摘要/Abstract
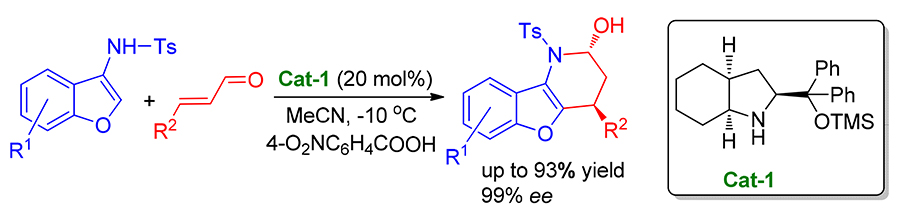
二苯基八氢吲哚醇硅醚可高对映选择性催化3-氨基苯并呋喃与 α, β-不饱和醛的[3+3]氮杂环化反应, 获得高收率(高达93%)、高非对映选择性( dr>20∶1)和高对映选择性(86%~>99% ee)的苯并呋喃衍生物. 该方法可获得克级规模的苯并呋喃衍生物.
关键词: 关键词 二苯基八氢吲哚醇硅醚, [3+3]氮杂环化αβ-不饱和醛, 3-氨基苯并呋喃
A highly enantioselective [3+3] aza-cyclization of α, β-unsaturated aldehydes with 3-aminobenzofuran promoted by diphenylperhydroindolinol silyl ether has been described, which afforded benzofuran derivatives in high yields (up to 93%), diastereoselectivities ( dr>20∶1) and enantioselectivities (86%~>99% ee). This method also enabled to obtain benzofuran derivatives in gram scale-up.
Key words: diphenylperhydroindolinol silyl ether, [3+3] aza-cyclizationαβ-unsaturated aldehydes, 3-aminobenzofuran
PDF全文下载地址:
点我下载PDF
