摘要/Abstract
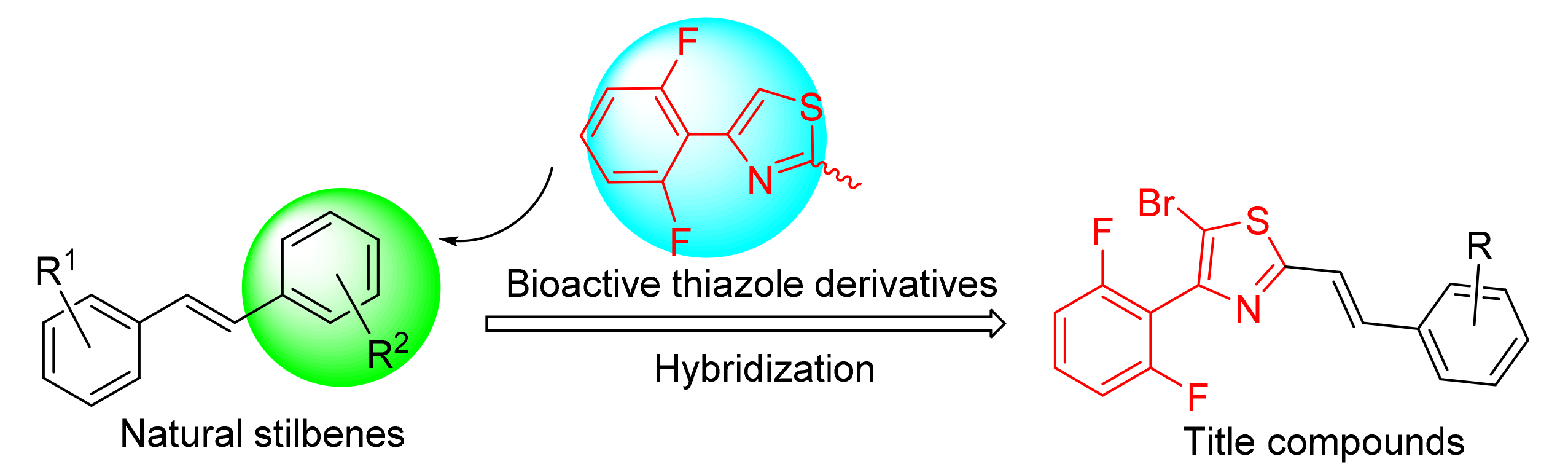
为了寻找新型结构的药物先导化合物,基于天然stilbenes骨架结构,将含氟苯基噻唑环结构与其相杂合,设计合成了一系列新型取代苯乙烯基噻唑类化合物.所有目标化合物结构均经1H NMR、13C NMR和ESI-HRMS表征确证.离体真菌抑制活性结果表明,在100 mg/L浓度下部分化合物对小麦赤霉病菌、玉米小斑病菌和黄瓜蔓枯病菌表现出中等抑制活性,其中(E)-5-溴-4-(2,6-二氟苯基)-2-(4-三氟甲基苯乙烯基)噻唑(6p)对小麦赤霉病菌的抑制率达到86.7%;采用Top1介导的DNA松散实验测试了化合物对拓扑异构酶I(Top1)的抑制活性,结果表明在50 μmol·L-1浓度下,所有化合物对Top1均表现出一定程度的抑制活性,其中(E)-5-溴-4-(2,6-二氟苯基)-2-(2-氯苯乙烯基)噻唑(6k)的抑制活性较好,其在0.2 μmol·L-1浓度下仍对Top1呈现出一定程度的抑制活性.
关键词: 二苯乙烯, 苯乙烯基噻唑, 合成, 生物活性
In order to find novel drug leads, a series of natural stilbene-inspired substituted styrylthiazole derivatives were designed and synthesized by hybridization of the structures of both bioactive 2,6-difluorophenylthiazole moiety and stilbene. The structures of the title compounds were confirmed by 1H NMR, 13C NMR and ESI-HRMS. The in vitro antifungal bioassay results indicated that some compounds showed moderate inhibition activity against FusaHum graminearum, Helminthosporium maydis and Mycosphaerella melonis at 100 μg/mL, and the inhibition rate of (E)-5-bromo-4-(2,6-difluorophenyl)-2-(4-tri-fluoromethylstyryl)thiazole (6p) against FusaHum graminearum reached 86.7%. These compounds were also screened for their topoisomerase I inhibitory activity using Top1-mediated relaxation assay. The results showed that all of them exhibited certain Top1 inhibitory activity at 50 μmol·L-1, and amongst them (E)-5-bromo-4-(2,6-difluorophenyl)-2-(2-chlorosty-ryl)thiazole (6k) displayed promising Top1 inhibitory activity, which still remained certain activity at 0.2 μmol·L-1.
Key words: stilbene, styrylthiazole, synthesis, biological activity
PDF全文下载地址:
点我下载PDF
