍催化的1,3-丁二烯、醛、炔和氢氯二茂锆的多组分偶联反应, 用于高效合成1,4-二烯产物. 该反应的原料均简单易得, 其中1,3-丁二烯更是大宗化工产品. 通过简单的炔烃氢锆化反应现场制备烯基锆试剂直接参与反应, 无需分离纯化. 反应以优秀的区域选择性和立体选择性合成了一系列(E,E)-1,4-二烯产物. 简单温和的反应条件使该方法具有广泛的底物适用范围和优秀的官能团兼容性. 该反应提供了一种合成1,4-二烯产物的高效且实用的方法.
关键词: 1,4-二烯, 镍催化, 丁二烯, 多组分反应, 偶联
The construction of skipped diene is a vital research area for organic synthesis, whose structure is found in many bioactive molecules. The synthesis of skipped diene from simple and readily available starting materials is highly desirable. Herein a nickel-catalyzed multicomponent coupling of 1,3-butadiene, aldehydes, alkynes, and Schwartz reagents for the preparation of skipped dienes is described. The reagents are common feedstock chemicals, especially 1,3-butadiene is an abundant feedstock produced from petroleum cracking. Moreover, the hydrozirconation of alkynes using Schwartz reagent was applied to in-situ prepared the alkenylzirconium reagents, which were used directly without further treatment. Various (E,E)-1,4-diene products were synthesized with excellent regio- and stereo-selectivity. The mild and straightforward reaction condition enables a broad substrate scope and good functional group tolerance. This protocol provides a useful and practical synthesis of skipped dienes.
Key words: skipped diene, nickel catalysis, butadiene, multicomponent reaction, coupling
PDF全文下载地址:
点我下载PDF
