摘要/Abstract
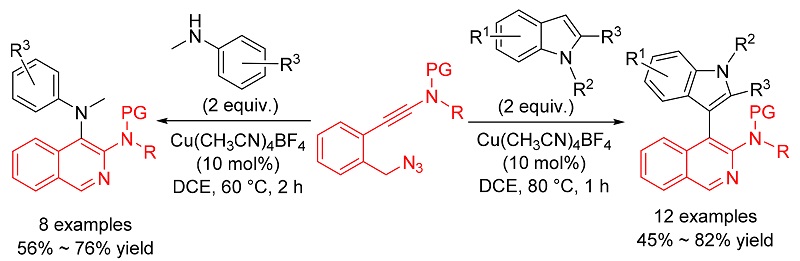
报道了利用铜催化叠氮-炔酰胺的环化反应合成异喹啉衍生物的方法. 首先通过铜催化分子内叠氮-炔酰胺环化反应生成α-亚胺铜卡宾中间体, 随后再分别被分子间的吲哚和苯胺捕获生成相应的C—H和N—H插入产物. 该方法操作简便、反应条件温和、底物普适性广, 为含有异喹啉-吲哚和异喹啉-苯胺骨架的天然产物和活性分子的合成提供了一条简便和高效的途径.
关键词: 环化反应, 铜催化, 铜卡宾, 炔酰胺
A copper-catalyzed azide-ynamide cyclization to synthesize isoquinoline derivatives is reported. First, α-imino copper carbene intermediate is generated via Cu(I)-catalyzed azide-ynamide cyclization, then this copper carbene can be captured by indoles and anilines to form C—H and N—H insertion products. The notable advantages of this method include a simple procedure, mild reaction conditions and widespread availability of the substrates. Thus, this protocol provides a highly convenient and efficient route for the preparation of natural products and active molecules which contain the isoquinoline-indole or isoquinoline-aniline skeletons.
Key words: cyclization reaction, copper catalysis, copper carbenes, ynamides
PDF全文下载地址:
点我下载PDF
