摘要/Abstract
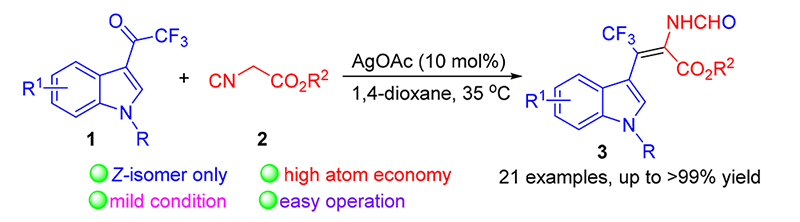
实现了银催化的3-三氟乙酰基吲哚与异氰酸酯的环化-重排反应, 以优秀的收率(up to >99%)得到了一系列单一构型的( Z)-β-三氟甲基脱氢色氨酸衍生物. 反应以克级规模进行时, 产率仍能达到98%, 这一结果证明了反应的实用性. 此外, 作者提出了可能的反应机理. 该反应具有原子经济性高、反应条件温和及操作简便等优点.
关键词: 3-三氟乙酰基吲哚, 异氰酸酯, 脱氢色氨酸
Catalytic cyclization-rearrangement reaction of 3-trifluoroacetyl indole and isocyanoacetate was achieved with AgOAc as catalyst in mild conditions. A serious of β-trifluoromethylated dehydrotryptophan derivatives were obtained with single Z-isomer in excellent yields (up to >99% yields). The large scale experiment proceeded smothly genereting desired products in up to 98% isolated yield, which demostrated the practicality of the method. The plausible mechanism was aslo proposed. This transformation has features of high atom economy, mild reaction condition and easy operation.
Key words: 3-trifluoroacetyl indole, isocyanoacetate, dehydrotryptophan
PDF全文下载地址:
点我下载PDF
