摘要/Abstract
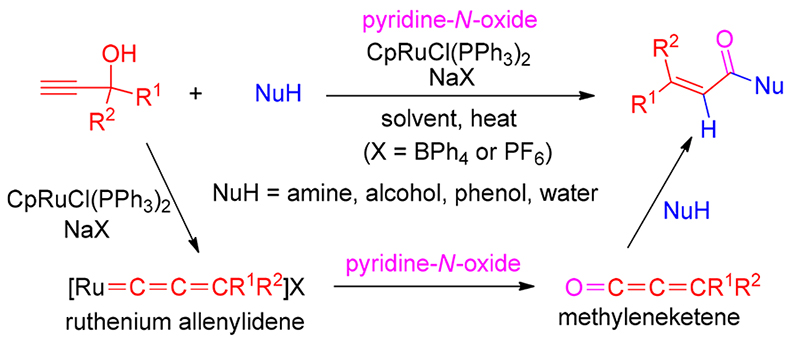
报道了钌催化末端炔丙醇经亚丙二烯基卡宾中间体氧化产生亚甲基烯酮合成 α, β-不饱和羧酸衍生物的高效方法. 机理研究实验表明, 催化剂CpRuCl(PPh3)2/NaBPh4和末端炔丙醇反应产生的钌亚丙二烯基卡宾与吡啶氧化物发生氧转移, 生成高活性的亚甲基烯酮中间体, 再发生亲核加成得到 α, β-不饱和产物. 该反应提供了一个机理上完全不同于传统方法的合成 α, β-不饱和羧酸衍生物的新策略, 是炔丙醇催化转化的一种新颖方法, 也是金属亚丙二烯基催化的一种新途径.
关键词: 钌, 亚丙二烯基, 炔丙醇, 氧转移, 亚甲基烯酮, 烯酮
A ruthenium-catalyzed oxygenative transformation of terminal propargyl alcohols to metheyleneketenes via allenylidene intermediates has been developed for the synthesis of a variety of α, β-unsaturated carboxylic acid derivatives. Mechanistic study experiments disclosed that oxygen transfer from pyridine- N-oxide to ruthenium allenylidene generated from the reaction of catalyst CpRuCl(PPh3)2/NaBPh4 with terminal propargyl alcohol resulted in the formation of reactive methyleneketene intermediate, which was trapped into nucleophilic addition reactions to afford α, β-unsaturated product. This reaction offers an attractive complementary strategy to the traditional approach for the synthesis of this class of unsaturated compounds, but in a distinct mechanism, which provides a novel method for the transformation of propargylic alcohols. The metal allenylidene-to-methyleneketene transformation also represents a new mechanistic modality for metal allenylidene-mediated catalysis.
Key words: Keywords ruthenium, allenylidene, propargyl alcohol, oxygen-transfer, methyleneketene, ketene
PDF全文下载地址:
点我下载PDF
