摘要/Abstract
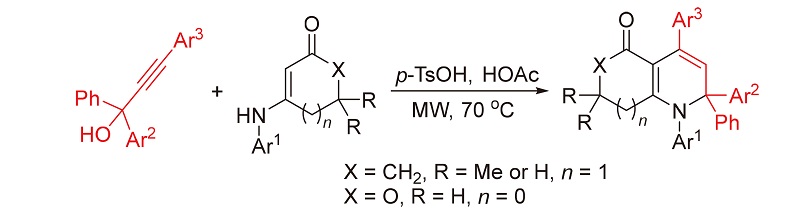
报道了一类新型的微波辅助对甲基苯磺酸促进的[3+3]环化反应. 利用烯胺酮或烯胺内酯可作为1,3-双亲核试剂及炔丙醇可作为1,3-双亲电试剂的特性, 使其在微波辐射及对甲苯磺酸促进条件下于冰醋酸中在70 ℃反应, 实现了[3+3]环化反应, 分别区域选择性地合成了2,2-二芳基取代四氢喹啉-5(1H)-酮衍生物和2,2-二芳基取代二氢呋喃并[3,4-b]吡啶-5-酮衍生物, 产率良好. 该反应利用微波合成技术促使反应, 在短时间内完成(30 min), 唯一副产物为水. 此外, 该方法具有原料简单易得、操作简单及底物普适性广等优点, 从而为具有潜在应用价值的稠合吡啶骨架的构建提供了一种绿色、经济且高效的合成策略, 符合绿色化学理念.
关键词: 微波合成, [3+3]环化, 喹啉-5(1H)-酮衍生物, 呋喃并[3,4-b]吡啶-5-酮衍生物, 绿色化学
A new microwave-assisted p-TsOH-promoted [3+3] cyclization was developed. By using the characteristics of enaminones or enamino lactones as 1,3-dinucleaphilic reagents and propargyl alcohols as 1,3-electrophilic reagents, p-TsOH- promoted [3+3] cyclization of these substrates at 70 ℃ was carried out in acetic acid under microwave irradiation, regioselectively affording 2,2-diaryl-substituted tetrahydroquinoline-5(1H)-ones and 2,2-diaryl-substituted dihydrofuro[3,4-b]pyridin- 5-ones in good yields. The reaction can be completed within a short period (30 min) by microwave synthetic technology, in which water was the sole by-product. This method features simple and available starting materials, simple operation and wide substrate scope, and provides a green, economic, and efficient synthetic strategy for the construction of fused pyridine skeleton with potential application, which is consistent with the concept of green chemistry.
Key words: microwave synthesis, [3+3] cyclization, quinoline-5(1H)-one, furo[3,4-b]pyridin-5-one, green chemistry
PDF全文下载地址:
点我下载PDF
