摘要/Abstract
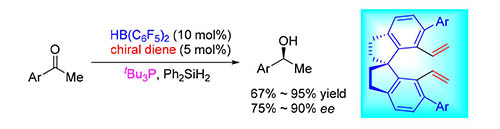
受阻路易斯酸碱对(frustrated Lewis pairs,FLPs)是目前合成化学的前沿挑战性研究领域之一,为非金属催化的氢化和Piers-type硅氢化反应提供了非常有效的途径.近年来,相关研究取得了重要的研究进展,但是相应的不对称反应发展比较缓慢.缺乏高效、高选择性的手性催化剂仍然是制约这一领域快速发展的重要因素.手性螺环是配体设计中的优势骨架.基于前期所发展的联萘骨架手性FLP催化剂及其在不对称催化氢化和硅氢化反应的应用,设计并合成了基于手性螺环骨架的手性双烯,通过与HB(C6F5)2的硼氢化反应原位制备了新型手性硼烷路易斯酸.利用其与三叔丁基膦形成的手性受阻路易斯酸碱对催化剂,成功地实现了简单酮的不对称Piers-type硅氢化反应,反应的对映选择性最高可达90%.
关键词: 不对称催化, 不对称硅氢化, 受阻路易斯酸碱对, 手性螺二烯, 酮
The chemistry of frustrated Lewis pairs (FLPs) is among the challenging frontiers of synthetic chemistry, which provides a powerful approach for metal-free catalytic hyrogenations and Piers-type hydrosilylations. In recent years, a significant progress has been made in this field. However, the deveopment of asymmetric reactions is still sluggish. The lacks of highly effective and enantioselective chiral FLP catalysts represent the key issue. C2-symmetric 1,1'-spirobiindane is one privileged framework in chiral ligands and catalysts. On the basis of chiral binaphthyl diene-derived frustrated Lewis pairs (FLPs) developed by our group, in this work, we designed and synthesized a novel class of chiral spiro dienes, which could further react with Piers' borane via the hydroboration reaction to generate chiral boranes in situ. With the combination of chiral borane and tri-tert-butylphosphine as an FLP catalyst, an asymmetric Piers-type hydrosilylation of simple ketones was successfully realized to give the desired secondary alcohols with up to 90% ee.
Key words: asymmetric catalysis, asymmetric hydrosilylation, frustrated Lewis pairs, chiral spiro dienes, ketones
PDF全文下载地址:
点我下载PDF
