摘要/Abstract
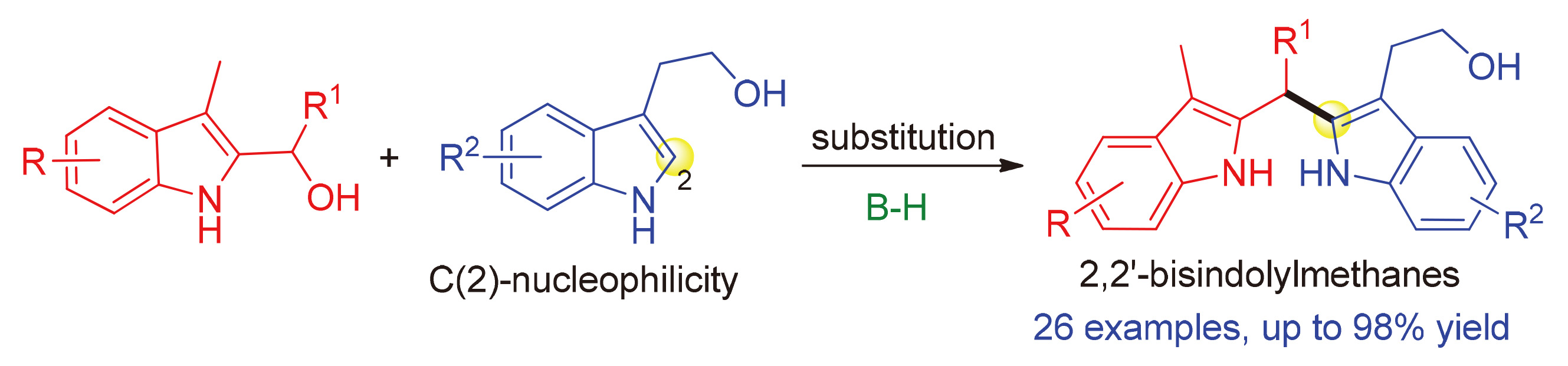
通过布朗斯特酸催化下2-吲哚甲醇与色醇的取代反应,化学选择性地合成了一系列2,2'-双吲哚甲烷衍生物,获得了高的收率(高达98%).该反应不仅为构建具有重要生物活性的2,2'-双吲哚甲烷骨架提供了有效的方法,而且实现了2-吲哚甲醇参与的取代反应,丰富了2-吲哚甲醇的化学性质.此外,该反应利用了色醇C(2)-位的亲核性,为实现色醇参与的化学选择性反应提供了一个良好的例子.
关键词: 2-吲哚甲醇, 色醇, 布朗斯特酸催化, 取代反应, 双吲哚甲烷
A Brønsted acid-catalyzed substitution reaction of 2-indolylmethanols with tryptophols has been established, which afforded a series of 2,2'-bisindolylmethanes in high yields (up to 98% yield) with chemoselectivity. This protocol not only provides an efficient method for constructing biologically important 2,2'-bisindolylmethane frameworks, but also has realized a substitution reaction of 2-indolylmethanols, which will enrich the chemical property of 2-indolylmethanols. Moreover, this approach has utilized the C(2)-nucleophilicity of tryptophols, which provided a good example for controlling the chemoselectivity in tryptophol-involved reactions.
Key words: 2-indolylmethanol, tryptophol, Brønsted acid catalysis, substitution, bisindolylmethane
PDF全文下载地址:
点我下载PDF
