摘要/Abstract
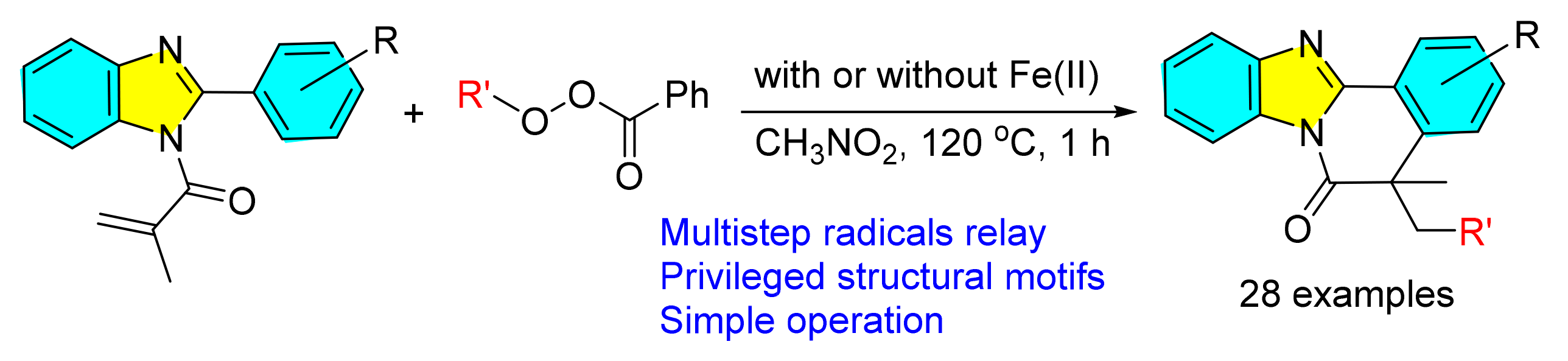
发展了一种过氧化物诱导的2-芳基苯并咪唑衍生物,在温和条件下经历自由基环化反应,一步合成了系列骨架多样性的苯并咪唑并异喹啉酮化合物的新方法.该反应具有底物范围宽泛、官能团兼容性好、步骤经济等特点.机理研究表明该反应经历了碳中心自由基历程.
关键词: 自由基, 碳环化, 多环化合物, 咪唑并异喹啉酮
In this paper, a new peroxide-induced carbon-centered radical relay carbocyclization reaction with 2-arylbenzo-imidazoles is described. This method provides an efficient route to a series of structurally diverse benzimidazole[2,1-a]iso-quinolines under mild conditions in a straightforward manner. The reaction is compatible with a wide substrate scope, excellent functional group tolerance and high step economy. Mechanistic studies suggest that the reaction proceeds through a carbon-centered radical pathway.
Key words: radical, carbocyclization, polycyclic compounds, benzimidazole[2,1-a]isoquinolines
PDF全文下载地址:
点我下载PDF
